Immunostimulatory Agent Evaluation: Lymphoid Tissue Extraction and Injection Route-Dependent Dendritic Cell Activation
Summary
Experimental procedures for the subsequent extraction of lymphatic tissues to test lymphoid dendritic cell activation are described after treatment of an immunostimulating nanomaterial.
Abstract
For evaluation of a new therapeutic agent for immunotherapy or vaccination, analysis of immune cell activation in lymphatic tissues is essential. Here, we investigated immunological effects of a novel lipid-DNA immunostimulant in nanoparticle form from different administration routes in the mouse: oral, intranasal, subcutaneous, footpad, intraperitoneal, and intravenous. These injections will directly influence the immune response, and harvesting lymphatic tissues and analysis of dendritic cell (DC) activation in the tissues are crucial parts of these evaluations. The extraction of mediastinal lymph nodes (mLNs) is important but quite complex because of the size and location of this organ. A stepwise procedure for harvesting the inguinal lymph node (iLN), mLN, and spleen and analyzing DC activation by flow cytometry is described.
Introduction
Advances in immunology and nanomaterials have led to an abundance of potential new therapeutic strategies for applications in biomedicine, including drug delivery and immunostimulation. Optimization of the administration route is a vital aspect affecting the efficacy of immunostimulatory agents. An immunostimulatory nanoparticle (INP) consisting of DNA is a newly developed nano-immune adjuvant self-assembled by microphase separation because of the amphiphilic structure of lipid-DNA1. Therefore, protocols for INP involving administration of the material1 in vivo via different routes, and three procedures for harvesting appropriate tissues such as the inguinal lymph node (iLN), mediastinal LN (mLN), and spleen, are described. Finally, these tissues were analyzed for dendritic cell (DC) activation, the most powerful antigen-presenting cells in the immune system. This protocol can also be applied for evaluating antigens, antibodies, or other immune adjuvants2.
We tested the INP formulation because it is an agent that has shown great promise. INP is a Toll-like receptor 9 (TLR9) adjuvant material that contains nucleic acids, for which assessment of immunostimulation efficacy is required to test different injection methods3. In this context, the stimulation of DCs is a potent endpoint for in vivo evaluation. After antigen or immunostimulatory molecules are phagocytosed by DCs in the peripheral tissues or blood, these cells migrate to lymphoid organs such as the spleen and LNs4,5. Thus, DC activation was analyzed in the spleen, iLN, and mLN of the injected animals. Properly harvesting of these tissues is therefore also crucial for evaluating the immune response to a novel adjuvant or pathogens5. Such tissue harvesting is also important for developing a novel immunological methodology as a cancer therapy. Furthermore, this protocol can be used to verify the efficiency of other drugs, such as anti-human immunodeficiency virus therapeutics6.
Protocol
All experimental procedures including animal handling, sacrifice, and organ isolation were performed in strict accordance with the rules of the International Animal Care and Use Committee at Shanghai Public Health Clinical Center and Asan Medical Center. The study protocol was approved by the respective committees on the Ethics of Animal Experiments at Shanghai Public Health Clinical Center (Mouse Protocol Number: SYXK-2010-0098) and Asan Medical Center (2016-02-168).
1. Preparation of Material
NOTE: A nano-adjuvant, INP, comprised of a self-assembled lipid-DNA carrier, namely U4T, and CpG-motif containing oligonucleotide, eCpG, was employed in the current study to test the appropriate injection route for immunotherapy and vaccination in mice2. Importantly, other potential therapeutic molecules such as antigens, antibodies, and adjuvant agents can be substituted for INP and tested using the same methodology described below.
- INP formulation2,4,7
- Anneal U4T (160 µM, carrier) with eCpG (80 µM, containing a TLR9 agonist sequence) in the presence of 1x phosphate-buffered saline (PBS, pH 7.4) and MgCl2 (2 mM). The standard volume for annealing is between 50 and 110 µL in a PCR tube. Heat the mixture to 95 °C for 10 min and then slowly cool (1 °C/16 min) to 25 °C using a thermocycler. The annealing protocol requires approximately 2 h.
CAUTION: The U4T-containing liquid is very foamy and must be carefully handled. - Make 50 µL aliquots of the INP preparation; use 50 µL of the material for each injection.
NOTE: In the case of 100 µL per injection, repeat step 1.1 with proper calculation of ingredients.
- Anneal U4T (160 µM, carrier) with eCpG (80 µM, containing a TLR9 agonist sequence) in the presence of 1x phosphate-buffered saline (PBS, pH 7.4) and MgCl2 (2 mM). The standard volume for annealing is between 50 and 110 µL in a PCR tube. Heat the mixture to 95 °C for 10 min and then slowly cool (1 °C/16 min) to 25 °C using a thermocycler. The annealing protocol requires approximately 2 h.
2. General Animal Procedures
Note: Alternative methods for handling the mice can be used depending on the laboratory requirements and approved animal protocols8. The type of mice used is 6-week-old C57BL/6 and female mice.
- List of materials
- Prepare the liquid anesthetic (isoflurane), anesthetic machine, carbon dioxide (CO2) inhalation chamber, sterilized gauze, forceps, mouse restrainer, gloves, hooked forceps, 1-inch dissecting scissors, 2-inch dissecting scissors, alcohol for sterilization, and insulin syringe.
- Inhalant anesthesia
NOTE: Anesthesia is mandatory for intranasal, subcutaneous, footpad, and intravenous injections of mice9. An induction chamber with a vaporizer machine and oxygen tank connected is used for this procedure.- Place the animal in the induction chamber and make sure to tightly close the lid.
- Flow the oxygen at 1 L/min and set the vaporizer setting to an induction level of 4% for isoflurane.
- Vary the exposure time of animals in the chamber depending on animal weight; however, more than 10 s of exposure and monitoring every 5 min is necessary for anesthesia10.
NOTE: No pedal reflex indicates successful anesthesia. - Turn off the isoflurane inductor and flow 1 L/min of oxygen to prevent personnel exposure to isoflurane gas.
- Sacrifice
- Prepare a chamber for CO2 inhalation sacrifice in accordance with the NIH "Guidelines for Euthanasia of Rodents Using Carbon Dioxide"11.
- Test gag response to confirm that the animal is fully sacrificed.
- Verify that the mouse is properly sacrificed by pinching the tip of its foot and confirming no gag response. If there is a response, repeat step 2.2.
- Disinfection
- Disinfect all surface areas used for injections or dissections with alcohol spray and sterilized gauze.
- Organ storage
- Use forceps to remove all fat and blood surrounding the harvested organs. Store these harvested tissues individually in a Petri dish filled with 3 mL of PBS. The container and media may differ depending on the subsequent procedure requirements.
3. Injection Routes
- Use the six injection methods to investigate injection-dependent immune responses: oral, intranasal, subcutaneous, footpad, intraperitoneal, and intravenous8,12,13.
4. Isolation of Lymph Nodes and Spleen
Note: Use young and healthy lean mice (6 weeks old) for these procedures because the fats that typically build up around the lymph nodes in older mice are difficult to remove and can prevent proper visualization of the organ.
- Pre-harvesting steps
NOTE: This section describes the post-injection harvesting of the iLN, spleen, and mLN from mice for analyzing immune stimulation. Refer to steps 2.3-2.5 for sacrifice, gag response, and disinfection methods.- Pin the limbs of the mouse to the surgical foam board and sterilize the ventral side of the mouse with 70% ethanol.
- Pick a location 2 cm above the genital area and use hooked forceps to pull up the outer skin cover like a tent. Make an approximately 1-cm cut at this location and slide the scissors (2-inch) underneath the skin, making further dissection cuts.
- For the integument, cut vertically through the outer skin with both arms of the scissors (2-inch). Expose the internal organs covered with peritoneum.
- Pin the outer layer of the skin.
- Isolation of the inguinal lymph node (iLN)
- Locate the iLN at the left side of the leg following the conjunction of the blood vessel going down the left leg, which appears as a tilted letter 'Y'.
NOTE: For subcutaneously injected mice, choose an iLN on the same side of the injection location. - Uncover the layer with two forceps to rip the covered lipid layer and harvest the pale yellow iLN (bean shape, 2 mm). For subsequent treatment and storage of the tissue, see step 2.6.
- Locate the iLN at the left side of the leg following the conjunction of the blood vessel going down the left leg, which appears as a tilted letter 'Y'.
- Isolation of the spleen14
- Make a tent at the center of the peritoneum using hooked forceps in one hand and use small thin scissors (1 inch) in the other hand to cut the peritoneum.
- Use hooked forceps in the left hand to grasp the intestine and flip it over to locate the red bean-like spleen (length of approximately 14 mm) attached to the left upper side of the mouse abdomen.
- Gently pull out the spleen with another set of forceps in the right hand while detaching other organs with the hooked forceps. Follow step 2.6 to store the spleen.
NOTE: Care is required when harvesting the spleen, as it is an easily damaged soft organ.
- Isolation of the mediastinal lymph node (mLN)
NOTE: The mLN is located approximately 1 cm beneath the ribs and is covered by other organs such as the heart and lungs. Because of its location and small size, it is important to carefully expose the surrounding area using forceps and scissors (1-inch).- Cut the diaphragm and detach it from the ribs. Hold the body of the ribs with forceps and then cut the left and right side of the ribs with scissors (1-inch). Pin the cutoff ribs over the mouse's shoulder on the right to expose the heart and lungs.
NOTE: Take care not to cut any of the blood vessels around the heart and lungs, as leaking blood makes it very difficult to locate the tiny translucent mLN. If any blood vessels are cut, gently absorb the blood in the area with gauze. - Using hooked forceps in the right hand, flip the heart and lungs gently to the right until the backbone is exposed. Use a pair of regular forceps in the left hand to remove the iLN with the hooked forceps in the right hand to assist with this harvesting.
- Grab the lungs and flip them until the backbone is widely exposed. The mLN (a translucent bean-shaped structure of approximately 2 mm in size) is located between the lungs and the spine.
- Utilize the hooked forceps to expose the area around the mLN and the other forceps to extract the tissue.
NOTE: The mLN is surrounded by numerous other tissues which must be removed carefully with forceps before harvest. - See step 2.7 for instructions on storing the harvested mLN.
- Cut the diaphragm and detach it from the ribs. Hold the body of the ribs with forceps and then cut the left and right side of the ribs with scissors (1-inch). Pin the cutoff ribs over the mouse's shoulder on the right to expose the heart and lungs.
5. Preparation of samples for flow cytometry
Note: To assay the activation of DCs in the harvested iLN, spleen, and mLN, single-cell suspensions of these tissues are stained with fluorescence-conjugated antibodies as DC-specific markers, co-stimulatory molecules, and major histocompatibility complex molecules and analyzed by flow cytometry.
- Preparation of single-cell suspensions: spleen
- Add 5 mL of fresh culture medium (CM) into 6-mm culture dish and place the samples on ice.
- Remove surrounding blood and fat tissues and place the tissue in the lid of the culture dish.
- Cut the tissues into small fragments (<1 mm) with curved scissors. Suspend the fragments in PBS followed by spinning down the tissue using a centrifuge at 646 × g for 5 min and removing the supernatant.
- Suspend the samples again in 3 mL of CM and digest the fragments with 200 µL of solution of 2% fetal bovine serum (FBS) supplemented with collagenase IV for 20 min.
- Gently shake and incubate the sample at 37 °C for 1 h. Filter the digested tissues through nylon mesh (100 nm) and spin them down. Wash the single cells in 5 mL of PBS and remove the supernatant by centrifugation.
- Re-suspend the cells in 5 mL of cell isolating solution. Re-load another layer of 5 mL of clear cell isolation solution to form an aqueous layer-by-layer stack.
- Centrifuge the cell preparation at 2012 × g for 10 min. Obtain the supernatant, which is the lighter density fraction (<1.077 g/cm3), for subsequent flow cytometry analysis.
- Count the number of cells with a hemocytometer.
- Preparation of single-cell suspensions: lymph node
- Follow step 5.1.1-5.1.2.
- Add 5 mL of CM using a 10-mL pipet to suspend the cells by pipetting 3-4 times. After thoroughly suspending the cells, use sterile nylon mesh to filter the cells for collection in a 15-mL conical tube.
- Spin the cells down at 646 ×g for 7 min at 4 °C and remove the supernatant.
- Add an additional 5 mL of CM into 10-mL pipet to better suspend the cells by pipetting.
- Wash with PBS and count the number of cells.
- Flow cytometry analysis
- Aliquot 0.5-1 × 106 cells into each fluorescence-activated cell sorting (FACS) tube.
- Centrifuge at 646 × g at 4 °C for 5 min and aspirate the supernatant.
- Add 5 µL of each diluted fluorescence-conjugated antibody preparation to the cells to re-suspend the cell pellet and vortex.
NOTE: It is important to shield the cell pellet from light exposure as much as possible because fluorescently labeled antibodies are light-sensitive. Generate an antibody master mix and always add Fc block. - Prepare FACS buffer using 100 µL of PBS, 1% of heat-inactivated regular FBS, and 0.1% sodium azide.
- Place the sample in the tube at 4 °C for 30 min. Rinse the cells with 2-4 mL of FACS buffer and centrifuge the sample at 1700 rpm for 7 min at 4 °C.
- Re-suspend the cells in 500 µL of FACS buffer in the dark. Analyze the cells by flow cytometry.
Representative Results
To evaluate appropriate injection routes of INP for lymphatic tissue DC activation, the DC population as lineage was defined as –CD11c+ cells in the spleen, iLN, and mLN and analyzed the expression levels of co-stimulatory molecules. Treatment of INP by subcutaneous (s.c.) and intravenous (i.v.) injection promoted substantial increases in CD40, CD80, and CD86 expression in the spleen and iLN DCs (Figure 2B and 2C). Footpad and intraperitoneal (i.p.) injection of INP also considerably up-regulated the expression levels of CD40, CD80, and CD86 in the spleen and iLN DCs compared to in the PBS-treated control (Figure 2B and 2C). In stimulated mLN DCs, intranasal (i.n.) injection of INP promoted the highest up-regulation of co-stimulatory molecules compared to that in the PBS-treated control (Figure 2D). i.p. and i.v. injection of INP also induced marked increases in co-stimulatory molecule expression in the mLN, while oral, s.c., and footpad injection of INP did not induce activation of mLN DCs (Figure 2D).
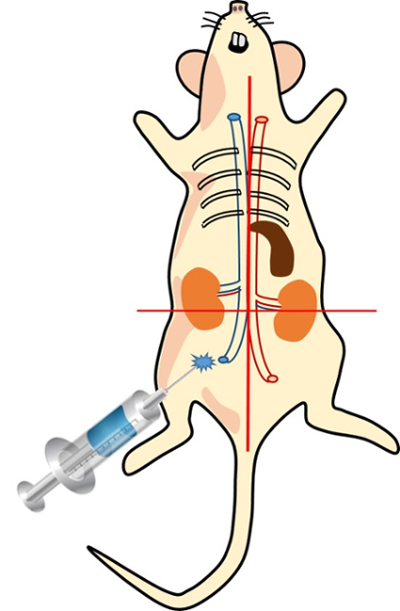
Figure 1: Location of intraperitoneal injection point Please click here to view a larger version of this figure.
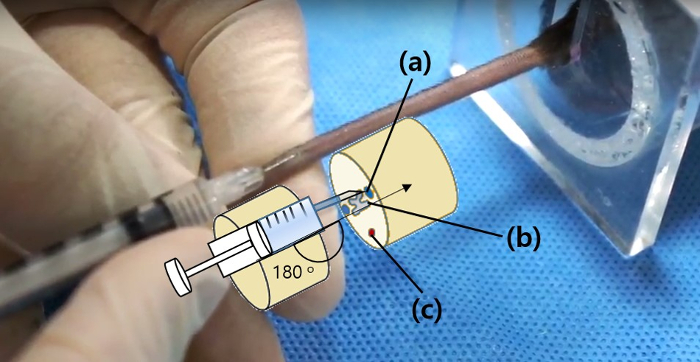
Figure 2: Accurate location of intravenous injection Please click here to view a larger version of this figure.
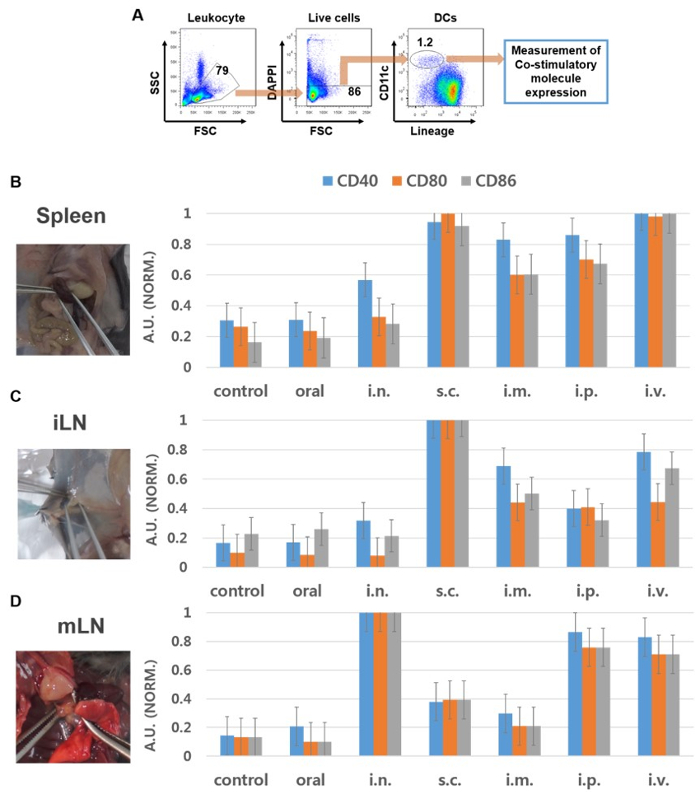
Figure 3: DC activation in lymphatic organs analyzed by flow cytometry. C57BL/6 mice were injected with INP by six different injection routes and 24 h after injection, the spleen, iLN and mLN were harvested. (A) The DC population in the lymphatic tissue cells was defined as lineage –CD11c+ cells in live leukocytes. (B-D) The expression levels of CD40, CD80, and CD86 were analyzed by flow cytometry in the spleen (B), iLN (C), and mLN (D). Data are averages from the analyses of six independent samples. Results are expressed as the means ± standard error of the mean (SEM). The statistical significance of the differences between experimental groups was calculated using analysis of variance with unpaired Student's t-test. p-values<0.05 were considered significant. * <0.05, **<0.01. Please click here to view a larger version of this figure.
Discussion
Many advances in nanotechnology and immunology have been achieved through therapeutic research of drug delivery and immunostimulation. Careful selection of the injection method is known to be important for immunostimulation, which was the focus of the present study.
Different injection routes were evaluated for a naturally non-toxic and biodegradable DNA-based material, INP (immunostimulatory nanoparticle), to determine which route yielded the best outcome. This approach is also relevant for delivering other therapeutic agents including antibodies, antigens, or other adjuvants.
To analyze the immune response to such injections, harvest of lymphatic tissue (spleen, iLN, and mLN) is required. Isolation of lymphatic tissues is the most crucial aspect of this protocol and required the use of a technique that has not been described previously in detail. Moreover, preparation of the single-cell suspension for further analyzing immune cells has not been well-described. This study focused on the extraction of the lymphatic tissues, particularly the iLN, mLN, and spleen, for analyzing DC activation. Systemic activation of the immune response mainly occurred through immune cells in the spleen. Additionally, the immune responses in the specific tissue were controlled by nearby lymph nodes. Evaluation of newly developed immune modulatory molecules should be conducted to determine whether the molecules can induce immune stimulation in the tissues. Therefore, the method for lymphatic tissue isolation and analysis of DC activation in the tissues can be used for newly developed immune stimulatory molecules.
To evaluate the immune stimulatory effect of INP, the iLN, mLN, and spleen were harvested and INP was shown to promote lymphatic DC activation. As determined previously, INP effectively targets TLR9 stimulation in DCs in mice2. Macrophages also express cytosolic TLR9 in mice. Therefore, INP injection may induce both DC and macrophage activation. However, macrophages reside in peripheral tissue that contribute to the phagocytosis of microbes in the tissue. Moreover, the antigen-presentation capacity and migratory effect to the lymphatic tissue of macrophages are comparably weaker than those in DCs. Therefore, evaluating the injection route of INPs for the immune stimulatory effect was suitable for examining DCs in the lymphatic tissue.
Therefore, the mode of immune adjuvant administration must be very carefully considered to achieve successful immunotherapy and vaccination using soft materials.
Divulgazioni
The authors have nothing to disclose.
Acknowledgements
This research was supported by Creative Materials Discovery Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT and Future Planning (NRF-2017M3D1A1039421) and by Marine Biotechnology Program funded by the Ministry of Oceans and Fisheries, Republic of Korea and a grant (20150220).
Materials
| Material | |||
| phosphate buffer saline | Corning | 21-040-CVR | Washing organs |
| (PBS, pH 7.4) | |||
| isoflurane solution | Aesica Queenborough limited | 26675-46-7 | Anesthesia process |
| Tuberculin 1mL syringe – | Junglim | N/A | Injection |
| 50 mL conical tube | S.P.L | 50050 | Anesthesia process |
| 1mL Insulin Syringe | (BD Ultra-FineTMII)_short needle | 324826 | Intramuscular Injection |
| DMEM High Glucose | Hyclone | SH30081.01 | Storing organs |
| Histopaque | Sigma-Aldrich | 10771 | FACS analysis |
| Ethyl alcohol anhydrous 99.5 % | Daejung | 4022-4110 | Disinfectant |
| Equipments | |||
| FineCycler C100 (Thermocycler) | Ssufine | – | Anealing |
| Centrifuge | Centrifuge | ||
| FACS tube | FALCON | 2052 | FACS analysis |
| Automated High-performance Flow Cytometer | BD (USA), FACSVerse | – | FACS analysis |
Riferimenti
- Park, H. . Preparation of dodecynyl modified DNA nanoparticles with unmethylated CpG adjuvant and ovalbumin for immunostimulation. , (2016).
- Jin, J. O., Park, H., Zhang, W., de Vries, J. W., Gruszka, A., Lee, M. W., Ahn, D. R., Herrmann, A., Kwak, M. Modular delivery of CpG-incorporated lipid-DNA nanoparticles for spleen DC activation. Biomaterials. 115, 81-89 (2017).
- Pal, I., Ramsey, J. D. The role of the lymphatic system in vaccine trafficking and immune response. Advanced Drug Delivery Reviews. 63 (10-11), 909-922 (2011).
- Jin, J. O., Kwak, M., Xu, L., Kim, H., Lee, T. H., Kim, J. O., Liu, Q., Herrmann, A., Lee, P. C. W. Administration of soft matter lipid-DNA nanoparticle as the immunostimulant via multiple routes of injection in vivo. ACS Biomaterial Science & Engineering. 3 (9), 2054-2058 (2017).
- Worbs, T., Hammerschmidt, S. I., Förster, R. Dendritic cell migration in health and disease. Nature Review Immunology. 17 (1), 30-48 (2017).
- Milling, S. W. F., Jenkins, C., MacPherson, G. Collection of lymph-borne dendritic cells in the rat. Nature Protocols. 1 (5), 2263-2270 (2006).
- Desormeaux, A., Bergeron, M. G. Lymphoid tissue targeting of anti-HIV drugs using liposomes. Methods in Enzymology. 391, 330-351 (2005).
- Machholz, E., Mulder, G., Ruiz, C., Corning, B. F., Pritchett-Corning, K. R. Manual restraint and common compound administration routes in mice and rats. Journal of Visualized Experiments. (67), (2012).
- Turner, P. V., Brabb, T., Pekow, C., Vasbinder, M. A. Administration of substances to laboratory animals: routes of administration and factors to consider. Journal of the American Association for Laboratory Animal Science. 50 (5), 600-613 (2011).
- . . General anesthesia of mice and rats. , (2016).
- . . Guidelines for the Euthanasia of Animals. , (2013).
- . JoVE Science Education Database. Lab Animal Research. Compound Administration I. Journal of Visualized Experiments. , (2018).
- . JoVE Science Education Database. Lab Animal Research. Compound Administration III. Journal of Visualized Experiments. , (2018).
- Mebius, R. E., Kraal, G. Structure and function of the spleen. Nature Review Immunology. 5 (8), 606-616 (2005).

