Imaging Ca2+ Responses During Shigella Infection of Epithelial Cells
Summary
Here, we present protocols to visualize calcium (Ca2+) responses elicited by HeLa cells infected by Shigella. By optimizing the parameters of bacterial infection and imaging with Ca2+ fluorescent probes, atypical global and local Ca2+ signals induced by bacteria over a large range of infection kinetics are characterized.
Abstract
Ca2+ is a ubiquitous ion involved in all known cellular processes. While global Ca2+ responses may affect cell fate, local variations in free Ca2+ cytosolic concentrations, linked to release from internal stores or an influx through plasma membrane channels, regulate cortical cell processes. Pathogens that adhere to or invade host cells trigger a reorganization of the actin cytoskeleton underlying the host plasma membrane, which likely affects both global and local Ca2+ signaling. Because these events may occur at low frequencies in a pseudo-stochastic manner over extended kinetics, the analysis of Ca2+ signals induced by pathogens raises major technical challenges that need to be addressed.
Here, we report protocols for the detection of global and local Ca2+ signals upon a Shigella infection of epithelial cells. In these protocols, artefacts linked to a prolonged exposure and photodamage associated with the excitation of Ca2+ fluorescent probes are troubleshot by stringently controlling the acquisition parameters over defined time periods during a Shigella invasion. Procedures are implemented to rigorously analyze the amplitude and frequency of global cytosolic Ca2+ signals during extended infection kinetics using the chemical probe Fluo-4.
Introduction
Ca2+ regulates all known cell processes, including cytoskeletal reorganization, inflammatory responses, and cell death pathways related to host-pathogen interactions1,2,3. Under physiological conditions, basal cytosolic Ca2+ concentrations are low, in the hundreds of nM range, but can be subjected to transient increases upon agonist stimulation. These variations often show oscillatory behavior through the action of pumps and channels at the plasma and endoplasmic reticulum membranes. The pattern of these oscillations is characterized by the period, duration, and amplitude of Ca2+ increases, and is decrypted by cells which, in turn, trigger specific responses in what is known as the Ca2+ code4,5. A sustained increase in the cytosolic Ca2+ concentration under pathological conditions may lead to cell death associated with the permeabilization of mitochondrial membranes and the release of pro-apoptotic or necrotic factors6,7.
Shigella, the causative agent of bacillary dysentery, invades epithelial cells by injecting effectors into host cells using a type III secretion system (T3SS)8,9. A Shigella invasion of host cells is associated with local and global Ca2+ signals elicited by the T3SS. As for pore-forming toxins, the T3SS translocon that inserts into host cell membranes and is required for the injection of T3SS effectors is likely responsible for the activation of PLC and the inositol (1, 4, 5) trisphosphate (InsP3)-dependent Ca2+ release. The combination of the localized PLC stimulation and the accumulation of polymerized actin at sites of a Shigella invasion result in an atypically long-lasting InsP3-dependent Ca2+ release10. The type III effector IpgD, a phosphatidyl 4,5 bisphosphate (PIP2)-4-phosphatase, limits the local amount of PIP2, thereby controlling the quantity of available substrate for PLC to generate InsP3, which contributes to the confinement of local Ca2+ responses at bacterial invasion sites11,12. These local Ca2+ responses likely contribute to the actin polymerization at Shigella invasion sites10. Global Ca2+ responses that are also elicited by Shigella, however, are dispensable for the bacterial invasion process but trigger the opening of connexin hemichannels at the plasma membrane and the release of ATP in the extracellular compartment. Released ATP acting in a paracrine manner, in turn, stimulates Ca2+ oscillatory responses in cells next to the infected cell. IpgD is also responsible for shaping global Ca2+ responses into erratic isolated responses with slow dynamics. Eventually, upon a prolonged bacterial infection, IpgD leads to the inhibition of InsP3-mediated Ca2+ signals. Through its interference with Ca2+ signaling, IpgD delays a Ca2+-dependent calpain activation leading to the disassembly of focal adhesion structures and the premature detachment of infected cells13.
While Ca2+ signals are involved in critical aspects of pathogenesis, the use of a microorganism raises a number of technical challenges that are not encountered in classical agonist studies. The protocols described here use the commonly used fluorescent Ca2+ chemical indicator Fluo-4 that we engineered to characterize local Ca2+ signals during a Shigella infection. Steps critical for the detection of these signals are discussed, as well as procedures implemented for their quantitative analysis that is required to characterize the role of bacterial effectors in Ca2+ signaling.
Protocol
1. Preparations
- Bacteria preparation
- Plate the bacteria—Shigella wild-type strain expressing the AfaE adhesin (M90T-AfaE)—onto a trypticase soy (TCS) agar plate containing 0.01% Congo red (CR) and incubate them for 18 h at 37 °C.
Note: To increase their reproducibility, the Shigella plates obtained in step 1.1.1 are stored at 4 °C and should be used within a week, because all colonies on CR medium will eventually turn red over time. - Inoculate TCS broth pre-culture by picking 3 red colonies from the streak plates.
Note: An inoculation with 3 colonies is performed to limit the probability of picking a single clone whose virulence plasmid has undergone a genetic recombination. - Grow liquid bacterial cultures in a shaking incubator for 16 h at 37 °C at 200 rpm. Add ampicillin at a final concentration of 75 mg/mL. Antibiotic concentrations should not exceed 3x the minimal inhibitory concentration, as an excess of antibiotic may lead to the loss of the large virulence plasmid.
Note: The maintenance of the Shigella virulence plasmid should be verified by plating the bacterial cultures on CR plates supplemented with appropriate antibiotic concentrations. The presence of white colonies indicates plasmid loss. - Inoculate the TCS culture with the bacterial pre-culture at a 1:100 dilution. Incubate it at 37 °C in a shaking incubator for 2 h at 200 rpm. Ensure that the optical density at 600 nm (OD600nm) is 0.2 – 0.4.
- Centrifuge the bacterial culture for 2 min at 13,000 x g at 21 °C and resuspend the pellet in an equivalent volume of EM medium (120 mM NaCl, 7 mM KCl, 1.8 mM CaCl2, 0.8 mM MgCl2, 5 mM glucose, and 25 mM HEPES, pH = 7.3).
- Dilute the bacterial suspension in an EM buffer at a final OD600nm of 0.1 and use it immediately or store it at 21 °C and use it within the next 60 min.
- Plate the bacteria—Shigella wild-type strain expressing the AfaE adhesin (M90T-AfaE)—onto a trypticase soy (TCS) agar plate containing 0.01% Congo red (CR) and incubate them for 18 h at 37 °C.
- Cell preparation
- Culture HeLa cells in DMEM with 1 g/L of glucose supplemented with 10% fetal calf serum (FCS) and grow them at 37 °C with 10% CO2. Grow TC-7 grown in DMEM with 4.5 g/L of glucose, containing 10% FCS and non-essential amino acids in a 37 °C incubator containing 10% CO2.
- For cell maintenance, split the cells regularly to avoid a growth-contact inhibition linked to confluent states; this is critical for a high Ca2+ probe loading/transfection efficiency.
- Plate HeLa cells onto sterile 25-mm diameter circular coverslips in 6-well plates at a density of 3 x 105 cells/well the day before the experiment for the HeLa cells.
- For TC-7 cells, trypsinize and count cells. Seed them at 4 x 105 cells/well and incubate them 5 – 7 days at 37 °C in a 10% CO2-incubator before the experiments to allow for a polarization.
- Refresh the cell culture medium every day until the day of the experiment.
Note: Glass-bottom 35-mm diameter disposable chambers may also be used as an alternative to the coverslips-containing wells. If the latter are used, temperature control through a heated stage or objective is not sufficient, and a thermostat incubation chamber mounted on the microscope stage is required.
- Refresh the cell culture medium every day until the day of the experiment.
- The day of the experiment, remove the medium and wash the cells 3x with 1 mL EM medium at 21 °C.
- Load the cells with 3 mM Fluo-4-AM14 in an EM buffer for 30 min at 21 °C.
Note: While the loading of sub-confluent HeLa cells does not raise major problems, the loading of polarized TC-7 cells is often heterogeneous and poorly efficient. For these cells, we found that the loading efficiency is enhanced by the addition of the dispersing agent—pluronic acid—at a final concentration of 0.1% and of the anion-transporter inhibitor bromosulfophtalein at a final concentration of 20 µM to the Fluo-4-AM-containing EM. The use of ratiometric chemical probes or genetically encoded-FRET probes requiring dual-acquisition is not compatible with the speed of acquisition required for the imaging of local Ca2+ responses. - Wash the samples 3x with EM buffer and further incubate them in 1 mL of EM buffer at 21 °C to allow for the hydrolysis of the AM moiety. Use Fluo-4 loaded cells immediately or within the next 90 min (maintained at 21 °C).
2. Infection and Image Acquisition of Local Ca2+ Responses
- Place the coverslip containing the Fluo-4 loaded cells in an imaging chamber or glass-bottom chamber. Wash the samples in the imaging chamber or glass-bottom disposable chamber 3x with EM buffer to remove compounds potentially resulting from cell lysis. Add 1 mL of EM buffer.
- Place the chamber on an inverted fluorescence microscope stage heated at 33 °C.
Note: This temperature is selected as a compromise between the optimal temperatures compatible with the Shigella invasion process and the slowing down of Ca2+ responses to visualize local responses. We use a 63X immersion oil objective (NA = 1.25) equipped with phase contrast rings. - Select a microscopy field and set up acquisition parameters, including exposure time and binning if necessary, to optimize the Fluo-4 fluorescent signal.
Note: The major challenges of local Ca2+ imaging are the low amplitude and the small duration of the responses, requiring acquisition at least every 30 ms. Several commercially available back-illuminated EM-CCD or C-MOS cameras present the required sensitivity to detect local Ca2+ responses using Fluo-4. Detection sensitivity is also an important issue to limit photodamage or artefacts such as spontaneous responses linked to the fluorescent excitation of Fluo-4 at a high frequency. Here, an LED-based illumination of 470 nm, with a 480 ± 40 nm band-pass excitation filter, a 505-nm dichroic filter, and a 527 ± 30 nm band-pass emission used at 5% of its maximal intensity combined with a 1.0 neutral density filter were used to minimize these problems under a stream of acquisitions of limited durations. An analysis of local Ca2+ signals by batches of 120 s streams was routinely performed. Under these low illumination conditions, fluorophore photobleaching was not observed during the 2 min-stream acquisitions. Successive acquisitions on the same sample can be performed, provided different fields are used. As a general rule, a first acquisition stream is performed on a control field in the absence of bacteria stimulation to ensure the absence of spontaneous responses. If spontaneous responses are observed, the samples are washed with EM buffer until no responses are observed. - To add bacteria to the sample, remove 500 µL of EM buffer from the chamber and add 500 µL of the bacterial suspension prepared in step 1.1.6 to obtain a final OD600nm of 0.05, with the appropriate care to avoid moving the selected microscopy field; the volumes ensure a proper mixing of the bacteria and a homogeneous distribution of the bacteria onto the cell samples.
- Perform an acquisition upon the bacterial addition. Alternatively, perform an acquisition 10 min following the bacterial addition, which corresponds to the time required for bacteria to sediment onto cells under the conditions used. At the end of the acquisition stream, acquire a phase-contrast image of the selected field to visualize the bacteria contacting the cells and the membrane ruffles associated with bacterial invasion sites.
- Repeat the acquisition procedure as in step 2.5, at intervals that are amenable to the duration of the image acquisitions, files savings, and selection of a new field to cover the whole process.
Note: For Shigella, we found that acquisition streams every 5 min for 20 min, following the 10 min bacterial sedimentation, allowed to cover the majority of the invasion events. - At the end of the acquisition procedure, add a final concentration of 2 µM of the Ca2+ ionophore ionomycin samples to determine the maximal amplitude of the Ca2+ signals. Acquire images every 3 – 5 s until signal stabilization, usually for less than 10 min.
- Follow by adding Ca2+ chelator EGTA at a final concentration of 10 mM to determine the fluorescence signal in the absence of Ca2+. Acquire images every 3 – 5 s until signal stabilization, usually for less than 10 min.
Note: Because of the relatively slow dynamics of the global Ca2+ variations, perform these acquisitions every 3 – 5 s (not in streaming mode), allowing for Ca2+ imaging on the same microscopic field during the successive treatments.
3. Analysis
- Express cytosolic Ca2+ variations over time as the percentage of ΔF/F0, where ΔF represents the variation of the average fluorescence intensity of the region of interest (ROI) and F0 the baseline fluorescence in the same ROI, to which a non-relevant region of the field devoid of cells is subtracted.
- Remove the basal fluorescence levels for each cell in different fields, corresponding to baseline levels; these levels can be unambiguously determined due to the low frequency and relatively short duration of local Ca2+ responses in a given stream acquisition.
Note: Quantify striking features of the responses related to the questions asked and the pathogenic model studied. Classically, various parameters are taken into account to analyze Ca2+ signals, including the frequency of cells showing local or global Ca2+ responses, as well as the duration, amplitude, and frequency of these responses. In the case of Shigella and local responses, another important aspect of the analysis is the spatial association of the responses with bacterial invasion sites.
- Remove the basal fluorescence levels for each cell in different fields, corresponding to baseline levels; these levels can be unambiguously determined due to the low frequency and relatively short duration of local Ca2+ responses in a given stream acquisition.
- To quantify the percentage of responsive cells showing global or local responses, draw ROIs in cells, in time-series corresponding to the variations of Fluo-4 intensity.
- Set strict parameters to identify local Ca2+ responses, such as an amplitude of three-fold above background variations of the average fluorescence intensity cell baseline, or a duration of at least 200 ms, to avoid scoring a false positive.
Note: As a general rule, even small local Ca2+ responses can be distinguished from any background noise by their profile. The ROIs should be large enough to integrate enough fluorescence signals to allow for the detection of local variations. Depending on the intensity of the Fluo-4 detection, these ROIs may correspond to circles with diameters ranging from 2 – 5 mM. - Measure the variations of the average Fluo-4 fluorescence intensity in 2 regions in a distinct area of the same cell, to determine whether the responses are local or global.
Note: The analysis of a large number of cells is facilitated by the use of imaging software permitting the on-line screen display of the variations of the average fluorescence intensity over time for the selected regions. Commercially available imaging software has such an option, but this can also be performed using the Icy freeware, using the ROI Intensity Evolution plugin. - Determine the percentage of cells showing local responses.
Note: This percentage is calculated from the total number of cells analyzed in the microscopy field, which should be in sufficient numbers to support a statistically significance using a non-parametric test. - Determine the duration of the local responses.
Note: Local Ca2+ responses can be further distinguished according to their duration ranges (i.e., less than 500 ms, between 5 and 10 s, or above 10 s) and their association with Shigella invasion sites.
- Set strict parameters to identify local Ca2+ responses, such as an amplitude of three-fold above background variations of the average fluorescence intensity cell baseline, or a duration of at least 200 ms, to avoid scoring a false positive.
- Quantify the amplitude of the responses. First, calibrate the maximal Ca2+ and cell background levels determined as in step 2.1.7. Since the Fluo-4 load may vary for each cell, perform these determinations for individual cells in the field.
- Express the amplitude of the responses as a relative percentage of the maximal response.
- Perform statistical testing using a normality test, followed by a parametric test to analyze potential differences in the amplitude of responses between samples.
- Determine the association of these responses relative to the bacterial invasion sites by their respective localization to the bacterial-induced membrane ruffles visualized in the corresponding phase contrast images.
Representative Results
Shigella invasion is associated with atypical long-lasting local Ca2+ responses:
Following the protocol mentioned above, Fluo-4-loaded HeLa cells were challenged with WT Shigella and stream acquisitions were performed to analyze Ca2+ signals. A representative experiment is shown in Figure 1, with time-lapse images series of the fluorescence intensity of the Fluo-4 probe averaged in a region of interest for a single cell, and the corresponding phase contrast image (Figure 1A, left panel). The Shigella invasion site is characterized by membrane ruffles detected in the phase contrast image (Figure 1A, arrow). Atypical local increases in free cytosolic Ca2+ are observed at the Shigella invasion site with a varying amplitude and durations ranging from 2.5 – 5 s (Figures 1A and 1B, arrows), followed by global increases in the infected cell (Figure 1B, arrowheads).
The type III effector IpgD regulates the transition from local to global Ca2+ responses induced by Shigella in HeLa cells:
The analysis of Ca2+ signals induced by wild-type Shigella and an isogenic mutant strain deficient for the type III effector IpgD, a phosphatidyl 4, 5 bisphosphate phosphatase, indicated that this latter strain induced more global and less atypical local responses with long durations (RATPs) with 11.3 ± 4.4 (SEM)% and 40.3 ± 7.5 (SEM)% of cells responsive with global responses in the case of a WT and ipgD mutant, respectively (Figure 1D)15. This ipgD mutant obtains local responses at a similar frequency as the WT strain.
Shigella inhibit InsP3-dependent global Ca2+ increases in HeLa cells during prolonged infection kinetics:
The imaging of global Ca2+ responses over extended infection kinetics pointed to a decrease in the frequency of responses 30 min following the challenge with wild-type Shigella (Figure 2A). A similar decrease in the frequency of Ca2+ responses was observed in TC-7 cells infected for 30 min with wild-type Shigella15. The quantification of dose-dependent cell responses to the Ca2+ agonist histamine illustrates the inhibition of an InsP3-dependent Ca2+ release at late stages of a Shigella infection. WT Shigella leads to atypical isolated Ca2+ responses during the first 30 min of the infection and after further incubation, a drastic decrease in the amplitude and frequency of Ca2+ responses was observed (Figure 2B).
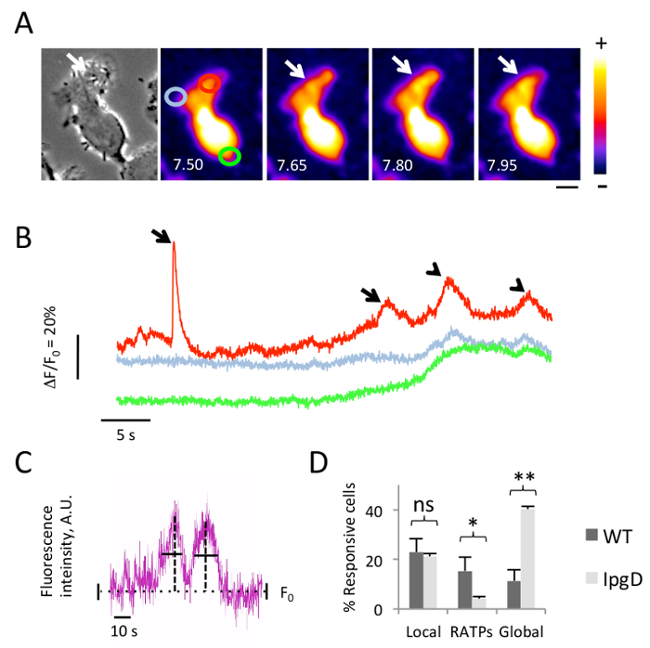
Figure 1. Local and global Ca2+ responses during Shigella invasion. HeLa cells were loaded with Fluo-4-AM and challenged with WT Shigella. (A) The left panel shows phase-contrast images. The right panel shows time series of Fluo-4 fluorescence average intensity with the color code depicted on the right. The time from the start of acquisition is indicated in seconds, 10 min after the bacterial challenge. The arrow indicates the invasion foci. The circles depicted correspond to the regions analyzed in (B) where the traces with the corresponding color represent the variations in Fluo-4 average fluorescence intensity over the baseline. The arrows indicate local responses. The arrowheads indicate global responses. (C) This is a scheme depicting the determination of the duration of the Ca2+ responses. The horizontal dotted line indicates the basal Ca2+ levels (F0); the vertical bars indicate background variations. The vertical dotted line indicates the maximal amplitude of the response; the horizontal bars indicate the duration of the response determined at the half-maximal amplitude. (D) This is the percentage of responsive cells ± SEM showing local responses and global Ca2+ responses induced 5 min post-infection with the WT Shigella (dark gray bars) or the ipgD mutant (light gray bars). RATPs are local Ca2+ responses that last for more than 5 s. N = 4; >60 cells for each time. Wilcoxon test, *P <0.05; **P <0.01. Please click here to view a larger version of this figure.
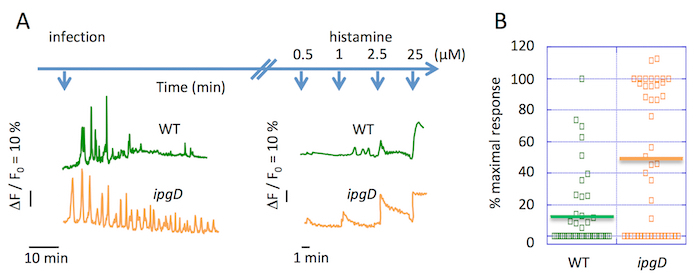
Figure 2. Inhibition of global Ca2+ responses during extended kinetics of Shigella infection. (A) The upper blue arrows represent the time scale of the bacterial and histamine challenge at the indicated concentrations. This panel shows the representative traces of single cell global Ca2+ variations following the infection by the WT Shigella (green) and ipgD mutant strain (orange). (B) This panel shows the percentage of the amplitude of Ca2+ responses relative to the maximal response, upon a stimulation at 0.5 μM histamine of the cells infected with the indicated strains for 90 min. The solid horizontal bar represents the average maximal percentage. Wilcoxon test, **P <0.01. Please click here to view a larger version of this figure.
Discussion
This manuscript describes the protocol that we engineered to follow local Ca2+ signals during the relatively short kinetics of a Shigella invasion, as well as global Ca2+ responses during the extended kinetics of Shigella. Below, key issues can be found that need to be addressed to optimize the detection of Ca2+ signals while minimizing any interference with the biological processes.
Chemical vs. genetically encoded Ca2+ probes:
To image local Ca2+ variations, we used the Fluo-4 chemical probe because of its high quantum yield and its fast response dynamics. The speed required for the acquisition also precludes the use of ratiometric probes, since a dual wavelength acquisition cannot be performed. The use of pathogenic microorganisms, however, may interfere with standard procedures using Ca2+ chemical probes. For example, a prolonged cell infection over 60 min by Shigella prevents any cell loading with chemical probes, presumably because of pathogen-induced alterations of the host cell plasma membranes. Consistently, a decrease of cell-associated Fluo-4 fluorescence is observed during long infection kinetics, due to the cytoplasmic loss of this probe. Hence, implementing a control such as the addition of ionomycin to determine the fluorescence at the maximal Ca2+ concentrations at the end of the acquisitions is critical to identify the responsive cells. Also, polarized intestinal epithelial cells relevant for a pathogen infection appear to be refractory to a loading of the Ca2+ probe and require specific loading procedures. While the use of a genetically encoded Ca2+ reporter (GECR) may help to resolve some of these issues, it also introduces other challenges. The transfection efficiency in host cell systems may represent a serious limitation, particularly if it superimposes with a poor infectious yield. Furthermore, the characteristics of a responsiveness to Ca2+ variations of most GECRs are not compatible with the fast kinetics required for local Ca2+ analysis. Finally, the expression of the GECR may interfere with pathogens-mediated processes. We will not discuss the use of GECR for the study of Ca2+ signaling during a host-pathogen interaction. The recent and on-going engineering of various "fast-responding" GECR would probably deserve researchers to revisit their use in future studies.
Timing acquisition of local Ca2+ responses during bacterial infection:
The imaging of local Ca2+ responses requires a high speed of image acquisition implicating a sustained fluorescence excitation of the Fluo-4 probe. Because of the photodamage linked to the high-frequency acquisition, it is critical that the intensity of the fluorophore excitation light is kept to the minimum required to obtain a sufficient signal-to-noise ratio with an exposure time not exceeding 30 ms. We had good results using a LED system at 5% of its maximal intensity, combined with a 1.0 optical density filter with the acquisition set-up as described in step 2.1.4. Even under these conditions, however, we found that the continuous illumination required for the stream mode acquisition did not allow for an acquisition period over 2 min. A stronger illumination or acquisitions period exceeding this limit may result in non-specific global Ca2+ responses and/or in the inhibition of the bacterial invasion processes, presumably linked to photodamage. While the imaging of local Ca2+ responses over longer kinetics may be possible with more sensitive detection means, at present state such imaging can only be performed over a limited time fraction of the 15 min Shigella invasion process. To cover the whole process, we performed successive series of 2-min streaming acquisitions. This time interval is sufficient to follow initial local and global Ca2+ responses at the onset of the infection process and to establish significant differences in the Shigella wild-type strain and its isogenic ipgD mutant16. The development of a highly sensitive camera may permit researchers to further reduce the intensity of the fluorophore illumination and perform local Ca2+ imaging over extended periods, thus improving the time resolution of more transient Ca2+ signals.
Spatial correlation between local Ca2+ responses and bacterial invasion sites:
Because of the local aspect of signaling events induced by microorganisms, it is important to study local Ca2+ signals with respect to their association with sites of the pathogen-host cell interactions. This raised two types of considerations. First, all cells may not be infected, and all microorganisms may not trigger signaling. For Shigella, only a minority of bacteria trigger an invasion, and all cells may not be infected. In our experiments, the MOI was adjusted so that one cell forms a 0.7 – 1 focus of the bacterial invasion. Higher numbers of foci/cell may lead to a possible interference for the analysis of individual invasion events and, conversely, lower numbers may render the analysis too complex, in particular when studying transiently transfected cells. We found that the transfection efficiency needs to reach at least 30% of the cells to bring down the number of replicate experiments to manageable numbers. Second, Shigella invasion sites can be readily detected by phase contrast microscopy. For other processes, other fluorescence-based detection methods could be used. An important aspect, however, is that in most experimental set-ups, the acquisition stream required for local Ca2+ imaging precludes other types of acquisition during the period. This implicates that the process analyzed should not show significant motion during this stream to permit their spatial correlation with local Ca2+ signals. While this is the case for Shigella invasion events that localize to the same cell area over several minutes, highly motile processes may not be manageable or would probably require shorter acquisition streams.
Scoring and analysis of local Ca2+ responses:
While global Ca2+ responses are easily detected, the detection of local Ca2+ responses of small amplitude and duration requires the optimization of the acquisition of fluorescent signals described above. It is also important that strict criteria are applied to differentiate the signals above the background variations. As a rule, we score as a Ca2+ signal an increase of the average Fluo-4 fluorescence intensity that reaches at least 3x the baseline variations in three consecutive 30 ms acquisitions. This response is considered as local if another region of the cell showing the same average fluorescence intensity does not show such an increase. The scoring of local Ca2+ responses in various samples should not cause major difficulties if these rules are systematically applied. In our hands, the low frequency of local Ca2+ signals associated with the bacterial invasion and ranging from 5 – 20% of the cell analyzed represented a major hurdle. Beyond the biological variations detailed in the previous paragraph, the fact that only a stream corresponding to a fraction of the analyzed invasion process also contributes to this low frequency. Because of this low frequency, establishing differences between samples may implicate performing a power test on pilot experiments to estimate the sample size to reach a statistical significance.
Future applications:
We believe that the protocols worked out to analyze local Ca2+ signals during a Shigella invasion will be helpful to image local Ca2+ responses for all processes that remain spatially confined during the acquisition period. While Ca2+ signaling is versatile, local Ca2+ signaling may be most relevant for processes occurring at the plasma or intracellular membranes where the initial point sources of signals reside. Thus, during microbial-host cell interactions, local Ca2+ signals may be relevant to adhesive or invasive processes at the plasma membrane or occurring at intracellular membranes of pathogen-containing vacuoles. On the other hand, the protocols that we designed to analyze global Ca2+ signals over extended infection kinetics may be relevant to processes affecting the general cell physiology during an infection, such as the regulation of a transcriptional program or cell death pathways by pathogens.
Divulgazioni
The authors have nothing to disclose.
Acknowledgements
We thank Jenny-Lee Thomassin for her help in editing the manuscript. The work was supported by the ANR grants MITOPATHO and PATHIMMUN, grants from the Labex Memolife and PSL IDEX Shigaforce. Chunhui Sun is a recipient of a Ph.D. grant from the China Scholarship Council. Laurent Combettes and Guy Tran Van Nhieu are recipients of a WBI-France exchange Tournesol program N°31268YG (Wallonie-Bruxelles International, Fonds de la Recherche Scientifique, Ministère Français des Affaires étrangères et européennes, Ministère de l'Enseignement supérieur et de la Recherche dans le cadre des Partenariats Hubert Curien).
Materials
| Fluo-4 AM | Invitrogen | F14201 | |
| Metamorph version 7.7 | Universal Imaging | ||
| CoolLED illumination system pE-2 | Roper Scientific | ||
| micro-dish 35 mm, high | IBIDI | 81156 | |
| Trypticase Soy (TCS) broth | Thermofisher | B11768 | |
| TCS agar | Thermofisher | B11043 | |
| Congo red | Sigma-Aldrich | 75768 | |
| M90T-AfaE | Sun et al. 2017 | Shigella flexneri serotype V. expressing the AfaE adhesin | |
| ipgD-AfaE | Sun et al. 2017 | isogenic ipgD mutant strain expressing the AfaE adhesin |
Riferimenti
- Ashida, H., Ogawa, M., Kim, M., Mimuro, H., Sasakawa, C. Bacteria and host interactions in the gut epithelial barrier. Nature Chemical Biology. 8 (1), 36-45 (2012).
- Berridge, M. J., Lipp, P., Bootman, M. D. The versatility and universality of calcium signaling. Nature Reviews Molecular Cell Biology. 1 (1), 11-21 (2000).
- Strehler, E. E. Plasma membrane calcium ATPases: from generic Ca(2+) sump pumps to versatile systems for fine-tuning cellular Ca(2). Biochemical and Biophysical Research Communications. 460 (1), 26-33 (2015).
- Muallem, S. Decoding Ca2+ signals: a question of timing. Journal of Cell Biology. 170 (2), 173-175 (2005).
- Uhlen, P., Fritz, N. Biochemistry of calcium oscillations. Biochemical and Biophysical Research Communications. 396 (1), 28-32 (2010).
- Carneiro, L. A., et al. Shigella induces mitochondrial dysfunction and cell death in nonmyleoid cells. Cell Host & Microbe. 5 (2), 123-136 (2009).
- Horng, T. Calcium signaling and mitochondrial destabilization in the triggering of the NLRP3 inflammasome. Trends in Immunology. 35 (6), 253-261 (2014).
- Galan, J. E., Lara-Tejero, M., Marlovits, T. C., Wagner, S. Bacterial type III secretion systems: specialized nanomachines for protein delivery into target cells. Annual Review of Microbiology. 68, 415-438 (2014).
- Ashida, H., Mimuro, H., Sasakawa, C. Shigella manipulates host immune responses by delivering effector proteins with specific roles. Frontiers in Immunology. 6, 219 (2015).
- Tran Van Nhieu, G., et al. Actin-based confinement of calcium responses during Shigella invasion. Nature Communications. 4, 1567 (2013).
- Niebuhr, K., et al. Conversion of PtdIns(4,5)P(2) into PtdIns(5)P by the S. flexneri effector IpgD reorganizes host cell morphology. The EMBO Journal. 21 (19), 5069-5078 (2002).
- Konradt, C., et al. The Shigella flexneri type three secretion system effector IpgD inhibits T cell migration by manipulating host phosphoinositide metabolism. Cell Host & Microbe. 9 (4), 263-272 (2011).
- Friedrich, P. The intriguing Ca2+ requirement of calpain activation. Biochemical and Biophysical Research Communications. 323 (4), 1131-1133 (2004).
- Thomas, D., et al. A comparison of fluorescent Ca2+ indicator properties and their use in measuring elementary and global Ca2+ signals. Cell Calcium. 28 (4), 213-223 (2000).
- Sun, C. H., et al. The Shigella type III effector IpgD recodes Ca2+ signals during invasion of epithelial cells. The EMBO Journal. 36 (17), 2567-2580 (2017).
- Allaoui, A., Menard, R., Sansonetti, P. J., Parsot, C. Characterization of the Shigella flexneri IpgD and IpgF genes, which are located in the proximal part of the mxi locus. Infection and Immunity. 61 (5), 1707-1714 (1993).

