A Method for the Measurement of Salivary Gland Function in Mice
Summary
Salivary gland hypofunction is a frequent consequence of autoimmune disease and radiation therapy. Reproducible evaluation of salivary gland function in mouse models of these diseases is a technical challenge. Here, a simple method for accurate and reproducible measurement of saliva production in mice is described.
Abstract
Patients with Sjögren's syndrome, an autoimmune disease affecting the exocrine glands, develop salivary gland inflammation and have reduced saliva production. Similarly, saliva production is severely compromised in patients receiving radiation treatment for head and neck cancers. Rodent models, developed to mimic these clinical conditions, facilitate an understanding of the disease pathogenesis and allow for the development of new therapeutic strategies. Therefore, the ability to accurately, reproducibly, and repeatedly measure salivary gland function in animal models is critical. Building on procedures previously described in the literature, a method was developed that meets these criteria and was used to evaluate salivary gland function in mice. An additional advantage of this new method is that it is easily mastered, and has little inter-operator variation. Salivary gland function is evaluated as the amount (weight or volume) or rate (mL/min) of saliva produced in response to pilocarpine stimulation. The collected saliva is a good source for the analyses of protein content, immunoglobulin concentrations, and other biomolecules.
Introduction
The salivary glands produce saliva in response to a variety of neurological and mechanical stimuli1. The stimuli are carried through the sympathetic and parasympathetic nervous system to the adrenergic and cholinergic receptors in the gland. Pilocarpine is a cholinergic, para-sympathomimetic agent that acts predominantly on muscarinic receptors. In the salivary gland, it induces the production of saliva by acting on the muscarinic acetylcholine receptor M31. Saliva production following pilocarpine administration is an indicator of the ability of the salivary glands to respond to stimulation and is commonly used as a measure of salivary gland function.
Accurate measurement of saliva production is critical in the study of salivary gland diseases including Sjögren's syndrome2 and radiation injury following head and neck cancer treatment3. Several different methods have been developed to measure saliva production in rodents. These include direct cannulation of the excretory salivary duct4, the collection of saliva from the oral cavity under vacuum5, and collection using glass capillaries6 or a micropipette7,8,9. Direct cannulation of the salivary duct provides the most accurate and pure saliva. However, this is a technically challenging procedure, and the potential for causing ductal injury precludes repetitive saliva collections from the same animal. Collecting saliva under vacuum can lead to variable results due to drying of the saliva from the tube. This loss is further exaggerated in mice with reduced salivation. Glass capillaries allow the collection of saliva for subsequent analyses, however, changes in viscosity of the saliva secreted in the diseased state prevents efficient filling of the capillary. Further, attempts to sweep the oral cavity to collect residual saliva can lead to injury. The pipet method allows for complete collection, and for the same operator, it is remarkably consistent between experiments. However, for unknown reasons, this method shows significant variation between different operators. Therefore, to allow appropriate comparisons, it becomes imperative that the same operator performs all the experiments related to a specific project. Clearly, this represents a major disadvantage for a laboratory.
To overcome these issues, a procedure was developed that combines saliva collection methods used for humans and rodents. The swab method, described below for measuring pilocarpine-induced saliva volume is simple, reproducible, not influenced by the operator, and can be performed repeatedly in the same animal. In addition, it allows collecting the saliva for subsequent analyses of proteins, immunoglobulins, or other biomolecules.
Protocol
The protocol described below was approved by the Institutional Animal Care and Use Committee and it follows the ethical guidelines established by the National Institutes of Health. All data presented in this report were generated by using female mice that were 10-12 weeks of age. The following strains of mice were used: C57BL/6, BALB/c, DBA1, 129S, and (B6XA/J) F1. All mice were housed in barrier cages (5 animals per cage) in specific pathogen-free conditions and provided feed and water ad libitum.
1. Preparation
- To prepare a stock solution of pilocarpine hydrochloride, weigh 10 mg of the compound and dissolve it in 1.776 mL of sterile isotonic saline by vortexing, to give a stock solution of 5.63 mg/mL. There is no need to sterilize this solution. But if desired, filter it through a 0.2 µM filter. Store this stock solution in multiple aliquots in a -80 oC freezer for up to 3 months. Each frozen stock is for single use. Once thawed, do not refreeze the pilocarpine solution.
- Take 0.6 mL microfuge tubes and carefully punch a small hole in the bottom of each tube with a heated 18-gauge needle. Set these tubes into 2 mL tubes. The tubes need not be sterile.
- Using a sharp, sterile razor blade, cut cylindrical absorbent swabs into pieces about 2 cm in length. Cut each 2 cm piece diagonally to give 2 conical shaped swabs. Place one swab in each of the 0.6 mL microfuge tubes.
- Weigh the 0.6 mL microfuge tube containing the dry swab.
- Transfer the mice to be studied into a new clean cage with water bottles. Keep the mice without food for at least 2 h prior to the start of saliva collection to prevent food particles from contaminating the saliva collected.
NOTE: It is not necessary to keep 1 mouse per cage for this procedure. However, the number of mice placed in each cage depends on the specific institution's rules and regulations for animal use. - Just before the end of 2 h, prepare the working pilocarpine solution (0.0563 mg/mL) by diluting the stock solution 100x in sterile saline. It is not necessary to further filter sterilize this solution. Always keep the working pilocarpine solution on ice.
NOTE: Table 1 gives the amount of pilocarpine to be injected for a range of mouse body weights to give a final dose of 0.375 mg/kg body weight.
2. Procedure
- Weigh each mouse and determine the dose of the anesthetic mix for each mouse using Table 1. Inject the first mouse with the appropriate volume of the anesthetic mix by the intraperitoneal route. Set the timer for 2 min (most mouse strains tested in this protocol go to sleep by 2 min). Ensure that the mouse is properly anesthetized by lack of walking when placed on a flat surface. If the mouse is still walking, wait for additional 2 min before proceeding to the next step. Apply a drop of lubricant ophthalmic ointment to both eyes to prevent drying.
NOTE: In Table 1, the dose of the anesthetic mix is 0.007 mL/g body weight. The optimal range for this procedure is 0.006 to 0.008 mL/g body weight. However, after 4 min, if the mouse is still walking or exhibiting jerky movements, consult with the institutional veterinarian for appropriately increasing the dose of the anesthetic. - Determine the dose of pilocarpine for the mouse using Table 1 and inject the appropriate amount by intraperitoneal route. Set the timer for 2 min and insert the mouse into the 50 mL restrainer tube until the head and ears stick out of the cut end. Place the tube at a 45 degree angle, with the head down, and the ventral surface facing upward. Tape the tube to the procedure board to keep it from moving.
NOTE: Table 1 provides dosage for mice more than 17 g, as this procedure has not been attempted on mice weighing below 17 g. - At the end of 2 min, gently insert a closed pair of micro dissecting forceps into the mouth and lift the lower jaw upwards to open the mouth. Push the forceps 1-2 mm further into the mouth, making sure that the tongue is seen resting on the top arm of the forceps. With another pair of fine forceps, hold the pre-weighed dry swab close to its conical tip.
- Gently slide the conical tip of the swab into the mouth. Withdraw the forceps while leaving the tip of the swab in the oral cavity.
- Grasp the wider end of the swab extending outside of the mouth and rotate it to allow the maximum area of contact with the mouth. Keep the swab in this position for 15 min.
NOTE: This ensures that the swab remains in the mouth for the duration of the collection. Note that the conical tip of the swab acts as a wick and the wider portion of the swab remains outside the mouth. - At the end of 15 min, gently rotate the swab to collect any saliva that has not been absorbed and place the wet swab in the 0.6 mL microfuge tube. Close the tube and set it in the 2 mL tube placed on ice.
NOTE: At this time, the oral mucosa will appear completely dry. The mouse can now be moved back into its cage for recovery from anesthesia. - Transfer the mouse in the cage. Place moistened food pellets in the cage after saliva collection to help faster rehydration.
NOTE: The mice wake up within 10 min after the end of the collection. Subcutaneous injection of 0.2 mL pre-warmed isotonic saline may also be given to accelerate recovery. - Proceed to the next mouse. All the mice should be monitored until they have completely recovered and are ambulatory.
3. Measurements
- At the end of all collections, weigh the 0.6 mL tubes with the wet swab. Calculate the difference between the wet weight and dry weight to get the weight of saliva produced. Place the 0.6 mL tube with saliva back into the 2-mL tube.
- Cut off the caps of the 2 ml tubes. Then, centrifuge the 2 mL tubes for 2 min at 7500 x g, at 4oC in a micro centrifuge to recover the saliva. Measure the volume of saliva obtained using a micropipette.
- Express the results as saliva weight (mg)10 over 15 min or as a ratio of saliva weight (mg)/mouse body weight (g).
NOTE: If the volume of saliva extracted out of the swab has been measured, express the results as the volume of saliva recovered (mL) or the ratio of saliva volume (mL)/mouse body weight (g).
Representative Results
In the experimental mouse model systems, the ability to produce saliva in response to pilocarpine stimulation is used as an indicator of salivary gland function. Saliva production was measured in 11-week-old C57BL/6 female mice by the swab method. In Figure 1A, the results are expressed as the amount of saliva (mg) collected following increasing doses of pilocarpine. At the dose of 1 mg/kg body weight, some of the mice started exhibiting distress (involuntary shaking and shivering). Hence higher doses of pilocarpine, beyond 1 mg/kg body weight were not tested in this study. In Figure 1B, the results are represented as the ratio of saliva weight (mg) to the mouse body weight (g). Collectively, these data show a good dose response relationship between pilocarpine amount and saliva production. The volume of saliva recovered from the swab was also measured. As shown in Figure 1C, there is a significant concordance between saliva weight and volume where 1 mg of saliva equals 0.001 mL.
The results obtained with the swab method are similar to those obtained with the pipette collection method. Figure 2A shows that the mean amount of saliva collected by the swab method and the pipet method is very similar and the differences are statistically not significant. Figure 2B, 2C and 2D show that the swab method did not impact estimation of biomolecules in saliva. In fact, in comparison with the pipet method, the swab method showed an overall higher trend in the mean levels of total salivary protein (Figure 2C), salivary lysozyme activity (Figure 2D) and salivary IgA (Figure 2E). However, these differences were statistically not significant.
The saliva collection method described here is able to detect differences in the amount of saliva produced by different mouse strains (Figure 3) and salivary gland hypofunction induced by different disease conditions (Figure 4). Figure 3A shows results of pilocarpine induced saliva production from 10-12-week-old female C57BL/6, BALB/c, DBA1/J and 129S6 mice. The 129S6 mice produced the lowest amount of saliva compared to the other strains. It should be noted that the differences in the body weights of these mice were not significantly different (Figure 3B). Next, the swab method was used to detect salivary gland hypofunction, and two examples are shown in Figure 4. Figure 4A shows a representative result of salivary gland hypofunction induced by the activation of innate immunity following injection of lipopolysaccharide (LPS) (10 µg/mouse, intraperitoneally). Control mice were injected with saline11. LPS-treated mice show a significant drop in saliva compared to controls. Passive transfer of antibodies reactive with Sjögren's syndrome antigen A/Ro52 induces salivary gland hypofunction, and this model mimics certain aspects of Sjögren's syndrome9. As shown in Figure 4B, mice injected with adjuvant alum plus anti-Ro52 antibodies together had significantly lower saliva production compared to untreated mice or mice treated only with alum.
The swab method is simple to implement and operator independent. Figure 5 shows saliva production from 10-13-week-old female C57BL/6 mice, in experiments performed at 6 different time points by 2 different operators. Operator I started with only 2 months of mouse handling experience while Operator II had 6 months of prior mouse handling experience, but was only introduced to the saliva collection method for one week. These data were generated in experiments carried out almost one year apart, using 0.375 mg/kg pilocarpine dose. The saliva ratios obtained by both operators show a range of readings (1.84 to 5.87). Operator I's experiments spanned a period of 10 weeks while Operator II's experiments were carried out over 3 consecutive days almost one year later (Figure 5A). Notably, the saliva ratios obtained over time were not significantly different (p=0.064; Kruskal-Wallis test). To further compare inter-operator variations, the ratios of saliva weight per g body weight from 3 experiments for each operator were pooled and are shown in Figure 5B. Saliva ratios obtained by Operator I (mean + SEM; 4.45 + 0.152, n=38) are not significantly different from Operator 2 (mean + SEM; 4.21 + 0.169; n=25; p=0.296 by Mann Whitney test).
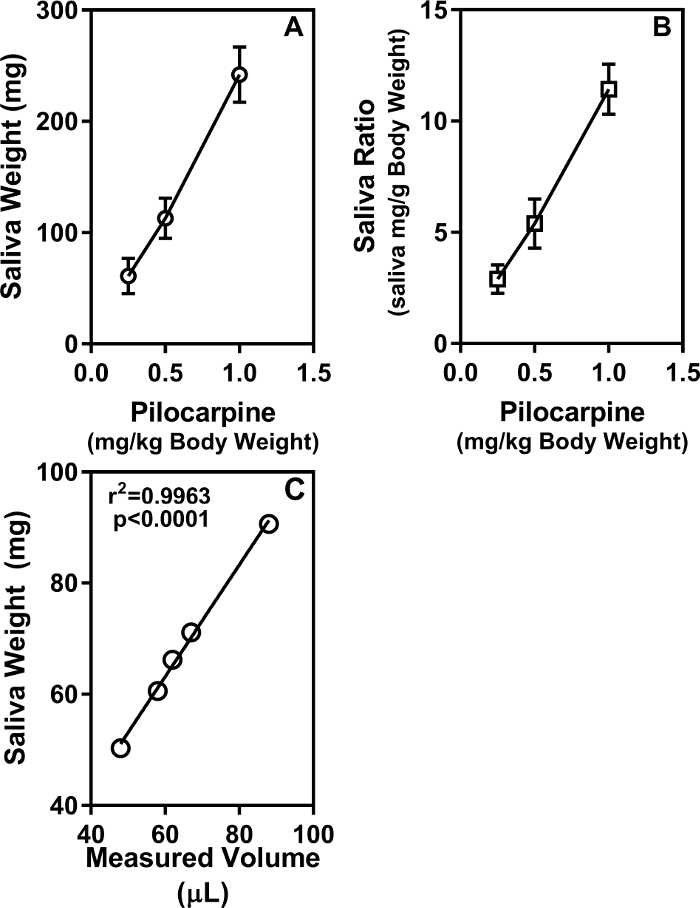
Figure 1: Saliva production is dependent on pilocarpine dose. C57BL6/J mice (11-week-old females, 5 per group) were injected with different doses of pilocarpine (0.25 mg/kg, 0.5 mg/kg and 1.0 mg/kg body weight) and saliva production was measured for 15 min. (A) Results are represented as mg saliva amount (mean ± SEM). (B) Data are represented as mean saliva ratio (mg saliva per g mouse body weight). (C) Saliva from 11-week-old female C57BL/6 mice (n=5) was collected by the swab method. Pilocarpine was used at a dose of 0.375 mg/kg body weight. The wet swabs were weighed to measure the amount of saliva produced in mg. The saliva in the swabs was then recovered by centrifugation, and the volumes of the recovered saliva were measured. The significant agreement is seen between the saliva weight in mg and saliva volume in µL. Please click here to view a larger version of this figure.
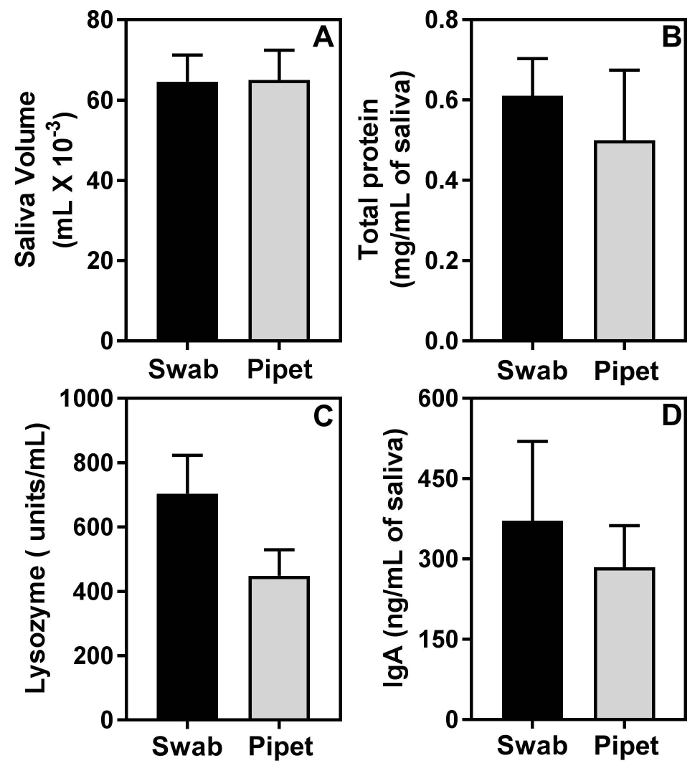
Figure 2: The swab method does not impact the analysis of biomolecules in saliva. Saliva from 2 groups of 11-week-old female C57BL/6 mice, (n=5 mice per group), was collected either by the swab method or by the micropipet method. The differences in mean volumes of saliva (A), mean amounts of protein (B), mean amounts of lysozyme activity (C), and mean amounts of IgA between the 2 groups are statistically not significant. Please click here to view a larger version of this figure.
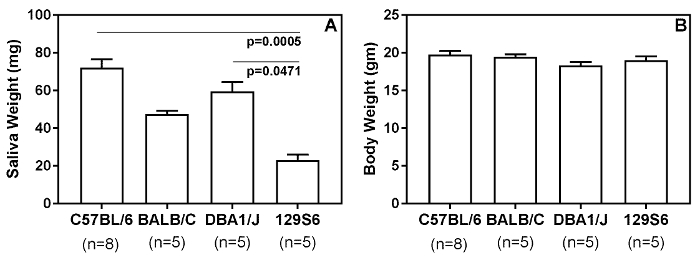
Figure 3: The swab method of saliva collection detects differences in base line saliva production by different strains of mice. (A) Saliva from female (10-12-week-old) mice were collected for 15 min by using a pilocarpine dose of 0.375 mg/kg body weight. (B) The mean body weights between the groups of mice were not significantly different. Statistical significance was analyzed by the Kruskal-Wallis test, followed by Dunn's multiple comparisons test. A p<0.05 was considered significant. Please click here to view a larger version of this figure.
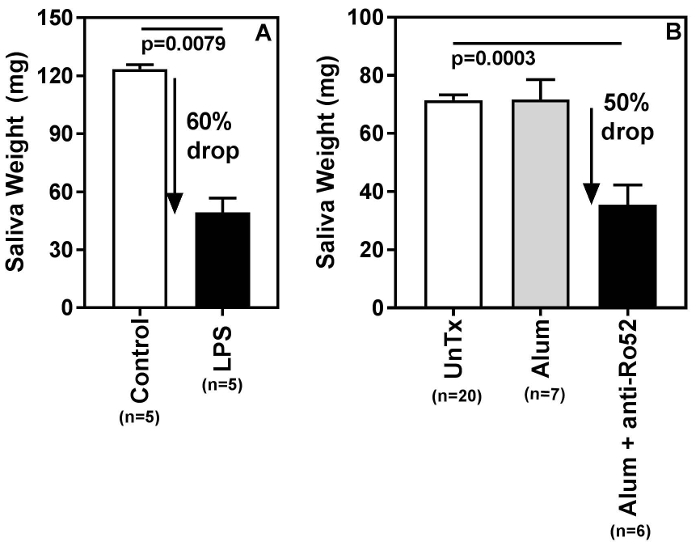
Figure 4: Swab method detects salivary gland hypofunction. (A) (B6 X A/J) F1 female mice were injected with either LPS solution (0.1 mg/mL, 0.1 mL per mouse, intraperitoneally) or saline, and saliva was measured 26 h later. A representative experiment shows a significant (p=0.0079) drop (60%) in saliva production in mice treated with LPS. (B) Salivary gland hypofunction was measured in an experimental mouse model system for Sjögren's syndrome. The previously published passive transfer model for induction of glandular dysfunction was used4 with few modifications. Female C57BL/6 mice (10-12 weeks old) were injected with alum adjuvant. On days 14 and 20 mice were injected with 0.05 mL of rabbit anti-Ro52 serum and saliva production was measured after 24 h. Note a significant (p=0.0003) drop (50%) in saliva production in mice treated with alum and anti-Ro52 serum. Statistical significance was analyzed by the Kruskal-Wallis test, followed by Dunn's multiple comparisons test. A p<0.05 was considered significant. Please click here to view a larger version of this figure.
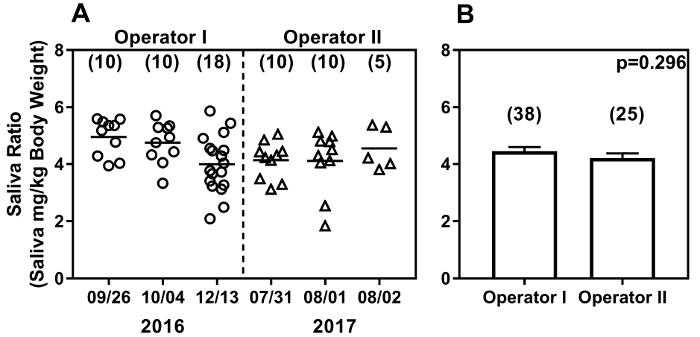
Figure 5: The swab method yields reproducible results over time (A) and between operators (B). The swab method shows comparable results between two operators with different lengths of mouse handling experience in experiments carried out over one year. Baseline saliva production in C57BL/6 female mice (10-13 weeks of age) was measured by each operator. Results are expressed as weight of saliva produced (mg/g body weight). Dates of saliva collection are on the X-axis and the results from data collected over time are shown (A). Each data point represents one mouse, and the number of mice analyzed at each time is shown in parenthesis. Baseline saliva ratios (mean + SEM) pooled from 3 experiments are shown (B), and are not significantly different between operators. Please click here to view a larger version of this figure.
Discussion
Saliva production is a complex process and is influenced by many factors. Therefore, measuring salivary gland function in laboratory animals can be a challenge. An additional challenge is that repeated measurements of salivary gland function in the same animal are required to establish the onset of disease or to demonstrate recovery following treatment.
The swab method described in this report is technically simple to perform with multiple options to represent the data. The results obtained are reproducible, with little inter-operator variation. The method can detect a reduction in saliva production in different models of salivary gland dysfunction. A significant advantage is the use of inert, highly absorbent polymers and efficient recovery of the saliva collected, allowing measurement of salivary biomolecules. Another advantage of this method is that it is possible to carry out this procedure simultaneously in up to 2 mice at a time by a single operator. This is significantly more efficient than some of the other methods, where it is performed in a single mouse at a time.
The variability in saliva production measurements have been the bane of all saliva measurement techniques. To minimize variability, it is important to adhere strictly to the protocol. Critical steps include: selecting the same time of the day for fasting the mice and collecting saliva, appropriate dosing of anesthetic and pilocarpine (table 1), and accurate weight records of dry and wet swabs. Careful time management is required to set up to 2 mice at a time for saliva collection.
As shown in Figure 3, the amount of saliva produced by mice is strain dependent. Thus, appropriate strain, age, and sex matched mice should be used as controls. Titration of the pilocarpine dosage, for the specific strain and the experimental condition being investigated, is highly recommended. Although a higher dose of pilocarpine beyond 0.5 mg/kg induces increased production of saliva, to evaluate glandular hypofunction, it is recommended to use a lower dose. This allows for the identification of even minor differences in saliva production between the diseased and control mice. Doses of 0.5 mg/kg body weight and 0.375 mg/kg body weight have been used successfully to demonstrate salivary gland hypofunction. However, it should be noted that certain experimental model systems may require shorter or longer periods of collection than that described in this protocol. This should be tested by each laboratory.
A limitation of this method, and most saliva measuring methods is the need for anesthesia. The report with the vacuum method does not include the use of anesthetic5. However, the absence of anesthesia induces varying levels of anxiety in the mice. They tend to struggle and bite the tubing, and that in turn, can influence saliva output and collection efficiency. Isoflurane anesthesia in rats induces a drop-in pilocarpine induced saliva production12. In contrast, ketamine increases bronchial and salivary secretions through sympathetic stimulation13. We and others have successfully used the ketamine and xylazine mixture as an anesthetic for measuring pilocarpine induced saliva production4,7,8,9. The recommended range for surgical anesthesia is 100-200 mg/kg of ketamine and 5-16 mg/kg of xylazine14. The dose of 0.006-0.008 mL/g body weight used in this method (corresponds to 60-80 mg/kg of ketamine and 6-8 mg/kg of xylazine) falls in the lower end of the recommended range and is sufficient for providing a consistent level of immobilization without deep anesthesia.
The swab method was established as an alternative to the routinely used micropipet method. The major disadvantage of the pipet method was the high inter-operator variability and the need to collect saliva from one mouse at a time. The swab method addresses both these problems. The benefits include limiting the number of animals needed to achieve power in statistical analyses and increased efficiency. Replacing cotton swabs with inert polymer swabs offer a better option since cotton swabs are known to impact quantitation of biologically active molecules15.
Given the simplicity of the swab method and its high reproducibility between different individuals, it is hoped that the swab method will facilitate better comparisons of mouse models between researchers in different laboratories and institutions.
Divulgazioni
The authors have nothing to disclose.
Acknowledgements
The study was supported by a grant from National Institutes of Health, National Institute of Dental and Craniofacial Research (DE025030).
Materials
| Chemicals for saliva collection | |||
| Pilocarpine hydrochloride | Alfa Aesar, Tewksbury, MA, USA | B21410 | Dilute in sterile isotonic saline. Store single use aliquots of 100X stock at -80oC |
| Name | Company | Catalog Number | Comments |
| Anesthesia Mix solution | Mix 1 mL ketamine hydrochloride + 0.5 mL xylazine + 8.5 mL sterile isotonic saline in a sterile vial. Can be used for 3 months. | ||
| Zetamine (Ketamine hydrochloride) | Vet One, Boise, Idaho, USA | C3N VT1 | Stock is 100 mg/mL |
| Anased (Xylazine) | Med-Vet International, Mettawa, IL, USA | RXANASED-20 | Stock is 20 mg/mL |
| Isotonic sterile saline | Vet One, Boise, Idaho, USA | 501032 | Used as diluent |
| Artificial tears (lubricant ophthalmic ointment) | Henry Schien, Dublin, OH, USA | 48272 | Used to prevent eyes from drying during the procedure |
| Name | Company | Catalog Number | Comments |
| Materials for saliva collection | |||
| SalivaBio Children's swab | Salimetrics, LLC Carlsbad, CA, USA | N/A | Individually wrapped swabs |
| 50 mL polypropylene tubes | VWR, Radnor, PA, USA | 89004-364 | Cut off the bottom 1 cm of the tube. Make sure that the cut edge is smooth. |
| Microcentrifuge tubes 0.6 mL | VWR, Radnor, PA, USA | 87003-290 | Make holes in the bottom of tube with heated 18 gauge needles |
| Microcentrifuge tubes 2 mL | VWR, Radnor, PA, USA | 87003-298 | |
| Insulin Syringes with permanently attached needles | Becton Dickinson and Company, Franklin Lakes, NJ, USA | 324702 | |
| 18 gauge regular bevel needles | Becton Dickinson and Company, Franklin Lakes, NJ, USA | 305195 | |
| Sterile scalpel blade #11 | Integra York Inc PA, USA | 4-311 | |
| Microdissecting forceps | Roboz, Gaithersburg, MD, USA | RS-5139 | Serrated angular 0.8 mm tip, 4" length |
| Label tape | Santa Cruz Biotechnology, Dallas, TX, USA | sc-224487 | |
| 3 channel timer | Amazon.com, Seattle, WA, USA | B06W2KCYVN | |
| Analytical Balance | Mettler Toledo, Columbus OH, USA | ||
| Name | Company | Catalog Number | Comments |
| Chemicals/ reagents for inducing salivary dysfunction | |||
| LPS | Invivogen, San Deigo, CA, USA | tlrl-b5lps | Dissolve in endotoxin free water, and store stock solution at 5 mg/mL . dilute in sterile HBSS 100 ug/mL – inject 100 uL/ moue ip |
| Imject Alum adjuvant | Thermo Scientific | 77161 | Dilute 1:1 in sterile saline. Inject intraperitoneally 0.1 mL/mouse |
| Rabbit anti-Ro52 antiserum | Generated in lab | Immunization of rabbits with recombinant mouse Ro52 | |
| Name | Company | Catalog Number | Comments |
| Chemicals/ Kits for saliva analyses | |||
| Salivary lysozyme estimation | |||
| EnzChek Lysozyme Assay Kit | Molecular Probes, Eugene, OR, USA | E-22013 | Used as per manufacturer's instructions |
| Name | Company | Catalog Number | Comments |
| Salivary IgA estimation by sandwich ELISA | |||
| Mouse IgA | Southern Biotech, Birmingham, AL, USA | 0106-01 | Standards for sandwich ELISA – range 30 ng/mL to 0.5 ng/mL |
| Goat anti- mouse IgA unlabeled | Southern Biotech, Birmingham, AL, USA | 1040-01 | Coat at 1 ug/mL in bicarbonate buffer |
| Goat anti- mouse IgA HRP | Southern Biotech, Birmingham, AL, USA | 1040-05 | Detection antibody used at 1:4000 dilution |
| TMB substrate | Becton Dickinson and Company, Franklin Lakes, NJ, USA | 555214 | Used as per manufacturer's instructions |
| Immulon 4HBX Microtiter 96 well plates | Thermo Scientific, Rochester, NY, USA | 3855 | |
| Name | Company | Catalog Number | Comments |
| Salivary protein estimation | |||
| Bio-Rad Protein Assay Dye Reagent Concentrate | BioRad, Hercules, CA, USA | 5000006 | For protein estimation as per manufacturer's instructions |
Riferimenti
- Proctor, G. B. The physiology of salivary secretion. Periodontol 2000. 70 (1), 11-25 (2016).
- Fox, R. I. Sjögren’s syndrome. Lancet. 366 (9482), 321-331 (2005).
- Eisbruch, A., Kim, H. M., Terrell, J. E., Marsh, L. H., Dawson, L. A., Ship, J. A. Xerostomia and its predictors following parotid-sparing irradiation of head-and-neck cancer. Int J Radiat Oncol Biol Phys. 50 (3), 695-704 (2001).
- Marmary, Y., Fox, P. C., Baum, B. J. Fluid secretion rates from mouse and rat parotid glands are markedly different following pilocarpine stimulation. Comp Biochem Physiol A Comp Physiol. 88 (2), 307-310 (1987).
- Lin, A. L., Johnson, D. A., Wu, Y., Wong, G., Ebersole, J. L., Yeh, C. K. Measuring short-term gamma-irradiation effects on mouse salivary gland function using a new saliva collection device. Arch Oral Biol. 46 (11), 1085-1089 (2001).
- Scofield, R. H., Asfa, S., Obeso, D., Jonsson, R., Kurien, B. T. Immunization with short peptides from the 60-kDa Ro antigen recapitulates the serological and pathological findings as well as the salivary gland dysfunction of Sjogren’s syndrome. J Immunol. 175 (12), 8409-8414 (2005).
- Deshmukh, U. S., Nandula, S. R., Thimmalapura, P. R., Scindia, Y. M., Bagavant, H. Activation of innate immune responses through Toll-like receptor 3 causes a rapid loss of salivary gland function. J Oral Pathol Med. 38 (1), 42-47 (2009).
- Deshmukh, U. S., Ohyama, Y., Bagavant, H., Guo, X., Gaskin, F., Fu, S. M. Inflammatory stimuli accelerate Sjögren’s syndrome-like disease in (NZB x NZW)F1 mice. Arthritis Rheum. 58 (5), 1318-1323 (2008).
- Szczerba, B. M., et al. Interaction between innate immunity and Ro52-induced antibody causes Sjögren’s syndrome-like disorder in mice. Ann Rheum Dis. 75 (3), 617-622 (2016).
- Takakura, A., Moreira, T., Laitano, S., De Luca Júnior, L., Renzi, A., Menani, J. Central muscarinic receptors signal pilocarpine-induced salivation. J Dent Res. 82 (12), 993-997 (2003).
- Yao, C., et al. Lipopolysaccharide-induced elevation and secretion of interleukin-1beta in the submandibular gland of male mice. Immunology. 116 (2), 213-222 (2005).
- Knudsen, J., Nauntofte, B., Josipovic, M., Engelholm, S. A., Hyldegaard, O. Effects of isoflurane anesthesia and pilocarpine on rat parotid saliva flow. Radiat Res. 176 (1), 84-88 (2011).
- Kohrs, R., Durieux, M. E. Ketamine: teaching an old drug new tricks. Anesth Analg. 87 (5), 1186-1193 (1998).
- Kozaki, T., Hashiguchi, N., Kaji, Y., Yasukouchi, A., Tochihara, Y. Effects of saliva collection using cotton swab on cortisol enzyme immunoassay. Eur J Appl Physiol. 107 (6), 743-746 (2009).

