Laminar Flow-based Assays to Investigate Leukocyte Recruitment on Cultured Vascular Cells and Adherent Platelets
Summary
Leukocytes avidly interact with vascular cells and platelets after vessel wall injury or during inflammation. Here, we describe a straightforward laminar flow-based assay to characterize the molecular mechanisms that underlie the interactions between leukocytes and their cellular partners.
Abstract
The recruitment of leukocytes upon injury or inflammation to sites of injury or tissue damage has been investigated during recent decades and has resulted in the concept of the leukocyte adhesion cascade. However, the exact molecular mechanisms involved in leukocyte recruitment have not yet been fully identified. Since leukocyte recruitment remains an important subject in the field of infection, inflammation, and (auto-) immune research, we present a straightforward laminar flow-based assay to study underlying mechanisms of the adhesion, de-adhesion, and transmigration of leukocytes under venous and arterial flow regimes. The in vitro assay can be used to study the molecular mechanisms that underlie the interactions between leukocytes and their cellular partners in different models of vascular inflammation. This protocol describes a laminar flow-based assay using a parallel-flow chamber and an inverted phase contrast microscope connected to a camera to study the interactions of leukocytes and endothelial cells or platelets, which can be visualized and recorded then analyzed offline. Endothelial cells, platelets, or leukocytes can be pretreated with inhibitors or antibodies to determine the role of specific molecules during this process. Shear conditions, i.e. arterial or venous shear stress, can be easily adapted by the viscosity and flow rate of the perfused fluids and the height of the channel.
Introduction
Upon injury, inflammation, or infection, leukocytes quickly respond to pathogen- or damage-associated molecular patterns (PAMPs, DAMPs), change into an activated state, and move out of the blood stream to sites of inflammation and tissue damage. The ability of leukocytes to interact with their cellular and molecular environment is essential for their correct function as immune cells, as highlighted by genetic disorders such as leukocyte adhesion deficiency1. Leukocyte adhesion has been the subject of intense investigation during the past decades and this has resulted in the concept of the leukocyte adhesion cascade in the early 1990s2,3. Leukocyte adhesion is initiated by the selectin-mediated capture of leukocytes to the endothelium, causing the cells to roll over the vascular surface. This rolling enables leukocytes to scan for endothelium-bound migratory cues, e.g., chemokines, which induce the activation of integrins. Subsequently, the activated integrins mediate the binding to endothelial ligands, resulting in firm leukocyte arrest. Leukocytes may subsequently prepare to extravasate by crawling and spreading, before penetrating the endothelial monolayer and transmigrating into the underlying tissue. The basic concept of the canonical leukocyte cascade has remained largely unchanged since its introduction, with some intermediate steps added4. Nevertheless, the exact molecular mechanisms and the roles of the many players involved in leukocyte recruitment have not been clarified thus far, and leukocyte recruitment remains an important subject in the field of infection, inflammation, and (auto-) immune research.
For example, during vascular inflammatory diseases such as atherosclerosis, increased leukocyte recruitment into the vessel wall drives plaque development. Unstable atherosclerotic plaques might rupture, leading to massive activation of platelets and the coagulation system, and subsequently to occlusion of the vessel5. This may result in severe cardiovascular outcomes such as myocardial infarction or stroke. In addition, endothelial denudation as it occurs clinically, e.g. after stenting of a coronary artery, leads to a multitude of interactions of leukocytes and platelets to the exposed vessel wall interior (e.g., matrix components and smooth muscle cells) and of leukocytes with platelets covering the vascular injury. These interactions are important for the further development of the disease as monocyte-platelet interactions might drive neointima formation6,7. In addition, platelet-leukocyte interactions mediated by leukocyte integrin Mac-1 (αMβ2) and platelet GPIbα have recently been identified as novel drivers of thrombosis in mice8.
Given the wide availability of human and animal blood as a source of leukocytes and platelets for research, and the broad spectrum of isolated matrix molecules and immortalized cell lines of leukocyte and vascular origin, it is feasible to simulate leukocyte interactions under flow in a laboratory setting, using specially designed flow perfusion chambers. Many variants have been designed over the past decades, ranging from vacuum-driven to self-adhesive perfusion chambers. All variants have in common that the immobile part (e.g., cultured vascular cells or matrix proteins) is assembled into a larger leak-proof chamber equipped with a pre-defined channel enabling perfusion of fluids over the immobile part. In addition, advances in molding technology enabled the development of custom-made solutions based on silica polymers9. The viscosity and flow rate of the perfused fluids and the height of the channel mainly determine the shear stress characteristics of the flow perfusion device10. In this article, we present an in vitro method to study underlying mechanisms of the adhesion, de-adhesion, and transmigration of leukocytes under venous and arterial flow regimes. The advantage of the methods presented here is that they can be performed using a common camera-connected fluorescence microscope, and do not require a experimenters to possess high technical proficiency. The in vitro assay can be manipulated in many ways (e.g., by adding inhibitors or blocking antibodies), and is thus applicable in different models of vascular inflammation and allows the investigation of adhesion protein functions or the evaluation of specific compounds.
Protocol
All methods described here have been approved by the Medical Ethical and Animal Ethical Boards of Maastricht University.
1. Flow-Based Assay with Human Cells
- Isolation of platelets from human blood
- Draw venous blood in citrate (3.2%) anticoagulant.
- Add 1/15 volume of Acid Citrate Dextrose (ACD: 80 mM trisodium citrate, 52 mM citric acid and 183 mM glucose) to the blood.
- Centrifuge at 350 x g without brake for 15 min to obtain platelet rich plasma (PRP).
- Transfer the supernatant to a 15 mL conical tube and add 1/20 volume of ACD.
- Centrifuge at 1,110 g without brake for 15 min to obtain platelet poor plasma (PPP).
- Discard the supernatant. Cut off the end of the pipet tip (P 1000), making it wide bore, which aids in the prevention of platelet activation, and carefully suspend the platelet pellet in the same volume as PRP in platelet buffer pH 6.6 (PB: 136 mM NaCl, 10 mM Hepes, 2.7 mM KCl, 2 mM MgCl2, 0.1% bovine serum albumin and 0.1% glucose). Add 1/20 volume of ACD and gently mix.
- Pellet the platelets at 1,110 g without brake for 15 min.
- Discard the supernatant and carefully suspend the platelets as above in 1 mL platelet buffer (pH 7.5).
- Measure the platelet concentration in a 1/10 dilution with an automated hematology analyzer.
- Adjust the volume with platelet buffer (pH 7.5) to achieve a count of 2 x 107/mL.
- Isolation of polymorphonuclear cells from human blood (adapted from Brinkmann et al.11 )
- Draw 24 mL of blood in heparin (10 U/mL) as anticoagulant.
- Add 6 mL of 1.077 g/mL density polysucrose-sodium diatrizoate medium (see Table of Materials) to a 15-mL conical tube, and carefully layer 5-6 mL whole blood on top.
- Centrifuge for 20 min at 800 x g without brake.
- Aspirate and discard the clear yellowish top layer and transfer the lower reddish phase containing granulocytes into fresh 15-mL conical tubes. Wash cells by adding PBS containing 2 mM EDTA to a final volume of 14 mL, and centrifuge for 10 min at 300 x g without brake.
- In the meantime, prepare a density gradient medium (e.g., percoll, see Table of Materials) working solution by mixing 18 mL density gradient medium with 2 mL 10x PBS stock solution. Dilute this working solution with PBS (1x) to obtain 4.25 mL each of 85%, 80%, 75%, 70%, and 65% gradient medium solutions.
- Prepare 2 conical tubes with the density gradient by layering 2 mL of every medium percentage on top of each other. Start with the highest concentration and continue in decreasing order. Mark the layers on the tube with a water-proof marker.
- Carefully layer 2 mL of the cell suspensions onto each of the two prepared gradients.
- Centrifuge for 20 min at 800 x g without brake in a carefully balanced centrifuge.
- After centrifugation, remove the top layer and most of the 65% layer (first ring) with PBMCs, and collect into new tubes the white remaining interphases until the 85% layer (second ring, PMN).
- Wash cells by filling up the tubes with PBS 2 mM EDTA, and centrifuge for 10 min at 300 x g without brake.
- Remove the supernatant and suspend the sedimented cells (typically ≥95% are PMN) in 1 mL of Hank's buffer (pH 7.45), containing 10 mM Hepes and 0.2% human albumin (Assay buffer).
- Count the cells using a hemocytometer and suspend cells at 1 x 106/mL.
- Continue with fluorescent labeling and perfusion of leukocytes (step 3.3).
- Preparation of adherent platelet- and vascular cell-monolayers
- Cell culture dish preparation (cultured cells)
- Pre-coat the 35 mm cell culture dishes with 1 mL of diluted rat tail collagen (30 µg/mL diluted in PBS filtered over a 0.2 µm filter).
- Incubate for 30 min at 37 °C, discard the collagen solution, and wash the dish once with PBS.
- Culture of vascular cells in culture dishes
- Detach primary (e.g., HUVEC, HAoEC, HAoSMC) or immortalized vascular cells (e.g., EA.Hy926, SVEC) grown to confluence in a T75 flask with 1.5 mL cell detachment solution (e.g., accutase). Add 4.5 mL of appropriate growth medium to neutralize the cell detachment solution and determine the cell concentration with a cell counting chamber. Suspend the cells at 0.5 x 105 cells/mL in appropriate growth medium (e.g., complete endothelial cell growth medium).
- Add 2 mL of cell suspension to each 35 mm dish. Culture the cells in a humidified incubator at 37 °C with 5% CO2 until confluence (taking 24–48 h, depending on the cell type).
- Glass coverslip preparation (adherent platelets)
- Immerse the glass coverslip once in HCl-EtOH (1.2 M / 50% v/v).
- Rinse the coverslip 2 times in ultrapure water.
- Dry the coverslip under N2.
- Pre-coat the glass coverslip with 100 µL, in accordance with the position of the channel of the perfusion chamber of diluted rat tail collagen type I (30 µg/mL diluted in PBS filtered over a 0.2 µm filter).
- Incubate for 30 min at RT, discard the collagen solution, and wash the coverslip 3x with PBS.
- Block the collagen coated coverslip with 1% BSA in HEPES for 0.5 h at RT.
- Wash and dry the coverslip and mount it into the perfusion chamber. Isolate platelets from human or mouse blood as outlined in step 1.1 or 2.1, respectively.
- Immobilization of platelets
- Add 70 µL of a 2 x 107/mL platelet suspension per channel, and incubate for 1.5 h at 37 °C and 5% CO2.
- Carefully replace non-adherent platelets with blocking solution (5% BSA in HEPES) for at least 30 min at RT. Continue with fluorescent labeling and perfusion of leukocytes (step 3.3).
- Stimulation of vascular cell monolayer
- Stimulate the vascular cells by replacing the media with fresh media containing 10 ng/mL tumor necrosis factor α (TNFα) for 4 h at 37 °C and 5% CO2.
- Cell culture dish preparation (cultured cells)
2. Flow-based Assay with Murine Cells
- Isolation of platelets from mouse blood
- Draw blood by cardiac puncture using a 21 G needle (~1 mL) from anesthetized (ketamine 80 mg/kg and medetomidine 0.3 mg/kg) 6–8 week-old mice (C57Bl/6) in citrate (3.2 %) as anticoagulant.
- Add 1/6 volume of ACD.
- Centrifuge at 220 x g, medium brake for 5 min to obtain platelet rich plasma (PRP).
- Transfer the supernatant (~600 µL) plus 1/3 of red blood cells to a new tube
- Centrifuge at 95 x g without brake for 6 min.
- Transfer the supernatant (PRP) and measure the platelet concentration in a 1/10 dilution in Tyrode's buffer (TH buffer: 136 mM NaCl, 5 mM Hepes, 2.7 mM KCl, 0.42 mM NaH2PO4*H2O, 2 mM MgCl2, 0.1% bovine serum albumin and 0.1% glucose) with an automated hematology analyzer.
- Wash the platelets by adding 1/25 volume of ACD + 0.1 U/mL apyrase, and centrifuge at 2,870 g, medium brake for 2 min.
- Discard the supernatant and add 600 µL TH buffer, 1/10 volume of ACD, and 0.1 U/mL apyrase. Cut off the pipet tip (P 1000), making it wide bore, which aids in the prevention of platelet activation, and gently suspend the pellet.
- Centrifuge at 2,870 g, medium brake for 2 min
- Discard the supernatant and gently suspend the platelets in TH buffer.
- Adjust the volume with TH buffer to achieve a count of 2 x 107/mL.
- Preparation of adherent platelet monolayers
- Prepare glass coverslip (adherent platelets) as per 1.3.3.
- Immobilization of platelets
- Add 100 µL of a 2 x 107/mL platelet suspension per channel, and incubate for 1.5 h at 37 °C and 5% CO2.
- Carefully replace non-adherent platelets with blocking solution (5% BSA in HEPES) for at least 30 min at RT. Continue with fluorescent labeling and perfusion of leukocytes (step 3.3)
- Stimulation of mouse platelet monolayer
- Prior to perfusion of leukocytes (step 3.3), stimulate the platelets by perfusion of thrombin (0.5 nM) in HHHSA buffer for 3 min at 37 °C.
3. Fluorescent Labeling and Perfusion of Leukocytes (PMN or THP-1 for Human and RAW264.7 or Primary Mouse Monocytes for Murine Flow-Based Assay)
- Fluorescent labeling
- Label the leukocytes (1 x 106/mL) with the cell-permeant green fluorescent nucleic acid stain (1 µM). Incubate the cells for 30 min at 37 °C.
- Wash the cells with PBS by centrifugation at 300 x g for 5 min, and suspend cells to a concentration of 0.5 x 106 cells/mL (5 mL per test condition, depending on flow rate) in assay buffer.
- Flow chamber assay preparation
- For leukocyte adhesion on a cell monolayer, discard the cell culture medium from the dish, and assemble it into the flow chamber. Connect tubing to the syringe. For leukocyte adhesion on immobilized platelets, connect the micro slide to the syringe. Pre-warm a water bath to 37 °C.
- Take a perfusion syringe (50 mL), install syringe on the syringe holder, and set the pump on withdraw mode. Set the pump volume to 0 mL, and set the diameter to 26.70 mm for the 50 mL syringe.
- Connect an elbow luer connector to one end of the tubing. Place a luer lock coupler to the syringe and connect the tubing with the elbow luer connector to the luer lock coupler. Connect the free tubing end to the flow chamber.
- Leukocyte perfusion and adhesion
- For leukocyte adhesion on a cell monolayer, discard the cell culture medium from the dish, and assemble it into the flow chamber. Connect tubing to the syringe. For leukocyte adhesion on immobilized platelets, connect the micro slide to the syringe.
- For leukocyte perfusion and adhesion over a vascular- or platelet monolayer, place the second tubing into a 50 mL conical tube containing assay buffer and fill tubing with 1 mL pipet, then squeeze the tubing and connect it to a flow chamber.
- Prior to leukocyte perfusion, add 3 mM CaCl2 and 2 mM MgCl2 to the cell suspension and incubate for 5 min at 37 °C.
- Perfuse assay buffer prior to perfusion of cells to remove possible air from the chamber and tubing.
- Close tube ends by squeezing them tight and switch tubing from assay buffer to cell suspension, preventing air bubbles from being trapped. Perfuse cells with appropriate flow rate/shear stress until the first cells arrive.
- Perfuse cells with appropriate flow rate/shear stress for 2 min, and capture at least 6 pictures between 2–6 min of rolling and adherent cells with an inverted phase contrast/fluorescence microscope (e.g., EVOS-FL) using 100X magnification connected to a digital CCD or CMOS camera.
NOTE: Flow rate/shear stress depends on flow chamber dimensions and viscosity of perfusate. For perfusion chamber used here (see Table of Materials):
τ=η*97.1*Φ
τ: shear stress (1 dyn/cm2)
η: viscosity (0.015 dyn*s/cm2)
Φ: Flow rate (0.67 mL/min)
- Leukocyte de-adhesion
- For de-adhesion, switch tubing from cell suspension to assay buffer by squeezing the tubing, preventing air bubbles from becoming trapped.
- Detach cells by perfusion with assay buffer with an appropriate flow rate/shear stress (See 3.3.7 Note).
- Leukocyte transmigration
- For leukocyte transmigration, perfuse leukocytes (step 3) until an adequate quantity of leukocytes adhere (~50 cells/view field).
- Replace leukocyte suspension with assay buffer.
- Record transmigrating cells by time lapse every 10–15 s for 30–60 min at 37 °C.
Representative Results
To study endothelial-leukocyte adhesion, fluorescently labeled THP-1 cells were perfused over a TNFα- or non-stimulated endothelial monolayer for 2 min at 3 dyne/cm2. The total number of adherent monocytic cells was determined after 2 min of perfusion by capturing 6 independent fields over a period of 2-6 min. The adherent cells were quantified in at least 6 pictures captured with an inverted phase contrast/fluorescence microscope (e.g., EVOS-FL) using 10X magnification connected to a digital CCD or CMOS camera and the average was expressed as adherent cells/mm2. Upon endothelial stimulation with TNFα, THP-1 arrest significantly increased compared to unstimulated endothelium. Representative micrographs and the quantification of firmly adherent THP-1 to non- or TNFα-stimulated endothelial cells is presented in Figure 1. Platelet-leukocyte adhesion was assessed by perfusion of fluorescently labeled neutrophils over a TRAP-6- or non-stimulated platelet monolayer for 2 minutes at 1 dyne/cm2. Adherent neutrophils were quantified as mentioned above. TRAP-6 stimulation significantly increased the adhesion of neutrophils relative to unstimulated platelets. Representative videos and the quantification of firmly adherent neutrophils to non- or TRAP-6-stimulated platelet monolayer is presented in Figure 1B.
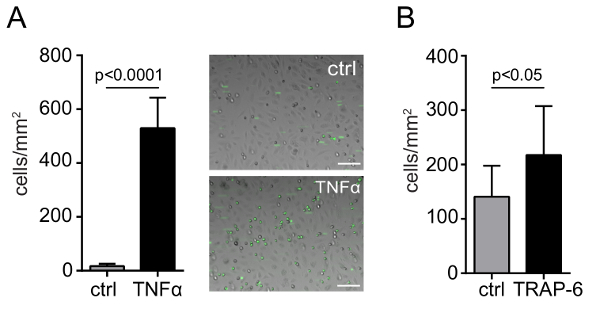
Figure 1: Endothelial- and platelet-leukocyte adhesion under flow conditions. Quantification and representative micrographs of adhesion of human monocytic cells (THP-1) to endothelial cells (HUVEC) seeded on collagen-coated culture dishes under flow conditions, with or without TNFα activation (10 ng/mL) (A). Quantification and representative micrographs of adhesion of human neutrophils to human platelets immobilized on collagen-coated glass slides under flow conditions, without or with TRAP-6 activation (50 µM) (B). P values were calculated by the unpaired t-test (n = 3; mean ± SD). Scale bar = 100 µm. Please click here to view a larger version of this figure.
Moreover, this assay can be implemented to study the role of adhesion molecules, such as platelet junctional adhesion molecule A (JAM-A). The adhesion of RAW264.7 mouse monocyte/macrophage to a platelet monolayer from JAM-A (trJAM-A+/+) and JAM-A-deficient (trJAM-A-/-) mice was assessed in our former study7. As observed therein, platelet JAM-A deficiency increased the adhesion of mouse monocytes to the platelet monolayer compared to wild type mice (Figure 2A-B). Alternatively, primary mouse neutrophils can be isolated from mice as described12.
To study underlying mechanisms, such as adhesion molecules involved in platelet-leukocyte interactions, specific surface receptors can be blocked. In the aforementioned study, blocking of platelet GPIbα and leukocyte αMβ2 significantly decreased monocyte adhesion, identifying a role for the GPIbα-αMβ2 axis in the increased monocyte recruitment to JAM-A-/- platelets (Figure 2A-B). Moreover, cell detachment experiments can be performed to investigate shear stress dependence of platelet-leukocyte interactions. For the measurement of flow-dependent detachment of monocytes, shear stress was incrementally increased from 0 to 20 dynes/cm2. In this study, the adhesion of RAW264.7 cells on JAM-A-/- platelets was more resistant to shear stress compared to JAM-A+/+ platelets (Figure 2C).
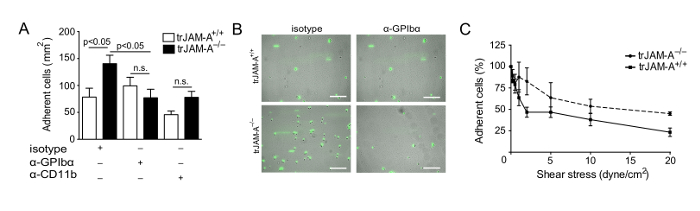
Figure 2: JAM-A-deficiency promotes flow-resistant monocytic cell adhesion. Adhesion of mouse monocyte/macrophage RAW264.7 cells to platelets from JAM-A+/+ and JAM-A-/- mice immobilized on collagen-coated glass slides under flow conditions, with or without thrombin activation (IIa, 0.5 nM) in the presence of indicated inhibitors (A). Representative micrographs of mouse monocyte/macrophage RAW264.7 cells adherent to JAM-A+/+ and JAM-A-/- platelets after treatment with isotype control or anti-GPIbα antibodies (B). Cell adhesion expressed as percentage of initially adherent cells under incrementally increased shear stress (dyne/cm2) on immobilized platelets after thrombin activation (IIa, 0.5 nM). P values were calculated by ANOVA with Tukey's post-test (n=3-5; mean ± SEM). Scale bar = 100 µm. This figure has been modified from Zhao et al.7 Please click here to view a larger version of this figure.
The prolonged recording time (30–60 min) of single fields following leukocyte perfusion and adhesion allows the study of leukocyte crawling and spreading behavior and final transmigration across the endothelial layer. Within this study, we have analyzed the transmigration of primary human monocytes (isolated with the monocyte isolation kit, according to the manufacturer's instructions as described13) across an endothelial layer. As presented in Figure 3, following firm arrest to the endothelium, monocytes prepare to extravasate by crawling and spreading until the preferred site for transmigration is reached. Subsequently, monocytes penetrate the endothelial cell barrier either by para- or transcellular migration. Transmigrating cells were defined as cells that spread out over endothelial cells, by extending their pseudopodia and passing through the endothelial barrier, thereby decreasing their brightness.
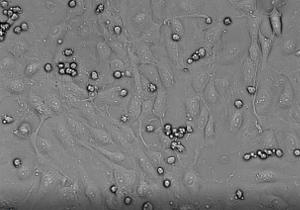
Figure 3: In vitro transmigration of primary monocytes across an endothelial monolayer. Monocyte crawling and spreading and final transmigration across the endothelium was recorded every 15 s for 30 min. Please click here to view this video. (Right-click to download.)
Discussion
This in vitro assay is a straightforward method to investigate underlying mechanisms of leukocyte recruitment during vascular inflammation, but there are some critical points to be noted. The first requirement for successfully performing this assay is the perfusion of leukocytes over an intact and confluent vascular or platelet monolayer. This can be achieved by prior coating of the surfaces with collagen type I. In general, when working with primary vascular cells, it is important to gently detach cells using the recommended collagenase-containing detachment solution (see the Table of Materials), which is less rigorous, but as effective as trypsin. For platelet monolayers, it is important to gently wash platelets during isolation to prevent platelet activation and aggregation.
Another critical consideration is maintaining the continuity of the pump withdrawal mode used for the perfusion, since a discontinuity leads to disturbed flow rate and shear conditions. Thus, this results in inaccuracy of actual shear stress and, subsequently, to misinterpretation of physiologically shear-dependent processes. This can be prevented by regular maintenance of the pump. Additionally, critical for the continuity during perfusion is the use of a fresh syringe for every experiment. Since the texture of the rubber within the syringe changes with increasing use and time in between experiments, it prevents a continuous withdrawal.
A further critical step is the prevention of air becoming trapped during the perfusion of leukocytes, since air will subsequently tear cells off from the surface and alter shear conditions. This can be prevented by rinsing all tubing prior to use with ultra-pure water and then with assay buffer. Also, when connecting tubing from assay buffer to cell suspension, or from one condition to the other, it is important to maintain liquid interfaces during connection. This can be achieved by squeezing the tubing or by not altering the liquid levels within tubing. Further, the fluid volume of the perfusate should always be sufficient to prevent the level falling below the tubing end and, thus, air being taken up in the system.
A possible limitation of this assay might be the use of cultured cells that are maintained in an artificial environment, i.e. cultured on a plastic surface surrounded by a medium that does not accurately model the in vivo state. Although the physiochemical and physiological environment can be precisely controlled, cultured cells have a rapid growth rate, which increases genetic variation and thus the heterogeneity of cells within a population. Moreover, the vascular- or platelet monolayer consists of a single cell type only. In particular, when investigating endothelial-leukocyte interactions, i.e., transmigration of leukocytes across the endothelial cell barrier, the physiologically relevant role of the underlying endothelial-cell basement membrane and pericytes cannot be taken into account. Alternatively, it might be interesting to create a multicellular environment by implementing 3D-cell culturing techniques in this assay.
To summarize, taking these points into account, the laminar flow-based assay can be efficiently applied in different models of vascular inflammation. In particular, it can be easily modified to address specific research questions, i.e. the adjustment of the shear stress by either varying the flow rate of the perfusate, the fluid viscosity, or the channel height and width. Particularly, the latter is of importance since changes in flow chamber dimensions reduce fluid volume or cells needed. Further, different fluorescence labeling of leukocytes, or of specific molecules of vascular or platelet monolayers can be used to study cell-cell interactions.
Divulgazioni
The authors have nothing to disclose.
Acknowledgements
We thank Drs. Martin M. Schmitt and Line Fraemohs. This work was supported by the Netherlands Foundation for Scientific Research (ZonMW VIDI 016.126.358, the Landsteiner Foundation for Blood Transfusion Research (LSBR Nr. 1638) and Deutsche Forschungsgemeinschaft (SFB1123/A2) awarded to R.R.K.
Materials
| Inverted fluorescence microscope e.g. EVOS-FL | Life Technologies Europe bv | ||
| Pump e.g. model: 210-CE | world precision instruments | 78-9210W | |
| Falcon 35 mm TC-Treated Easy-Grip Style Cell Culture Dish | corning | 353001 | |
| 50 mL syringe | Becton Dickinson | 300137 | |
| Silicone tubing | VWR | 228-0700 | |
| Elbow Luer Connector Male | Ibidi | 10802 | |
| Female Luer Lock Coupler | Ibidi | 10823 | |
| Flow chamber | University Hospital RWTH Aachen | Patent DE10328277A1: Baltus T, Dautzenberg R, Weber CPD. Strömungskammer zur in-vitro-Untersuchung von physiologischen Vorgängen an Zelloberflächen. 2005. | |
| Ibidi sticky slide VI 0.4 | Ibidi | 80608 | |
| Coverslips for sticky slides | Ibidi | 10812 | |
| Collagen Type I, rat tail | Life Technologies Europe bv | A1048301 | |
| Recombinant Human TNF-α | Peprotech | 300-01A | |
| Thrombin Receptor Activator for Peptide 6 (TRAP – 6) | Anaspec / Eurogentec | 24191 | |
| SYTO 13 green fluorescent nucleic acid stain | Life Technologies Europe bv | S7575 | |
| Hanks’ Balanced Salt Solution 10x | Sigma-Aldrich Chemie | H4641 | |
| HEPES buffer solution 1M, liquid | Life Technologies Europe bv | 15630049 | |
| BUMINATE Albumin, 25% | Baxter | 60010 | |
| Water for injection | B. Braun | 3624315 | |
| Calcium chloride dihydrate | Merck | 102382 | |
| Magnesium chloride hexahydrate | Sigma-Aldrich Chemie | M2670 | |
| Apyrase | Sigma-Aldrich Chemie | A6535 | |
| Primary Human Umbilical Vein Endothelial Cells (HUVEC) | Promocell GmbH | C-12203 | |
| Endothelial Cell Growth Medium (Ready-to-use) | Promocell GmbH | C-22010 | |
| Primary Human Aortic Endothelial Cells (HAoEC) | ATCC | PCS-100-011 | |
| Vascular Cell Basal Medium | ATCC | PCS-100-030 | |
| Endothelial Cell Growth Kit-BBE | ATCC | PCS-100-040 | |
| Primary Human Aortic Smooth Muscle Cells (HAoSMC) | ATCC | PCS-100-012 | |
| Vascular Smooth Muscle Cell Growth Kit | ATCC | PCS-100-042 | |
| Human endothelial cell line, EA.hy926 | ATCC | CRL-2922 | |
| Dulbecco's Modified Eagle's Medium (DMEM) | ATCC | 30-2002 | |
| Mouse endothelial cell line, SV 40 transformed (SVEC4-10) | ATCC | CRL-2181 | |
| Human Monocytic cell line, THP-1 | DSMZ | ACC-16 | |
| RPMI 1640 with Ultra-Glutamine | Lonza | BE12-702F/U1 | |
| Mouse monocyte/macrophage RAW264.7 | ATCC | TIB-71 | |
| Dulbecco's Modified Eagle's Medium (DMEM), high glucose | Gibco | 11965092 | |
| Accutase | Promocell GmbH | C-41310 | |
| Percoll | Sigma-Aldrich Chemie | P1644 | |
| Histopaque 1119 | Sigma-Aldrich Chemie | 11191 | |
| 10x PBS | Life Technologies Europe bv | 14200075 | |
| BD Vacutainer Plastic Blood Collection Tubes with Sodium Heparin | Becton Dickinson | 367876 | |
| VACUETTE TUBE 9 ml 9NC Coagulation sodium citrate 3.2% | Greiner Bio | 455322 | |
| HCl/EtOH mixture (1.2 mol/L HCl and 50% ethanol) in water | Sigma-Aldrich Chemie | Prepare by mixing 0.3L HCl (4 mol/L) with 0.7L of 70% v/v ethanol in a fume hood |
Riferimenti
- Dinauer, M. C. Primary immune deficiencies with defects in neutrophil function. American Society of Hematology. 2016 (1), 43-50 (2016).
- Butcher, E. C. Leukocyte-endothelial cell recognition: three (or more) steps to specificity and diversity. Cell. 67 (6), 1033-1036 (1991).
- Springer, T. A. Traffic signals for lymphocyte recirculation and leukocyte emigration: the multistep paradigm. Cell. 76 (2), 301-314 (1994).
- Nourshargh, S., Alon, R. Leukocyte migration into inflamed tissues. Immunity. 41 (5), 694-707 (2014).
- Legein, B., Temmerman, L., Biessen, E. A. L., Lutgens, E. Inflammation and immune system interactions in atherosclerosis. Cellular and Molecular Life Sciences. 70 (20), 3847-3869 (2013).
- Wang, Y., Sakuma, M., et al. Leukocyte engagement of platelet glycoprotein Ibalpha via the integrin Mac-1 is critical for the biological response to vascular injury. Circulation. 112 (19), 2993-3000 (2005).
- Zhao, Z., Vajen, T., et al. Deletion of junctional adhesion molecule A from platelets increases early-stage neointima formation after wire injury in hyperlipidemic mice. Journal of cellular and molecular medicine. , (2017).
- Wang, Y., Gao, H., et al. Leukocyte integrin Mac-1 regulates thrombosis via interaction with platelet GPIbα. Nature communications. 8, 15559 (2017).
- Fujii, T. PDMS-based microfluidic devices for biomedical applications. Microelectronic Engineering. 61-62, 907-914 (2002).
- Son, Y. Determination of shear viscosity and shear rate from pressure drop and flow rate relationship in a rectangular channel. Polymer. 48 (2), 632-637 (2007).
- Brinkmann, V., Laube, B., Abu Abed, U., Goosmann, C., Zychlinsky, A. Neutrophil extracellular traps: how to generate and visualize them. Journal of visualized experiments : JoVE. (36), e1724 (2010).
- Swamydas, M., Lionakis, M. S. Isolation, purification and labeling of mouse bone marrow neutrophils for functional studies and adoptive transfer experiments. Journal of visualized experiments : JoVE. (77), e50586 (2013).
- Schmitt, M. M. N., Megens, R. T. A., et al. Endothelial junctional adhesion molecule-a guides monocytes into flow-dependent predilection sites of atherosclerosis. Circulation. 129 (1), 66-76 (2014).

