Retrospective MicroRNA Sequencing: Complementary DNA Library Preparation Protocol Using Formalin-fixed Paraffin-embedded RNA Specimens
Summary
Formalin-fixed paraffin-embedded specimens represent a valuable source of molecular biomarkers of human diseases. Here we present a laboratory-based cDNA library preparation protocol, initially designed with fresh frozen RNA, and optimized for the analysis of archived microRNAs from tissues stored up to 35 years.
Abstract
–Archived, clinically classified formalin-fixed paraffin-embedded (FFPE) tissues can provide nucleic acids for retrospective molecular studies of cancer development. By using non-invasive or pre-malignant lesions from patients who later develop invasive disease, gene expression analyses may help identify early molecular alterations that predispose to cancer risk. It has been well described that nucleic acids recovered from FFPE tissues have undergone severe physical damage and chemical modifications, which make their analysis difficult and generally requires adapted assays. MicroRNAs (miRNAs), however, which represent a small class of RNA molecules spanning only up to ~18–24 nucleotides, have been shown to withstand long-term storage and have been successfully analyzed in FFPE samples. Here we present a 3' barcoded complementary DNA (cDNA) library preparation protocol specifically optimized for the analysis of small RNAs extracted from archived tissues, which was recently demonstrated to be robust and highly reproducible when using archived clinical specimens stored for up to 35 years. This library preparation is well adapted to the multiplex analysis of compromised/degraded material where RNA samples (up to 18) are ligated with individual 3' barcoded adapters and then pooled together for subsequent enzymatic and biochemical preparations prior to analysis. All purifications are performed by polyacrylamide gel electrophoresis (PAGE), which allows size-specific selections and enrichments of barcoded small RNA species. This cDNA library preparation is well adapted to minute RNA inputs, as a pilot polymerase chain reaction (PCR) allows determination of a specific amplification cycle to produce optimal amounts of material for next-generation sequencing (NGS). This approach was optimized for the use of degraded FFPE RNA from specimens archived for up to 35 years and provides highly reproducible NGS data.
Introduction
miRNAs are remarkably well conserved in formalin-fixed paraffin-embedded (FFPE) specimens1,2,3. Previous work has demonstrated that the expression of these short regulatory non-coding single stranded RNA molecules can be successfully evaluated using total RNA from FFPE samples and provide relevant gene expression data when compared to the original fresh tissues4,5,6,7,8. When compared to large-size messenger RNAs, which have been shown to be critically affected by FFPE tissue processing (formaldehyde, heat, desiccation, etc.), endogenous RNases, and the age of the specimens, the small size of miRNAs (~18–24 nucleotides) appears to make them resistant to degradation and resilient to long-term storage, also demonstrated through miRNA expression studies that outperform high-throughput mRNA studies in archived specimens9. miRNA expression studies using archived clinical specimens, which have mostly been performed in small-scale analyses, have demonstrated that single or multiplexed quantitative PCR assays, different types of microarray technologies, and most recently NGS can be used to assess the expression of preserved miRNAs after optimization of these assays10,11,12,13,14.
Given that dysregulation of miRNA expression has been associated with the development of a variety of human malignancies and that there is potentially an enormous supply of clinically annotated archived specimens, it has become apparent that these small RNA molecules represent a promising source of potential cancer biomarkers15,16,17,18. The use of a high-throughput gene expression technology such as NGS has the advantage of providing a global evaluation of all miRNA transcripts when compared to targeted technologies such as PCR and/or microarrays19. For this reason, an optimized, affordable, and easily applicable protocol for cDNA library preparation of small RNAs from older archived specimens for NGS was optimized to enable large-scale retrospective studies20.
We previously established a simultaneous RNA/DNA extraction protocol for separate recovery of RNA and DNA from older archived specimens, which we found to outperform contemporary commercial kits21. Using this extraction protocol, to obtain total RNA from FFPE tissues archived for extended period of times, we optimized the preparation of cDNA libraries for NGS of miRNAs preserved in clinical specimens for up to 35 years. Furthermore, in a recently published study where we prepared cDNA libraries from clinically classified ductal carcinoma in situ (DCIS) specimens, we identified differentially expressed miRNAs that were validated by quantitative PCR, which indicated that specific miRNA expression changes may be detectable in DCIS lesions from patients who develop breast cancer when compared to DCIS lesions from patients who do not develop breast cancer.
Considering the cost of commercial kits for preparation of small RNA cDNA libraries, the potential for their discontinuation, as well as the use of copyright/patent-protected reagents that cannot be optimized, we decided to adapt a previously published laboratory-based and kit-free 3' barcoded cDNA library preparation protocol for NGS of small RNAs archived in FFPE specimens, allowing simultaneous analysis of 18 samples22. This protocol provides an ideal and robust step-by-step procedure with visual and technical evaluation checkpoints, which were critical for adaptation to FFPE RNA specimens, and has a strong potential for application to other sources of compromised or difficult to use RNA material. The original protocol's applicability was improved by replacing radioactively labeled size markers with fluorescent (e.g., SYBR Gold) detectable RNA size markers used during selection of ligated libraries on large polyacrylamide gels. This optimized protocol relies on the ligation of 3' barcoded adapters to 18 individual FFPE RNA specimens, which are then pooled together to undergo 5' adapter ligation, reverse-transcription, and a pilot PCR analysis for tailored amplification of the final cDNA library prior to large-scale PCR amplification, purification, and NGS on a high throughput sequencer.
Protocol
1. Preparation of All Reagents and Primers
- Order all primers and adapters as described in Figure 1.
- Prepare the calibrator cocktail stock, which will be spiked in each individual ligation.
- Resuspend the carrier oligonucleotide (Figure 1) to 0.5 µM with RNase-free water. Resuspend the 10 calibrator oligonucleotides (Figure 1) to 100 µM using the 0.5 µM carrier oligonucleotide solution.
- Combine 10 µL of each of the 10 calibrators in a siliconized microcentrifuge to obtain a 100 µM cocktail calibrator stock, and prepare a 0.026 nM solution to store at -20 °C.
- Dilute the 20 unique, lyophilized, adenylated 3' adapters with RNase-free water to a final concentration of 50 µM (Figure 1).
NOTE: It is recommended to prepare 2.5 µL aliquots from each adapter in individual siliconized tubes and store them at -80 °C for up to 2 years. - Resuspend the desalted 19 nt-3' adapter and 24 nt-3' adapter size marker oligonucleotides to 250 ng/mL (Figure 1).
- Resuspend the HPLC purified 5' adapter to 100 µM with RNAse-free water. Resuspend the 5' and 3' PCR primers to 100 µM using RNase-free water (Figure 1).
NOTE: Aliquot all oligonucleotides in amounts necessary for 1–2 experiments and store at -80 °C. - Prepare the polyacrylamide denaturing (PAA) gel loading dye by combining 98.8% formamide, 1% (v/v) 0.5 M Na2H2 EDTA, pH 8.0, and 0.2% bromophenol blue, and set 1 mL aliquots at -80 °C.
- Prepare the 0.5 M Na2H2 EDTA solution by adding 18.6 g of Na2H2 EDTA powder to 50 mL of nuclease-free water. Add NaOH pellets to reach pH 8.0. Adjust the volume to 100 mL to obtain a 0.5 M Na2H2 EDTA, pH 8.0 stock solution.
- Weigh 15 mg of bromophenol blue in a separate 15 mL tube. Add 600 µL of nuclease-free water. Add 14.25 mL of de-ionized formamide. Add 150 µL of the 0.5 M Na2H2 EDTA, pH 8.0 solution.
- Aliquot the PAA final solution of 15 mL in 1 mL aliquots at -80 °C.
- Prepare the 5x agarose gel loading dye by combining 0.2% bromophenol blue, 0.2% xylene cyanol FF, 50 mM Na2H2 EDTA, pH 8.0, and 20% Ficoll Type-400.
- Resuspend 1.86 g of Na2H2-EDTA in 50 mL of nuclease-free water in a 400-mL beaker on a magnetic stirrer at room temperature (RT). Add NaOH pellets to reach a pH 8.0. Add nuclease-free water to reach 100 mL to obtain a 50 mM Na2H2 EDTA solution.
- Weigh 20 mg of bromophenol blue powder, 20 mg of xylene cyanol FF powder, and 2 g of Ficoll Type-400 powder, and resuspend in 5 mL of 50 mM Na2H2 EDTA.
- Vortex the tube containing the three dyes and add 5 mL of 50 mM Na2H2 EDTA. Mix the 5x agarose gel loading dye, prepare 1 mL aliquots, and store at -80 °C.
2. Set Up the 3' Barcoded Adapter Ligations with 18 Individual RNA Samples
- Identify 18 individual RNA specimens (pre-aliquoted at 100 ng in 9.5 µL of nuclease-free water in 1.5 mL siliconized microcentrifuge tubes), and set the tubes on ice to defrost for 10 min.
- Prepare the 10x RNA Ligase Buffer (without ATP) fresh.
- In a 1.5 mL siliconized microcentrifuge tube, combine 343 µL of RNase-free water, 500 µL of Tris 1 M pH 7.5, 100 µL of 1 M MgCl2, 50 µL of 20 mg/mL bovine serum albumin, and 7 µL of 14 M 2-mercaptoethanol. Mix by flicking the tube, centrifuge for 2 s at 2,000 x g and RT, and set on ice.
NOTE: All steps in this protocol that indicate "centrifuge for 2 s" are performed in a benchtop microcentrifuge at RT and a top speed of 2,000 x g to collect solutions to the bottom of the tubes.
- In a 1.5 mL siliconized microcentrifuge tube, combine 343 µL of RNase-free water, 500 µL of Tris 1 M pH 7.5, 100 µL of 1 M MgCl2, 50 µL of 20 mg/mL bovine serum albumin, and 7 µL of 14 M 2-mercaptoethanol. Mix by flicking the tube, centrifuge for 2 s at 2,000 x g and RT, and set on ice.
- Prepare 50% aqueous DMSO stock by adding 1 mL of RNase-free water to 1 mL of DMSO in a 2 mL siliconized microcentrifuge tube, wrap the tube in aluminum foil, and store at RT.
- Defrost the 0.026 nM calibrator cocktail on ice for 10 min. Prepare the Ligation Master Mix by combining in the following order in a 1.5 mL siliconized microcentrifuge tube: 40 µL of 10x RNA Ligase Buffer and 120 µL of 50% aqueous DMSO using a 200 mL pipette, and add 10 µL of 0.026 nM calibrator cocktail using a 10 µL pipette. Flick the 1.5 mL tube to mix, centrifuge for 2 s, and set it on ice.
- Add 8.5 µL of Ligation Master Mix to each of the 18 individually aliquoted FFPE RNA samples, gently flick the tubes, centrifuge for 2 s, and store on ice.
- Defrost 2.5 µL aliquots from each of the 18, 3' barcoded adapters and place them on ice to defrost for 20 min.
- Use a 3-µL pipette to transfer 1 µL of each adapter to the corresponding FFPE RNA samples containing RNA and Ligation Master Mix (i.e., 9.5 µL + 8.5 µL).
- Do not pipette up and down, simply dispense into the liquid and pull the tip out. Make sure to change tips between FFPE RNA samples. Close each tube, flick to mix, centrifuge for 2 s, and set on ice.
- Denature the reactions by placing the 18 tubes on a heat block at 90 °C for 1 min and then immediately place on ice.
- Prepare the ligation enzyme solution by diluting 10 µL of Truncated K227Q T4 RNA Ligase 2 with 10 µL of RNase-free water into a fresh 1.5 mL siliconized microcentrifuge tube.
- Use a 3 µL pipette to transfer 1 µL of the diluted ligation enzyme into each of the 18 RNA samples (do not pipette up and down, and change tips between each tube). Set the 18 ligations on ice and place in a 4 °C cold room for an overnight incubation of 18 h.
3. Purification of the Ligated Small RNAs
- Deactivate the ligation reactions by placing the 18 tubes for 1 min on a heat block at 90 °C, and then return to ice for at least 2 min to cool down.
- Prepare the Precipitation Master Mix by adding 1 µL of blue dye covalently linked to glycogen to 26 µL of 5 M RNase-free NaCl into a fresh siliconized microcentrifuge tube.
- Transfer 1.2 µL of the Precipitation Master Mix into each of the 18 tubes. Add 63 µL of 100% ethanol to each of the 18 tubes. Close each tube, flick to mix, centrifuge for 2 s, and place the tubes on ice. Combine the contents of all 18 tubes into a single 1.5 mL siliconized tube.
- Close the tube, invert three times to mix, centrifuge for 2 s, and place on ice for 60 min to precipitate.
- Prepare a large (16 x 20 cm2) 15% polyacrylamide gel for PAGE.
- Cast two siliconized glass plates (treated with a safe alternative to silane coatings) with 0.1 cm spacers.
- Combine 9 mL of system diluent, 18 mL of system concentrate, 3 mL of system buffer, 240 µL of APS (9%), and 12 µL of TEMED in a 50 mL tube.
- Transfer the PAGE gel solution in between glass plates using a 30-mL pipette, insert a 14-well comb (0.1 cm thick), and let the gel solidify at RT for 30 min.
- Centrifuge the 1.5-mL tube with the pooled ligations at 16,000 x g and 4 °C for 60 min.
- Remove the comb. Clean the wells abundantly with RNase-free water using a squirt bottle with a 200 µL tip at its end to reach inside the wells and force un-polymerized acrylamide out (one well at a time) above a sink. Set the 15% PAGE on a gel apparatus, fill the reservoirs with 0.5x Tris-Borate EDTA (TBE) solution, and pre-run the gel for 30 min at 450 V.
- Recover the tube with pooled ligations from the centrifuge and carefully dry the RNA pellet.
- Remove the supernatant with a 1-mL pipette tip but leave some liquid at the bottom. Tilt the tube and use a 20 mL pipette to remove the remaining supernatant without touching the pellet. Vacuum suction the tube using a Pasteur pipette with a 10-µL tip on its end, without touching the pellet.
- Resuspend the RNA pellet in 20 µL of RNase-free water by flicking the tube. Add 20 µL of PAA gel loading solution to resuspend the RNA, flick to mix, centrifuge for 2 sat RT, and set the tube at 90 ºC for 1 min and then immediately place on ice.
- Replace the 0.5x TBE of the gel apparatus with fresh 0.5x TBE, and load the ladder, size markers, and ligated miRNAs in the center of the gel, leaving 2-empty wells on both sides (Figure 2).
- Run the gel at 450 V (35 mA) for 60 min, pause the run for 10 min to cool down, and run again at 520 V (25 mA) for another 60 min. Uncast the 15% PAGE by removing one of the glass plates.
- Lightly spray the gel sitting on the glass plate with fluorescent dye solution (10 µL) in 25 mL of 0.5x TBE, and let it sit flat for 5 min in the dark.
- Lay the glass with the gel on a blue light transilluminator, and align both 19 nt and 24 nt size markers with a ruler to direct the excision of the ligated small RNAs (Figure 2). Excise the gel.
- Place the excised gel in a 0.5 mL tube, designed to fragment gel slices, securely positioned into a 1.5-mL siliconized microcentrifuge tube. Centrifuge at 16,000 x g for 3 min at RT. Resuspend the fragmented gel with 300 µL of 400 mM NaCl solution, close the tube, and seal it with the paraffin film.
- Set the tube on agitation on a thermomixer at 1,100 rpm in a 4 °C cold room for an overnight incubation (16-17 h).
4. Ligation of the 5' Adapter
- Transfer the solution and fragmented gel to a 5 µm filter tube sitting in a 1.5 mL siliconized tube, seal with paraffin film, and centrifuge for 5 min at 2,300 x g and RT.
- Add 950 µL of 100% ethanol to the filtered solution, close the tube, invert to mix, centrifuge for 2 s, seal the tube with paraffin film, and set on ice for 1 h.
- Prepare a 12% PAGE gel as described in step 3.3, but combine 12.6 mL of diluent, 14.4 mL of concentrate, 3 mL of buffer, 240 µL APS, and 12 µL of TEMED into a 50 mL tube. Pre-run the 12% PAGE gel for 30 min at 450 V (~ 35 mA) in 0.5x TBE.
- Centrifuge the purified small RNA solution at 16,000 x g for 60 min at 4 °C.
- Carefully pipette out the supernatant using a 1 mL pipette to remove the bulk of the solution and use a 200-mL pipette to remove the remaining solution, without touching the pellet.
- Using a Pasteur pipette with a 10 mL tip at its end connected to a vacuum flask, carefully dry the pellet without touching it.
- Resuspend the pellet in 9 µL of RNase-free water without pipetting up and down, but by lightly flicking the tube, centrifuge for 2 s, and set the tube on ice.
- Prepare the 10x RNA Ligase Buffer (with ATP) fresh by combining 500 µL of 1 M Tris pH 7.5, 100 µL of 1 M MgCl2, 50 µL of 20 mg/mL acetylated BSA, 200 µL of 10 mM ATP, 7 µL of 2-mercaptoethanol 14 M, and 143 µL of RNase-free water.
- Mix the 10x RNA Ligase Buffer (with ATP) by flicking the tube, and centrifuge for 2 s to collect the solution at the bottom of the tube.
- Set up the 5' adapter ligation by adding 2 µL of 10x RNA Ligase Buffer (with ATP), 1 µL of 100 µM 5' adapter, and 6 µL 50% aqueous DMSO to the 9 µL, 3' barcoded small RNA solution.
- Flick the tube lightly to mix, centrifuge for 2 s to collect the solution, place the tube on a heat block at 90 °C for 1 min, and back on ice for at least 2 min.
- Add 2 µL of T4 RNA Ligase 1 into the solution (do not pipette up and down), flick the tube, centrifuge for 2 s, and sit the tube in floating rack in a 37 °C water bath for 60 min.
- Simultaneously, set up two ligations between the 19 nt-3' adapter and the 5' adapter, and two ligations between the 24 nt-3' adapter and the 5' adapter.
- Combine 2 µL of the 19nt-3' adapter (250 ng/µL) or 24 nt-3' adapter (250 ng/µL) with: 2 µL of the 5' adapter (100 µM), 2 µL of 10x RNA Ligase Buffer (with ATP), 6 µL of 50% aqueous DMSO, 6 µL of RNase-free water, and 2 µL of T4 RNA Ligase 1 in a 1.5 mL siliconized microcentrifuge tube.
- Flick the tubes to mix, centrifuge for 2 s, and set the tubes at 37 °C for 60 min.
- Add 20 µL of PAA Denaturing Buffer to all ligations (purified small RNAs, 2 ligations with 19nt-3' adapter, and 2 ligations with 24nt-3' adapter).
- Mix by flicking the tubes, centrifuge for 2 s, and incubate the tubes on a heat block at 90 °C for 1 min. Transfer all tubes to ice, and prepare a ladder (3 mL of ladder with 20 µL of PAA).
- Empty the upper reservoir of the gel apparatus with the pre-run 12% PAGE gel, and add fresh 0.5x TBE solution below the level of the wells (wells are emptied with a long, thin tip pipette).
- Load the samples with a thin pipette tip, as described in Figure 3.
- Use fresh 0.5x TBE to fill individual wells to the top of the gel.
- Add fresh 0.5x TBE to the reservoir above the wells and start the electrophoresis of the 12% PAGE for 60 min at 450 V (~35 mA).
- Pause the run by switching off the generator for 10 min to cool the gel down. Restart the generator and run the gel for 60 min at 520 V (~25 mA).
- Stop the generator, empty the reservoirs by inverting the apparatus above a sink, and uncast the 12% PAGE by removing one of the glass plates.
- Sit the glass with the gel flat, spray the gel with 10 µL of the fluorescent dye in 25 mL 0.5x TBE, and incubate in the dark for 5 min. Lay the glass with the gel on a blue light transilluminator, and align both the 19 nt and both the 24 nt size markers with a ruler to direct the excision of the ligated small RNAs (Figure 3).
- Excise the gel and transfer the excised gel piece into a 0.5 mL gel breaker tube. Close the cap of the gel breaker tube, set into a 1.5 mL siliconized microcentrifuge tube, and secure with paraffin film. Remove the gel breaker tube, add 300 µL of 300 mM NaCl, and 1 µL of 100 µM 3' PCR primer to the crushed gel pieces in a 1.5 mL tube.
- Seal the tube with paraffin film on agitation on a thermomixer at 1,100 rpm at 4 °C, overnight (17–18 h) in a cold room.
5. Reverse Transcription of the 5' Ligated and 3' Barcoded Purified Small RNAs
- Retrieve the tube from the thermomixer and filter purify the solution with a 5 µM filter tube.
- Use a 1 mL pipette to transfer the solution onto a 5 µM filter tube inserted into a 1.5 mL siliconized RNase-free collection tube. Centrifuge the tube for 3 min at 2,300 x g and RT.
- Discard the filter tube and add 950 µL of 100% ethanol to the 1.5 mL microcentrifuge collection tube containing the filtered solution. Invert the tube to mix, centrifuge for 2 s, and place on ice for 60 min. Precipitate the RNA pellet by centrifuging the tube at 16,000 x g and 4 °C for 1 h.
- Dry the RNA pellet.
- Open the cap and carefully remove the supernatant with a 1-mL pipette, leaving some liquid at the bottom.
- Use a 20 µL pipette to remove all the remaining supernatant without touching the pellet. Tilt the tube to allow any solution to sit on the opposite side of the pellet.
- Use a Pasteur pipette with a 10 mL unfiltered pipette tip to aspirate the supernatant and dry the pellet using a vacuum.
- Resuspend the RNA pellet in 5.6 µL of RNase-free water.
- Defrost the reverse transcription reagents on the ice for 15 min. Set up a reverse transcription reaction by adding 3 µL of 5x Buffer, 4.2 µL of 10x deoxynucleotide triphosphate (dNTPs) (each 2 mM), and 1.5 µL of dithiothreitol (DTT) to the 5.6 µL of RNase-free resuspended pellet.
- Gently flick the tube to mix the reaction, and centrifuge for 2 s to collect the solution. Set up the reaction on a heat block at 90 °C for exactly 30 s and then transfer directly onto a thermomixer at 50 °C.
- Leave the tube on the thermomixer for 2 min so that the temperature equalizes. Add 0.75 µL of the reverse transcription enzyme directly into the solution, flick gently to mix, and set the tube back on the thermomixer at 50 °C for 30 min immediately.
- After 30 min, transfer the tube to a heat block at 95 °C for 1 min to stop reverse transcription. Add 95 µL of RNase-free water directly to the reverse transcription, flick the tube to mix, and set it directly on ice for 2 min.
- Label the tube with the cDNA Stock Library and library ID.
6. Pilot PCR and Large-scale PCR Amplification
- Prepare fresh 10x PCR buffer by adding 304 µL of RNase-free water, 125 µL of 2 M KCl, 50 µL of 1 M Tris pH 8.0, 10 µL of 1 M MgCl2, 5 µL of 1% Triton X-100, and 6 µL of 1 M 2-mercaptoethanol in a siliconized microcentrifuge tube, and keep at RT.
- Assemble the Pilot PCR reaction by combining 67 µL of RNase-free water, 10 µL of 10x PCR Buffer, 10 µL of 10x dNTPs, 0.5 µL of 100 µM 5' PCR primer, 0.5 µL of 100 µM 3' PCR primer, 10 µL of cDNA Stock Library, and 2 µL of 50x Taq Polymerase.
- Set up two PCR cycles on a thermocycler as follows: File 1: 94 °C for 45 s, 50 °C for 85 s, and 72 °C for 60 s for 10 cycles, hold at 4 °C; File 2: 94 °C for 45 s, 50 °C for 85 s, and 72 °C for 60 s for 2 cycles, hold at 4 °C. Set up the Pilot PCR reaction in the thermocycler and start File 1.
- At the end of File 1, open the PCR tube, and using a 20 µL pipette, transfer 12 µL from the Pilot PCR reaction into a 1.5 mL microcentrifuge tube containing 3 µL of 5x gel loading dye, mix by pipetting up and down, close the tube, and label it "10 cycles".
- Close the Pilot PCR tube and start File 2 on a thermocycler. At the end of File 2, open PCR tube and use a 20 mL pipette to transfer 12 µL of solution into 1.5-mL microcentrifuge tube with 3 µL of 5x gel Loading dye, and label it "12 cycles".
- Repeat the steps described in steps 6.2.2 and 6.2.3 to collect 12 µL PCR products from the Pilot PCR reaction at PCR cycles 14, 16, 18, and 20. Add 3 µL of 5x gel loading dye to each of the 12 µL PCR aliquots, and to the 20 nt ladder containing 3 µL of ladder and 9 µL of RNase-free water.
- Prepare a 2.5% agarose gel with 0.5x TBE.
- Weigh 2.5 g of agarose in a clean beaker and add 100 mL of 0.5x TBE. Heat up the solution in a microwave until it boils. Remove the solution from the microwave, swirl the bottle to mix the agarose, add 4 μL of ethidium bromide (10 mg/mL), and transfer the solution to a 7 x 10 cm2 electrophoresis tray, closed on both ends with tape.
- Set an 8-well comb and let the agarose gel solidify at RT. Transfer the solidified agarose gel into an electrophoresis apparatus filled with 0.5x TBE.
- Load the ladder and PCR samples on the agarose gel, and run it for 30 min at 120 V.
- Evaluate the gel on a UV box to identify the optimal PCR cycle (Figure 4A).
NOTE: The size of the anticipated PCR bands is shown in Figure 4B.- Observe the two PCR bands in each of the different wells (upper band: DNA library, lower band: primer dimers).
- Identify the adequate PCR cycle for the cDNA amplification by selecting the cycle where the cDNA library is visible, but the primer dimers are barely observable.
NOTE: Generally, the adequate PCR cycle is selected between 12–16 cycles. On Figure 4A, the adequate PCR cycle for this library is 14.
- Set up the large-scale PCR reactions (6x 100 µL) for agarose gel library purification.
- Prepare a fresh tube of 10x PCR Buffer following step 6.1.
- Prepare the large-scale PCR Master Mix in a 1.5 mL tube by combining 435.5 µL of RNase-Free water, 65 µL of 10x PCR buffer, 65 µL of 10x dNTPs, 3.25 µL of 5' PCR primer, 3.25 µL of 3' PCR primer, and pipetting up and down to mix.
- Transfer 88 µL of the PCR Master Mix into six 0.5 mL PCR tubes. Transfer 10 µL of cDNA Stock Library (stored on ice), add 2 µL of 50x Taq polymerase into each of the 6 PCR tubes, and pipette to mix.
- Prepare a negative PCR reaction tube by combining 77 µL of RNase-free water, 10 µL of 10x PCR buffer, 10 µL of 10x dNTPs, 0.5 µL of 5' PCR primer, 0.5 µL of 3' PCR primer, and 2 µL of 50x Taq polymerase, and pipette to mix.
- Place the PCR tubes in the thermocycler using the PCR cycle described in step 6.2.1 and set the optimal number of amplification cycles. Prepare a 2.5% agarose gel using 0.5x TBE, as described in step 6.3.
- After PCR amplification, transfer 9 µL from each PCR tube into six 1.5 mL microcentrifuge tubes containing 3 µL of 5x gel loading dye.
- Prepare 20 nt ladder by combining 3 mL of ladder into 9 µL of RNase-free water and adding 3 µL gel loading dye into a 1.5 mL tube.
- Load the ladder, negative PCR control, and 6 PCR products onto the agarose gel, and run the gel for 30 min at 120 V.
- Verify the uniformity of the PCR amplifications between lanes on a UV box (take an image).
- Combine 3 PCR reactions into two 1.5 mL siliconized microcentrifuge tube (3x 91 µL), add 27 µL of 5 M NaCl and 950 µL of 100% ethanol. Invert the tubes to mix and set them at -20 °C overnight to precipitate the PCR products.
7. cDNA Library Purification and Evaluation
- Centrifuge both tubes with the PCR reactions at 16,000 x g for 60 min at 4 °C.
- Remove the supernatant with a 1 mL pipette, then tilt the tube with the pellet on the upper side and liquid on the lower side, and use a Pasteur pipette connected to a vacuum flask with a tub and aspirate liquid with a 10 mL tip at the end of the Pasteur pipette.
- Set up the PmeI digest.
- Resuspend one of the PCR pellets with 17.5 µL of nuclease-free water, and transfer the resuspended pellet to the second PCR pellet. Add 2 µL of 10x Buffer and 0.5 µL of PmeI enzyme, and set the digest in a water bath for 2 h at 37 °C.
- Prepare a 2.5% agarose gel using 0.5x TBE (step 6.3). Add 6 µL of 5x gel loading dye to the digest. Transfer 13 µL of each digest into two adjacent wells of the agarose gel, load the 20 nt size ladder at the end wells, and run the gel for 90 min at 150 V (Figure 4D).
- Excise the upper PCR bands from the gel on the UV box, transfer the gel pieces into a freshly weighed 1.5 mL microcentrifuge tube, and weigh the gel pieces on a scale.
- Purify the excised PCR amplified cDNA library using a gel extraction kit following the manufacturer's instructions. Quantify the cDNA library using a DNA quantification assay and instrument, following the manufacturer's instructions.
- Evaluate the size and purity of the cDNA library with a high-sensitivity DNA chip on a Bioanalyzer (Figure 5). Sequence the cDNA library using a high-throughput system. Transfer the FASTQ data file to the RNAworld pipeline for adapter trimming and alignment to the human genome to identify the miRNA sequences.
Representative Results
As described in the method here, a total of 18 individual FFPE RNA samples (100 ng each) are set up in separate tubes to undergo 3' adenylated barcoded oligonucleotide T4 ligation overnight. The next day, the enzymatic reactions are heat-deactivated, combined, and precipitated in a single tube. The RNA pellet is resuspended and the ligated RNA molecules are separated on a 15% denaturing polyacrylamide gel (PAGE), where RNA oligonucleotide size markers that migrated in adjacent wells of the PAGE gel, are used to select the appropriately sized 3' barcoded small RNAs (Figure 2). The excised gel piece is incubated in a NaCl solution overnight to elute the ligated RNA molecules. The next day, the eluted RNA is precipitated, and a 5' adapter ligation is performed. Then, the 5' adapter ligated small RNA molecules are migrated and separated on a 12% acrylamide gel, where again migrated RNA size marker oligonucleotides allow size excision of the small RNAs containing both the 3' barcoded oligonucleotides and the 5' adapter (Figure 3). The excised gel is incubated overnight in a NaCl solution to allow elution of the RNA. The next day, the ligated small RNA molecules are precipitated, and the pellet is resuspended in RNase-free water, followed by reverse-transcription; an aliquot of the cDNA molecules undergoes a pilot PCR reaction (Figure 4A). Large-scale PCR reactions using the same input of cDNA libraries are set up and evaluated on a 2.5% agarose gel to verify that all reactions were adequately PCR amplified (Figure 4B), prior to pooling and overnight ethanol precipitation. The next day, the amplified cDNA library containing all 18 individual libraries for the 18 unique FFPE RNA specimens, is migrated on a 2.5% agarose gel, and the top PCR band, running at 100 nt is excised and purified (Figure 4C). The cDNA library purification is then evaluated on a high sensitivity DNA chip (Figure 5) to determine that the purified PCR product does not contain an excess of primer dimers or other byproducts of the PCR reaction. The PCR product is then analyzed on a high-throughput sequencing system. The adapter trimming and generation of 18 individual files for each of the 18 specimens are performed using the RNAworld pipeline (access was provided to us by Dr. Thomas Tuschl). Biostatistical analyses are then performed to evaluate the miRNA contents of the FFPE RNA specimens.
To validate this optimized procedure, matched fresh frozen and FFPE breast tumor specimens were used for the analyses (Figure 6). Two similar invasive ductal breast carcinoma (IDC) tumors were selected to evaluate the sensitivity of the procedure and determine if miRNA expression differences identified between the two fresh frozen tissues could also be detected in the matched archived FFPE RNA specimens. For this experiment, the quality of the total RNA obtained from the 2 fresh frozen and the matched two FFPE RNA samples was evaluated (Figure 6A). As anticipated, the RNA size and the quality of the FFPE specimens was severely decreased when compared to the matched fresh frozen RNAs (compare lanes 1 and 3, and lanes 2 and 4). One of the FFPE RNA had been archived at RT for 4 years (Invasive Breast Cancer 1, (IBC1)) and the other had been archived at RT for 8 years (IBC2), while the fresh frozen counterparts had been stored at -80 °C. The four individual RNA specimens were analyzed in a single library, using 4 individual barcodes, and the miRNA read distribution plots are displayed in Figure 6B. The two top panels display miRNA expression correlation between the two-fresh frozen tumor RNAs and their specific FFPE RNA specimen counterparts. The plots between matched fresh frozen and FFPE miRNAs indicate that the cDNA library preparation provides a good reproducibility as a high correlation can be observed between the miRNAs detected in specimens processed differently (Frozen vs. FFPE). The two lower panels display the correlation between miRNA expression data from the two different frozen tumors and between the two different FFPE tumors. As indicated in Figure 6C, the miRNA expression differences identified between the two fresh frozen tumors were correlated with the differences detected between the two matched FFPE tumor specimens. Significance miRNA expression differences were detectable both in fresh frozen and FFPE tumors.
To further evaluate the sensitivity of this approach, miRNA expression data from 12 archived FFPE specimens and 4 fresh frozen RNA specimens were used (Figure 6D). The 16 different RNA specimens were individually 3' barcoded and all used for the preparation of a single cDNA library. The RNA samples used in this library included two breast cell lines, the MCF10A (normal-like cell line) and the MCF7 (breast cancer cell line) with RNA from fresh cells and from their archived FFPE counterparts23, matched fresh frozen and FFPE RNA samples from the two breast cancer samples analyzed independently (IBC1 and IBC2 in Figure 6A and 6B), and matched fresh frozen and FFPE RNA samples from normal cervical samples (Cx). Additionally, archived FFPE specimens from normal (normal Br1, normal Br2, and normal Br3), and cancer breast tissues (IBC 5, IBC 6, and IBC 7), without their fresh frozen counterparts were analyzed. As observed on the heatmap, regardless of fresh frozen or FFPE RNA origin, miRNA expression profiles of the same cells (MCF10 or MCF7), or the same tissues (IBC1, IBC2, or Cx) clustered together. Additionally, as noted on the unsupervised cluster, from left to right, normal breast cells and tissues clustered together while breast tumors and tumor cells clustered on the right. The cervical tissue, which displayed a different miRNA expression profile, clustered on the right of the heatmap.
Considering that most of the clinically archived FFPE specimens do not have fresh frozen counterparts but that they can be retrieved after different storage duration, it was sought to determine if the optimized cDNA library preparation protocol was applicable and reproducible with increasingly older FFPE specimens. As displayed in Figure 7, miRNA expression profiles from FFPE tissues archived for 18, 20, 22, 27, 30, and 35 years were obtained. The RNA was extracted using the optimized simultaneous RNA/DNA procedure21, and quadruplet RNA aliquots from each individual FFPE specimen were prepared on the same day and stored at -80 °C prior to library preparations. A total of 9 different FFPE specimens were analyzed in duplicate, where each individual RNA aliquot was ligated with different barcoded oligonucleotides (18 barcodes total), within the same library. This experiment was repeated during two consecutive weeks (week 1 and week 2). This allowed evaluation of the cDNA library preparation reproducibility with the same RNA specimens using two different barcodes within a single library, and between two different libraries with a one-week interval. As observed on Figure 7, the correlation coefficient remained above 0.96 regardless of the age of the specimen or the library preparation week; therefore, the optimized cDNA library preparation protocol provides a robust tool for reproducible analysis of FFPE specimens regardless of their archival time, for example, the 35-year-old archived FFPE RNA (see Breast #9) displayed high reproducible measures equivalent to those noted with the 20-year-old FFPE RNA samples (see Breast #3).

Figure 1: Oligonucleotides. All oligonucleotide sequences, their corresponding chemical modifications, and concentrations used in this protocol are described. The types of chemical modifications are described in the modification box and the abbreviated modifications displayed in the oligonucleotide sequences. The calibrator cocktail displays the list of the 10 individual RNA oligonucleotide calibrators, which were resuspended in a solution containing the carrier oligonucleotide (0.5 µM). The eighteen 3' barcoded oligonucleotide adapters are detailed with grey shading over the barcode's sequence. The RNA sequence of the 5' adapter, and the DNA sequences of the 3' PCR and 5' PCR primers are detailed. The RNA sequences of the two size marker oligonucleotides, namely referenced in the protocol as 19 nt-3' adapter and 24 nt-3' adapter size markers, are provided. All DNA and RNA oligonucleotides were commercially purchased. Please click here to view a larger version of this figure.
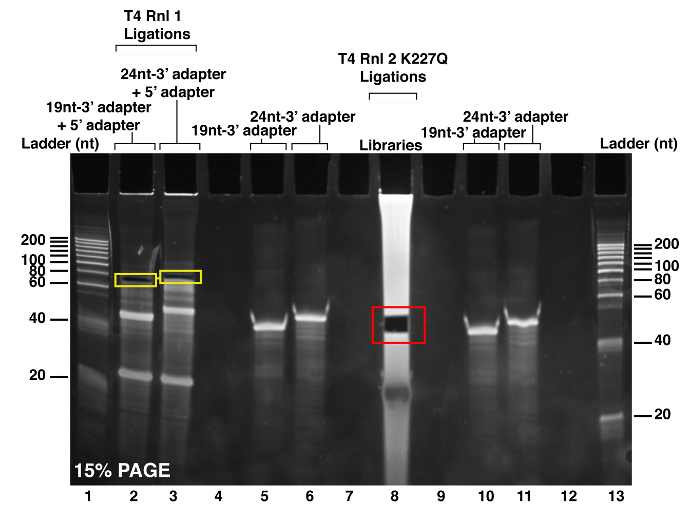
Figure 2: PAGE purification of the 3' barcoded RNA samples. After pooling and precipitating the 18 barcoded RNA samples, the resuspended RNA pellet is migrated and separated by electrophoresis on a 15% acrylamide gel (see well 8). The red square highlights the area containing the 3' barcoded microRNAs, which was excised with a scalpel blade and transferred into a nuclease-free siliconized microcentrifuge tube. A total of four wells, with two containing the 19 nt-3' adapter and two containing the 24 nt-3' adapter was set a well away, on each side of the library (see wells 5, 6, and 10, 11, respectively). As a test for the T4 RNA ligase 1 and for purification of the ligated size markers to be completed the next day, ligation reactions containing the 19 nt-3' adapter size marker with the 5' adapter and the 24 nt-3' adapter size marker with the 5' adapter were run in wells 2 and 3, respectively. The yellow squares display the excised bands representing the ligated size marker RNA oligonucleotides with the 5' adapter. These bands are purified, precipitated, and run during on the 12% PAGE purification (see Figure 3 below). Wells 1 and 13 contain the 20 nt size ladder, which helped confirm the anticipated sizes of the ligated products. Please click here to view a larger version of this figure.
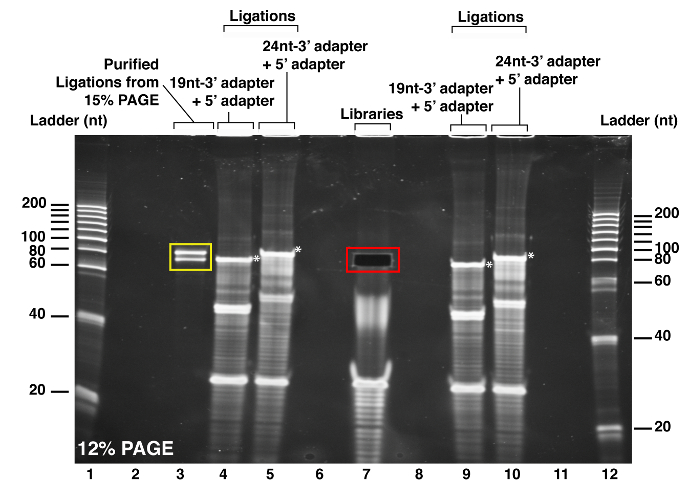
Figure 3: PAGE purification of the purified library after ligation of the 5' adapter. The purified RNA library was ligated with the 5' adapter and the expected product size was excised from the 12% PAGE using the ligated size markers (see red square). The products of the T4 RNA ligations between the 19 nt-3' adapter and the 5' adapter (wells 4 and 9) and between the 24 nt-3' adapter and the 5' adapter (wells 5 and 10) were run in parallel, on each side of the well containing the RNA library. The highest bands (see white asterisks) are the products of the 5' adapter ligation and used as the guide for the gel band excision containing the 5' adapter ligated RNA library. The ligation size marker ligation products purified on the previous PAGE are run in well 3. As observed in wells 1 and 12, the 20 nt size ladder was also run to validate the anticipated size of the RNA library. Please click here to view a larger version of this figure.
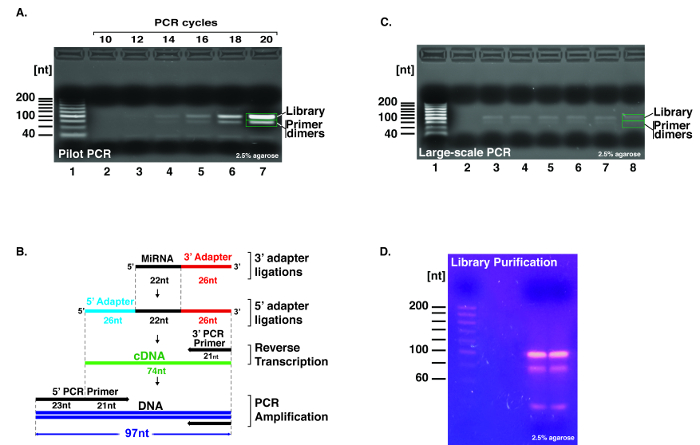
Figure 4: Pilot PCR and large-scale amplification of the cDNA library. The size and ratio of the PCR products were evaluated on a 2.5% agarose gel in the presence of ethidium bromide. (A) After reverse transcription of the barcoded RNA library, an aliquot of the cDNA library was amplified using the 5' and 3' PCR primers in a single pilot PCR reaction. Aliquots of the pilot PCR reactions were obtained at 10, 12, 14, 16, 18, and 20 cycles and migrated on a 2.5% agarose gel. As observed between wells 1 and 7, the presence of the cDNA library and adapter dimers was exponentially observable (see green rectangles). For this library, the PCR cycle selected for amplification of the cDNA library was 15. (B) This schematic displays the position and length of the different oligonucleotides and the resulting RNA and DNA products, which are identifiable on the PAGE and agarose gels. (C) Aliquots of the 6 large-scale PCR reactions (identified in A) were analyzed separately on a 2.5% agarose gel (see wells 3 to 8). The two types of PCR products, namely the library (upper band) and the primers dimers (lower band) are visible on this gel (see green rectangles). The blank PCR reaction without cDNA displays no PCR amplification (see well 2). The 20 nt size ladder allows verification of the product sizes (see well 1). (D) Gel image on a blue light transilluminator of the 2.5% agarose gel containing the pooled PCR reactions ran in two adjacent wells. As observed, the highest band in both wells is within the expected library size and the adapter dimer band is located below. The upper PCR bands were excised and purified with a gel extraction kit. Please click here to view a larger version of this figure.
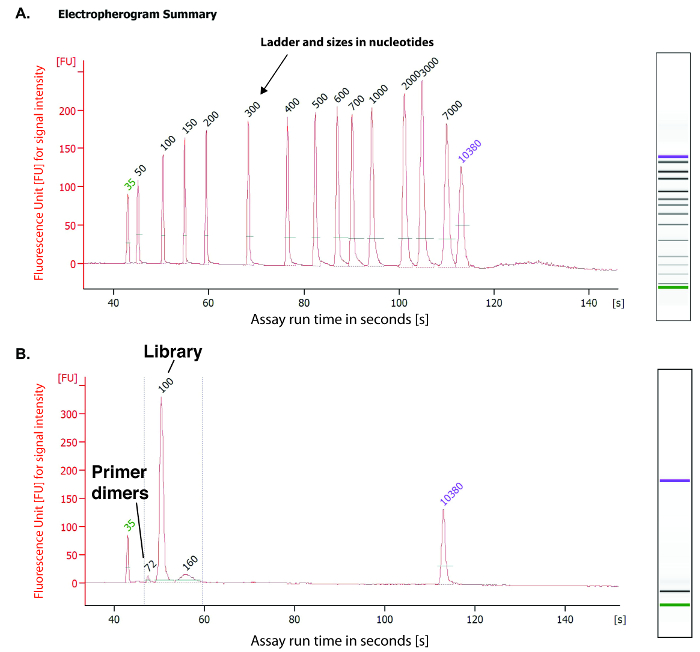
Figure 5: Evaluation of the purified PCR amplified DNA library. A small aliquot (1 µL) of the PCR amplified cDNA library is analyzed using a high sensitivity DNA chip on a microfluidics-based platform. (A) This panel displays the migration of the size markers for calibration of the instrument. (B) This panel displays the migration of the purified PCR amplified cDNA library. The highest peak is measured at a size of 100 bp (see asterisk) and represents the cDNA library. The small peak evaluated at 72 bp by the instrument represents the primer adapter dimers. The 100 bp peak detected by microfluidics based platform reveals the estimated size for the amplified cDNA library, which contains 18 individual barcoded RNA specimens for subsequent NGS on a high-throughput sequencing system. Please click here to view a larger version of this figure.
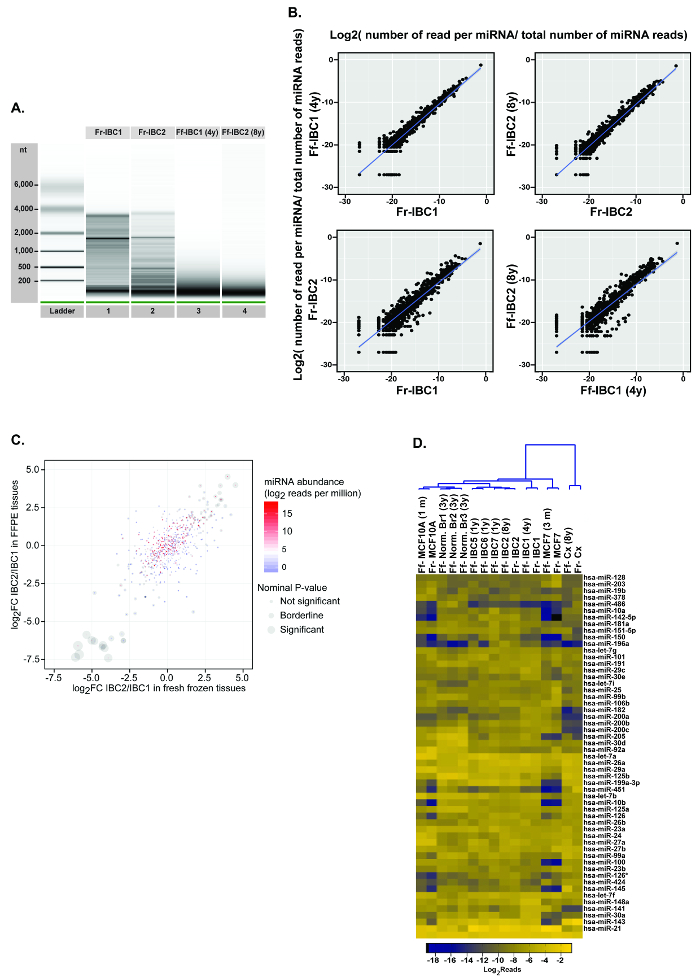
Figure 6: cDNA library preparation and next-generation sequencing using matched fresh frozen and formalin-fixed paraffin embedded specimens. (A) Total RNA extracted from matched fresh frozen and FFPE invasive ductal carcinoma (IDC) tumors was analyzed on a total RNA chip on a microfluidics based platform. (B) Total RNA from matched fresh frozen and FFPE specimens (IBC 1 and IBC2) underwent the cDNA library preparation protocol and the miRNA sequencing data were plotted. (C) DifferentialmiRNA expression between IBC1/IBC2 specimens and correlation between frozen and FFPE paired samples. miRNA expression is displayed in log count per million (CPM), from high to low reads (red to blue color). The significance of the expression difference between the two tissue pairs, per miRNA, is displayed by the gray circles, with high and low expression miRNAs identified in fresh and FFPE samples. (D) Total RNA extracted from matched fresh and FFPE RNA specimens of breast cell lines (MCF10A and MCF7), human invasive breast cancer (IBC 1 and IBC2), cervical tissue (Cx), archived normal breast tissues (normal Br1, normal Br2, and normal Br 3), and archived invasive breast cancer (IBC 5, IBC 6, and IBC7) underwent the cDNA library preparation within the same run. The NGS data of the miRNAs detected in the libraries are displayed in a heat-map configuration. This figure has been modified from Loudig et al.20 Please click here to view a larger version of this figure.
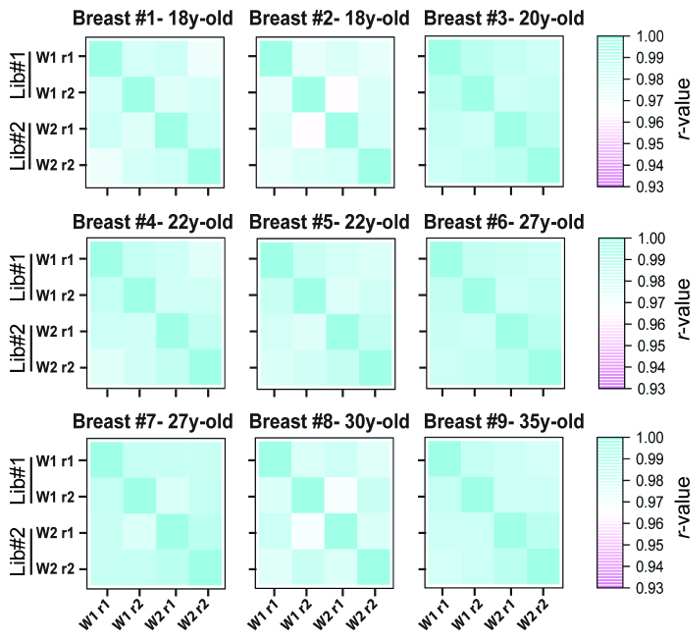
Figure 7: cDNA library preparation and miRNA expression correlation between replicates using older archived FFPE specimens. Total RNA from 18, 20, 22, 27, 30, and 35-year-old archived FFPE breast tissue specimens underwent our optimized cDNA library preparation protocol. Replicate RNA samples from 9 different FFPE specimens were ligated with different 3' barcoded oligonucleotides and analyzed within the same library on week 1 (w1). The same experiment was repeated within a one-week interval (week 2 or w2). Reproducibility measures of duplicated miRNA expression data between the same archived RNA specimens are displayed in the heatmaps and with correlation coefficients evaluated between 0.93 and 0.99. This figure has been modified from Loudig et al.20 Please click here to view a larger version of this figure.
Discussion
A highly reproducible and robust cDNA library preparation protocol for NGS of small RNAs archived in FFPE RNA specimens is presented in this protocol, which is a modified and optimized version of the procedure described by Hafner et al.22
All steps of this protocol have been optimized for use with older archived and compromised total RNA recovered from FFPE specimens. The key step of this protocol, for processing small amounts of FFPE RNA, resides in the pooling of all RNA samples (i.e., 18 individual 100 ng FFPE RNA samples) after individual ligations with the 18 unique 3' barcoded adapters. This critical step allows for the 18 FFPE RNA samples to uniformly undergo all subsequent biochemical and enzymatic steps necessary for preparation of the small RNA cDNA library. Additionally, this step maximizes the amount of RNA undergoing precipitations and gel purifications by enhancing the carrier effect of the small RNA molecules and by facilitating pellet observation and gel selections. Another key feature of this protocol, which further highlights its versatility when working with small amounts of FFPE RNA, is that a pilot PCR reaction is used to identify the optimal amplification cycle of the cDNA library. This step also provides insights into the dynamics of the library amplification versus primer dimer amplification, which is critical when preparing the large-scale PCR reaction and purifying the small RNA library on the agarose gel. The data added to this protocol demonstrate the reproducibility of this approach between matched frozen and FFPE specimens, and also highlight its applicability to older FFPE RNA samples from specimens archived for up to 35 years.
This protocol was modified from its original version, so it is optimized for preparation of small RNA libraries made from lower quality and quantity of chemically modified and compromised FFPE RNA. One aspect of this procedure that was modified to successfully ligate and amplify small RNAs from FFPE specimens relates to the amount of calibrator cocktail spiked during the initial 3' barcoded adapter ligation, for which input was reduced to 0.026 nM to prevent competition with the ligation of low abundancy small RNAs present in FFPE RNA samples. Another important modification to the original procedure was the removal of all radioactively labeled size-markers used during selection of the ligated small RNAs on the PAGE gels. Use of these radiolabeled size-markers not only restricted the applicability of this approach to laboratories certified for use of radio-isotopes but also prevented observation of the RNA products on the gels. Instead, in this optimized protocol, size markers are run in wells adjacent to the library, on the PAGE gel, and visualized directly with a fluorescent dye to direct excision of the ligated small RNAs. Importantly, the fluorescent dye is lightly sprayed on the gel to prevent diffusion of the RNA products and then visualized on a blue light box. Subsequently, as a measure of precaution and to improve the diffusion of the ligated small RNAs contained in the excised PAGE gel fragments, gel-breaker tubes are used to uniformly crush the gel prior to incubation in a NaCl solution overnight at 4 °C under agitation. All these steps were carefully evaluated to work with smaller amounts of material.
This protocol was applied to FFPE RNA samples from different sources (organs and institutions) that had been stored for different amounts of time. The analyses revealed the high reproducibility of sequencing small RNAs from fresh and frozen specimens, when using this optimized procedure. Additional analyses using older archived FFPE specimens, stored for up to 35 years, further demonstrated the protocol's vast applicability to clinical specimens. The minimum FFPE RNA input requirement for FFPE specimens was shown to be 100 ng. This low requirement allows applicability of this protocol to a variety of lesions of different sizes and availability, but it would not allow the analysis of single cell FFPE RNA. It is important to note, however, that this optimized protocol has also been used with total RNA extracted from circulating exosomes with inputs of less than 1 ng and shown to provide highly reproducible small RNA expression profiles (data not shown). This low input suggests that small RNAs in FFPE samples, although representative of the original fresh tissue, are present in much lower proportions than in total RNA from circulating exosomes.
In recent work, where this optimized protocol was applied to clinically classified DCIS FFPE specimens, it was found that miRNA expression differences identified by NGS of the cDNA library could be validated by quantitative PCR. This work demonstrated the feasibility of using DCIS lesions from archived tissues from different institutions for large-scale retrospective studies [20]. This study also highlighted the robustness of this cDNA library preparation when performed at different times (at intervals of several weeks), without compromising reproducibility and sensitivity of the assay.
Considering the vast amount of clinically classified archived FFPE specimens, this optimized protocol provides a robust tool for preparation of cDNA libraries in large-scale retrospective analyses and for the potential identification of miRNA biomarkers associated with cancer development21.
Divulgazioni
The authors have nothing to disclose.
Acknowledgements
We thank Dr. Thomas Tuschl, head of the laboratory for RNA molecular biology, as well as members of his laboratory for their support and for sharing the technology developed in his laboratory and providing access to the RNAworld pipeline. We also thank Dr. Markus Hafner for sharing his protocol and providing detailed descriptions on all biochemical and enzymatic steps used in his initial procedure.
Materials
| 1% Triton x-100 | Invitrogen | HFH10 | |
| 10mM ATP | Ambion | AM8110G | |
| 10X dNTPs | Ambion | AM8110G | |
| 10x TBE | Thermofisher Scientific | 15581044 | |
| 14M Mercaptoethanol | Sigma | O3445I-100 | |
| 20 nt ladder | Jena Bioscience | M-232S | |
| 20mg/ml Bovine Serum Albumine | Sigma | B8894-5ML | |
| 50X Titanium Taq | Clontech Laboratories | 639208 | |
| Ammonium Persulfate | Fisher Scientific | 7727-54-0 | |
| BRL Vertical Gel Electrophoresis System with glass plates and combs | GIBCO | V16 | |
| Dimethyl sulfoxide (DMSO) | Sigma | D9170-5VL | |
| Eppendorf microcentrifuge 5424R | USA scientific | 4054-4537Q | |
| Eppndorf Thermomixer | USA scientific | 4053-8223Q | |
| Fisherbrand™ Siliconized Low-Retention Microcentrifuge Tubes 1.5ml | Fisher Scientific | 02-681-320 | |
| Gel Breaker Tube 0.5 ml | IST Engineering Inc, | 3388-100 | |
| Gel electrophoresis apparatus 7cm x10cm- Mini-sub Cell GT with gel trays and combs | Biorad | 1704446 | |
| Glycoblue | Ambion | AM9516 | |
| Jersey-Cote | LabScientific, Inc | 1188 | |
| KcL 2M | Ambion | AM9640G | |
| MgCl2 1M | Ambion | AM9530G | |
| Minifuge dual rotor personal centrifuge | USA scientific | 2641-0016 | |
| Model V16 polyacrylamide gel electrophoresis apparatus, glasses, combs, and spacers | Ciore Life Science | 21070010 | |
| Oligonucleotides | IDT | Defined during order | |
| Owl EasyCast B2 mini electrophoresis system- with gel trays and combs | Thermofisher Scientific | B2 | |
| Qiaquick Gel Extraction kit | Qiagen | 28704 | |
| Restriction enzyme PmeI | NEB | R0560S | |
| RNase-free water | Ambion | AM9932 | |
| Safe Imager 2.0 | Life Technologies | G6600 | |
| Safe Imager 2.0 blue light transilluminator | Thermofisher | G6600 | |
| SeaKem LE agarose | Lonza | 50002 | |
| Superscript III reverse transcription kit | Invitrogen | 18080-044 | |
| SybrGold | Life Technologies | S11494 | |
| T4 RNA Ligase 1 | NEB | M0204S | |
| T4 RNA Ligase 2 Truncated K227Q | NEB | 0351L | |
| TEMED | Fisher Scientific | O3446I-100 | |
| Themocycler with heated lid | Applied Biosystem | 4359659 | |
| Tris 1M pH 7.5 | Invitrogen | 15567027 | |
| Tris 1M pH8.0 | Ambion | AM9855G | |
| UltraPure Sequagel system concentrate, diluent, and buffer | National Diagnostics | EC-833 |
Riferimenti
- Peiró-Chova, L., Peña-Chilet, M., López-, J. A., García-Gimenez, J. L., Alonso-Yuste, E., Burgues, O., et al. High stability of microRNAs in tissue samples of compromised quality. Virchows. Arch. 463, 765-774 (2013).
- Klopfleisch, R., Weiss, A. T., Gruber, A. D. Excavation of a buried treasure–DNA, mRNA, miRNA and protein analysis in formalin fixed, paraffin embedded tissues. Histol. Histopathol. 26, 797-810 (2011).
- Kakimoto, Y., Kamiguchi, H., Ochiai, E., Satoh, F., Osawa, M. MicroRNA Stability in Postmortem FFPE Tissues: Quantitative Analysis Using Autoptic Samples from Acute Myocardial Infarction Patients. PLoS One. 10 (6), e0129338 (2015).
- Giricz, O., Reynolds, P. A., Ramnauth, A., Liu, C., Wang, T., Stead, L., et al. Hsa-miR-375 is differentially expressed during breast lobular neoplasia and promotes loss of mammary acinar polarity. J. Pathol. 226, 108-119 (2012).
- Hui, A. B., Shi, W., Boutros, P. C., Miller, N., Pintilie, M., Fyles, T., et al. Robust global micro-RNA profiling with formalin-fixed paraffin-embedded breast cancer tissues. Lab. Invest. 89, 597-606 (2009).
- Zhang, X., Chen, J., Radcliffe, T., Lebrun, D. P., Tron, V. A., Feilotter, H. An array-based analysis of microRNA expression comparing matched frozen and formalin-fixed paraffin-embedded human tissue samples. J. Mol. Diagn. 10, 513-519 (2008).
- Kolbert, C. P., Feddersen, R. M., Rakhshan, F., Grill, D. E., Simon, G., et al. Multi-platform analysis of microRNA expression measurements in RNA from fresh frozen and FFPE tissues. PLoS One. 8, e52517 (2013).
- Meng, W., McElroy, J. P., Volinia, S., Palatini, J., Warner, S., Ayers, L. W., et al. Comparison of microRNA deep sequencing of matched formalin-fixed paraffin-embedded and fresh frozen cancer tissues. PLoS One. 8 (5), e64393 (2013).
- Liu, A., Tetzlaff, M. T., Vanbelle, P., Elder, D., Feldman, M., Tobias, J. W., et al. MicroRNA expression profiling outperforms mRNA expression profiling in formalin-fixed paraffin-embedded tissues. Int. J. Clin. Exp. Pathol. 2, 519-527 (2009).
- Dijkstra, J. R., Mekenkamp, L. J., Teerenstra, S., De Krijger, I., Nagtegaal, I. D. MicroRNA expression in formalin-fixed paraffin embedded tissue using real time quantitative PCR: the strengths and pitfalls. J Cell Mol Med. 16, 683-690 (2012).
- Siebolts, U., Varnholt, H., Drebber, U., Dienes, H. P., Wickenhauser, C., Odenthal, M. Tissues from routine pathology archives are suitable for microRNA analyses by quantitative PCR. J. Clin. Pathol. 62, 84-88 (2009).
- Jang, J. S., Simon, V. A., Feddersen, R. M., Rakhshan, F., Schultz, D. A., Zschunke, M. A., Lingle, W. L., Kolbert, C. P., Jen, J. Quantitative miRNA expression analysis using fluidigm microfluidics dynamic arrays. BMC Genomics. 12, 144 (2011).
- Andreasen, D., Fog, J. U., Biggs, W., Salomon, J., Dahslveen, I. K., Baker, A., Mouritzen, P. Improved microRNA quantification in total RNA from clinical samples. Methods. 50 (4), S6-S9 (2010).
- Kelly, A. D., Hill, K. E., Correll, M., Wang, Y. E., Rubio, R., Duan, S., et al. Next-generation sequencing and microarray-based interrogation of microRNAs from formalin-fixed, paraffin-embedded tissue: preliminary assessment of cross-platform concordance. Genomics. 102, 8-14 (2013).
- Visone, R., Croce, C. M. MiRNAs and cancer. Am. J. Pathol. 174, 1131-1138 (2009).
- Lu, J., Getz, G., Miska, E. A., Alvarez-Saavedra, E., Lamb, J., Peck, D., et al. MicroRNA expression profiles classify human cancers. Nature. 435, 834-838 (2005).
- Cho, W. C. MicroRNAs: potential biomarkers for cancer diagnosis, prognosis and targets for therapy. Int. J. Biochem. Cell. Biol. 42, 1273-1281 (2010).
- Hui, A., How, C., Ito, E., Liu, F. F. Micro-RNAs as diagnostic or prognostic markers in human epithelial malignancies. BMC Cancer. 11, 500 (2011).
- Tam, S., de Borja, R., Tsao, M. S., McPherson, J. D. Robust global microRNA expression profiling using next-generation sequencing technologies. Lab. Invest. 94, 350-358 (2014).
- Loudig, O., Wan, T., Ye, K., Lin, J., Wang, Y., Ramnauth, A., et al. Evaluation and Adaptation of a Laboratory Based cDNA Library Preparation Protocol for Retrospective Sequencing of Archived MicroRNAs from up to 35-Year-Old Clinical FFPE Specimens. Int J Mol Sci. 18, e627 (2017).
- Kotorashvili, A., Ramnauth, A., Liu, C., Lin, J., Ye, K., Kim, R., et al. Effective DNA/RNA co-extraction for analysis of microRNAs, mRNAs, and genomic DNA from formalin-fixed paraffin-embedded specimens. PLoS One. 7 (4), e34683 (2012).
- Hafner, M., Renwick, N., Farazi, T. A., Mihailović, A., Pena, J. T., Tuschl, T. Barcoded cDNA library preparation for small RNA profiling by next-generation sequencing. Methods. 58 (2), 164-170 (2012).
- Loudig, O., Brandwein-Gensler, M., Kim, R. S., Lin, J., Isayeva, T., Liu, C., et al. Illumina whole-genome complementary DNA-mediated annealing, selection, extension and ligation platform: assessing its performance in formalin-fixed, paraffin-embedded samples and identifying invasion pattern-related genes in oral squamous cell carcinoma. Hum. Pathol. 42 (12), 1911-1922 (2011).

