Bio-inspired Polydopamine Surface Modification of Nanodiamonds and Its Reduction of Silver Nanoparticles
Summary
A facile protocol is presented to functionalize the surfaces of nanodiamonds with polydopamine.
Abstract
Surface functionalization of nanodiamonds (NDs) is still challenging due to the diversity of functional groups on the ND surfaces. Here, we demonstrate a simple protocol for the multifunctional surface modification of NDs by using mussel-inspired polydopamine (PDA) coating. In addition, the functional layer of PDA on NDs could serve as a reducing agent to synthesize and stabilize metal nanoparticles. Dopamine (DA) can self-polymerize and spontaneously form PDA layers on ND surfaces if the NDs and dopamine are simply mixed together. The thickness of a PDA layer is controlled by varying the concentration of DA. A typical result shows that a thickness of ~5 to ~15 nm of the PDA layer can be reached by adding 50 to 100 µg/mL of DA to 100 nm ND suspensions. Furthermore, the PDA-NDs are used as a substrate to reduce metal ions, such as Ag[(NH3)2]+, to silver nanoparticles (AgNPs). The sizes of the AgNPs rely on the initial concentrations of Ag[(NH3)2]+. Along with an increase in the concentration of Ag[(NH3)2]+, the number of NPs increases, as well as the diameters of the NPs. In summary, this study not only presents a facile method for modifying the surfaces of NDs with PDA, but also demonstrates the enhanced functionality of NDs by anchoring various species of interest (such as AgNPs) for advanced applications.
Introduction
Nanodiamonds (NDs), a novel carbon-based material, have attracted considerable attention in recent years for use in various applications1,2. For instance, the high surface areas of NDs provide excellent catalyst support for metal nanoparticles (NPs) because of their super-chemical stability and thermal conductivity3. Furthermore, NDs play significant roles in bio-imaging, bio-sensing, and drug delivery due to their outstanding biocompatibility and nontoxicity4,5.
To efficiently extend their capabilities, it is valuable to conjugate functional species on the surfaces of NDs, such as proteins, nucleic acids, and nanoparticles6. Although a variety of functional groups (e.g., hydroxyl, carboxyl, lactone, etc.) are created on the surfaces of NDs during their purification, the conjugation yields of the functional groups are still very low because of the low density of each active chemical group7. This results in unstable NDs, which tend to aggregate, limiting further application8.
Currently, the most common methods used to functionalize NDs, are covalent conjugation by using copper-free click chemistry9, covalent linkage of peptide nucleic acids (PNA)10, and self-assembled DNA11. The non-covalent wrapping of NDs has also been proposed, including carbohydrate-modified BSA4, and HSA12coating. However, because these methods are time consuming and inefficient, it is desirable that a simple and generally applicable method can be developed to modify the surfaces of NDs.
Dopamine (DA)13, known as a natural neurotransmitter in the brain, was widely used for adhering and functionalizing nanoparticles, such as gold nanoparticles (AuNPs)14, Fe2O315, and SiO216. Self-polymerized PDA layers enrich amino and phenolic groups, which can be further utilized to directly reduce metal nanoparticles or to easily immobilize thiol/amine-containing biomolecules on an aqueous solution. This simple approach was recently applied to functionalize NDs by Qin et al. and our laboratory17,18, although DA derivatives were employed to modify NDs via Click Chemistry in earlier studies19,20.
Here, we describe a simple PDA-based surface modification method that efficiently functionalizes NDs. By varying the concentration of DA, we can control the thickness of a PDA layer from a few nanometers to tens of nanometers. In addition, the metal nanoparticles are directly reduced and stabilized on the PDA surface without the need for additional toxic reduction agents. The sizes of the silver nanoparticles depend on the initial concentrations of Ag[(NH3)2]+. This method allows the well-controlled deposition of PDA on the surfaces of NDs and the synthesis of ND conjugated AgNPs, which dramatically extends the functionality of NDs as excellent nano-platforms of catalyst supports, bio-imaging, and bio-sensors.
Protocol
1 . Preparation of Reagents
CAUTION: Please read and understand all relevant material safety data sheets (MSDS) before use. Some of the chemicals are toxic and volatile. Please follow special handling procedures and storage requirements. During the experimental procedure, use personal protective equipment, such as gloves, safety glasses, and a lab coat to avoid potential hazards.
- Preparation of Tris-HCl buffer
- Dissolve 30.29 g of Tris powder in 100 mL of deionized H2O, ensure that the powder dissolves completely, and then transfer the solution to a 250 mL-volumetric flask.
- Add deionized H2O to the scale of 250 mL in the volumetric flask to give 1.0 M of Tris buffer.
- Dilute the 1.0 M Tris buffer 100 times to give 0.01 M Tris buffer and adjust the pH to 8.5 by using 1.0 M HCl standard solution.
- Use a pH-meter to calibrate the pH value of the 0.01 M Tris-HCl buffer.
- Preparation of ND suspensions
- Dilute 100 nm of monocrystalline ND suspensions (1.0 mg/mL) 50 times with the 0.01 M Tris-HCl buffer to give 0.02 mg/mL of ND suspensions.
- Preparation of dopamine solution
- Dissolve 20 mg of dopamine hydrochloride in 2.0 mL of 0.01 M Tris-HCl buffer to give 10 mg/mL DA solution.
NOTE: The DA solution must be freshly prepared and used within 15 min.
- Dissolve 20 mg of dopamine hydrochloride in 2.0 mL of 0.01 M Tris-HCl buffer to give 10 mg/mL DA solution.
- Preparation of Ag[(NH3)2]OH solution
- Dissolve 100 mg of AgNO3 solid in 10 mL of deionized H2O to give 10 mg/mL AgNO3 solution.
- Add 1.0 M ammonium hydroxide (NH3·H2O) dropwise to the AgNO3 solution until yellow precipitate forms, then continue to add the NH3·H2O solution until the precipitation disappears.
NOTE: Make the minimum volume required; prepare immediately before use and dispose immediately after use.
CAUTION: Add NH3·H2O in fume hood with face shields, gloves, and goggles.
2 . Synthesis PDA Layer on the Surface of NDs (PDA-NDs)
- Add the freshly prepared DA solution (10 mg/mL) to the ND suspensions to give varied final concentrations of 50, 75, 100 µg/mL of DA. Adjust the total reaction volume to 1.0 mL, transfer it to a 10 mL-test tube, and vigorously stir at 25 °C, in the dark for 12 h.
- Centrifuge the PDA-NDs solution for 2 h at 16,000 x g, remove the supernatant and wash three times with deionized water for 1 h at 16, 000 x g each time.
- Re-disperse the PDA-NDs in 200 µL of deionized water with sonication for 30 s. The PDA- coated NDs will be ready for further use.
3 . Reduction of AgNPs on the Surface of PDA-NDs (AgNPs-PDA-NDs)
- Dilute 40 µL of the pre-synthesized PDA-NDs in Step 2.3 two times with deionized water. Add Ag[(NH3)2]OH solution to give various final concentrations of Ag[(NH3)2]+ (0.08, 0.16, 0.24, 0.40, and 0.60 mg/mL).
- Adjust the final volume to 100 µL in a 1.5 mL-centrifuge tube by adding deionized water, followed by sonication for 10 min.
- Centrifuge the AgNPs-PDA-NDs for 15 min at 16,000 x g to remove the free silver ions, discard the supernatant after centrifugation, add 100 µL of deionized water, and wash three times with deionized water at 16,000 x g for 5 min each time.
- Re-disperse the AgNPs-PDA-NDs in 100 µL of deionizedwater with sonication for 30 s to prepare for further use.
4 . Analysis of PDA-NDs and AgNPs-PDA-NDs Clusters
- Ultraviolet-visible (UV) spectra
- Use the UV spectra to monitor the average size distribution of AgNPs on PDA-ND surfaces. Transfer the AgNPs-PDA-NDs samples prepared in Step 3.4 with varied concentrations of Ag[(NH3)2]OH in 1 cm-quartz cuvette and monitor the absorption at a scan wavelength of 250 to 550 nm.
- Transmission election microscopy (TEM)
- Place the carbon coated copper grids on a glass slide wrapped with parafilm to keep the grids in place. Insert the glass slide with attached TEM grids into the plasma cleaner. Turn on the plasma cleaner and the vacuum pump. After 5 min, turn on the plasma and discharge the grids with a medium power level for 3 min.
- Deposit 5 µL of the samples on the carbon film coated Cu-grids for 3 min. Use filter paper to wick off the extra sample from the edge of grid. Then, deposit a drop of deionized water on the grid for 15 s to remove salts, then wick off the water with filter paper. Repeat the washing procedure twice and allow the grid to air dry for further use.
- Visualize the samples by TEM, typically at 38,000X magnification. Operate at 200 KV.
Representative Results
The formation of PDA layers on ND surfaces were analyzed by TEM (Figure 1). Different thicknesses of PDA layers were observed as higher concentrations of DA led to thicker PDA layers. In addition, after an encapsulating reaction, the color of the NDs solution changed from colorless to dark, while the higher the initial concentration of DA was, the darker the solution became.
Figure 2 describes the reduction of Ag[(NH3)2]+ to AgNPs on the surface of 100 nm PDA-ND surfaces. The size distribution of AgNPs, calculated by TEM, were used to determine the dependence of the original concentration of Ag[(NH3)2]+ on the sizes of the AgNPs.
The flow chart in Figure 3 presents the two-step procedure for functionalizing the surface of NDs by PDA and for reducing metal ions into NPs on the PDA-ND layers.
The formation of AgNPs on the ND surface was monitored by UV-vis spectra (Figure 4). The intensity of the peaks at ~ 400 nm increased, along with an increase in the concentration of silver solution while the peaks showed a red-shift, indicating the formation of AgNPs with increased sizes.
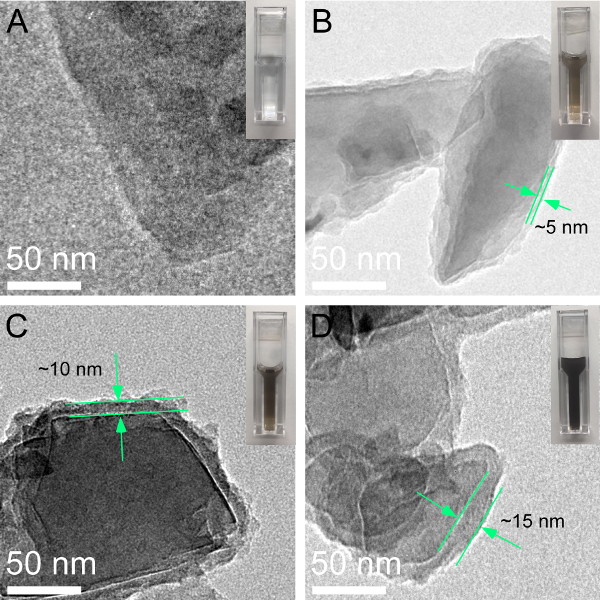
Figure 1. Characterization of the thicknesses of PDA layers on the surfaces of 100 nm NDs with varied concentrations of DA (0, 50, 75, and 100 μg/mL) and their corresponding TEM images. The average thicknesses of each PDA layer are ~5 nm (B), ~10 nm (C), and ~15 nm (D), respectively. The inset photograph shows the colorimetric change in the corresponding samples. Please click here to view a larger version of this figure.
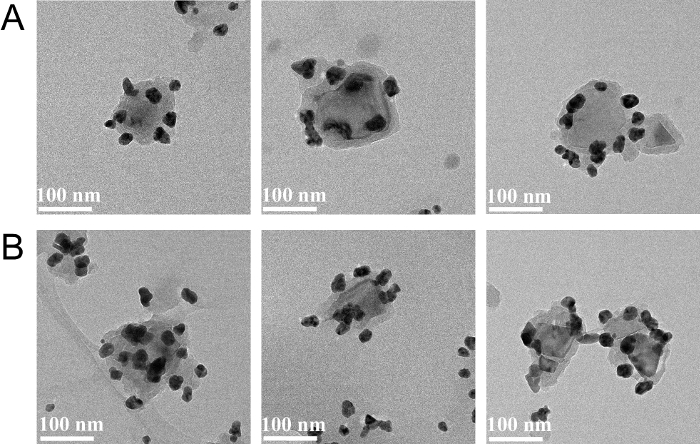
Figure 2. Characterization of AgNPs-PDA-NDs. TEM images of AgNP-PDA-NDs and the size distribution of AgNPs by adding 0.4 mg/mL (A), and 0.6 mg/mL (B) of [Ag(NH3)2]+, respectively. Please click here to view a larger version of this figure.
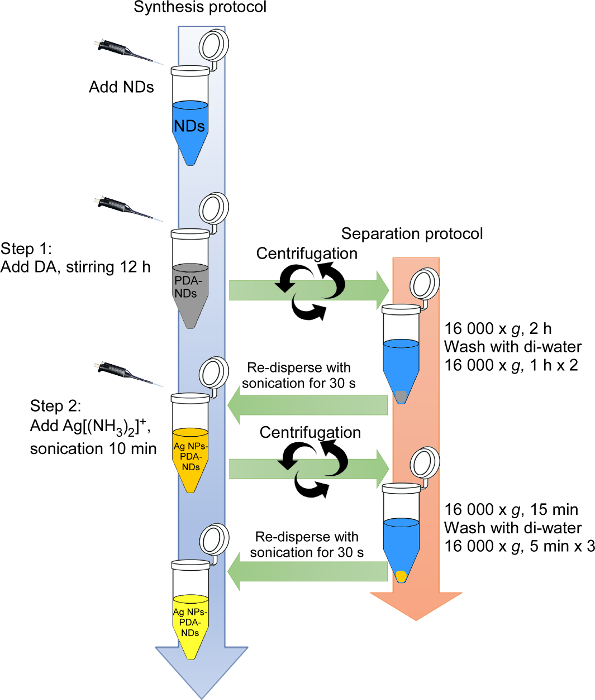
Figure 3. Wall chart diagram of the surface functionalization of NDs. Two-step functionalization of the surface of NDs: (1) the surface coating of NDs with DA polymerization; (2) the reduction of metal ions into NPs on the PDA layer. Please click here to view a larger version of this figure.
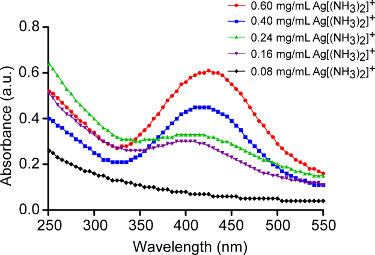
Figure 4. Characterization of reduced AgNPs on the surfaces of NDs, via UV-vis spectroscopy. This figure has been modified and reprinted by permission from Zeng et al.17. Please click here to view a larger version of this figure.
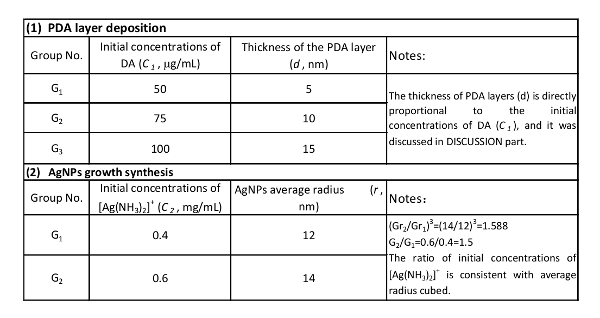
Table 1. The thickness of PDA layer and the size of reduced AgNPs. The validation of the mathematical models with experimental data. The thickness of PDA layer is determined by the initial concentration of DA, and the ratio of initial concentrations of [Ag(NH3)2]+ is consistent with the average radius cubed of AuNPs.
Discussion
This article provides a detailed protocol for the surface functionalization of NDs with self-polymerized DA coating, and the reduction of Ag[(NH3)2]+ to AgNPs on PDA layers (Figure 3). The strategy is capable of producing various thicknesses of PDA layers by simply changing the concentration of DA. The size of the AgNPs can also be controlled by altering the original concentration of metal ion solution. The TEM image in Figure 1A displays the uncoated 100 nm NDs which tended to form microclusters and aggregates. When NDs were encapsulated with PDA, the PDA layers showed as a thin ring round the NDs. The thicknesses of the PDA layers, as measured in TEM images, were around 5 nm, 10 nm, and 15 nm, which corresponded to the final DA concentrations of 50 µg/mL, 75 µg/mL, and 100 µg/mL, respectively. The color of the NDs suspension was observed to change from colorless to dark following PDA coating, indicating the successful wrapping of PDA on ND surfaces and showing that the thickness of PDA was dependent on the concentration of DA. Please note: the critical factor that influences the DA polymerization is the pH condition (the most favorable value is 8.523). The accurate pH value of a solution is beneficial for controlling the thicknesses of PDA layers. In addition, fast agitation during polymerization is necessary for the disaggregation of NDs and the formation of a uniformed PDA layer. Therefore, this method is not effective for any particles that are unstable in alkaline solutions.
To describe the influential factors that contributed to the thickness of the PDA, we introduce equation (1) to describe the formation of a PDA layer on ND surfaces. This is based on the kinetic equation of PDA deposition on nanoparticles from previous reports21,22. The initial concentrations of DA (C1, m/v), reaction time (t), and the thickness of the PDA layer (d), are as follows:
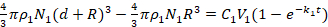 (1)
(1)
R is the radius of NDs (assuming NDs are spheres), p1 is the density of PDA, V1 is the reaction volume, N1 is the number of NDs, and k1 is a constant related to pH values, partial pressure of O2, ambient temperature and luminous intensity23. Therefore, the thickness of a PDA layer can be written as equation (2)
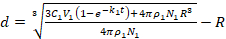 (2)
(2)
Or if we rewrite equation (1) a (3):
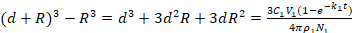 (3)
(3)
Then, eliminate d3 and 3d2R because d is far less than R (d<<R).
At last, the d can be expressed as equation (4)
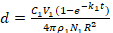 (4)
(4)
The coating process required 12 h, with the DA being completely consumed and monitored by UV-vis spectra. Therefore, 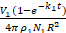 was a constant, and the value of d was directly proportional to the initial concentrations of DA (C1), which were confirmed by our experimental results (Table 1). Please note, along with the increase in the thicknesses of the PDA layers, accumulation speeds of the layers were slower because of the increases in the surface areas of the NDs-PDA.
was a constant, and the value of d was directly proportional to the initial concentrations of DA (C1), which were confirmed by our experimental results (Table 1). Please note, along with the increase in the thicknesses of the PDA layers, accumulation speeds of the layers were slower because of the increases in the surface areas of the NDs-PDA.
The presence of the catechol groups in PDA has been shown to directly induce the growth of the nanoparticles upon the reduction of metal precursors and their immobilization on a PDA-coated surface24,25,26,27. After coating 100 nm NDs with a PDA layer (~ 15 nm), the resulting PDA-NDs were used as a substrate to synthesize AgNPs from a metal ion solution, with the assistance of sonication. As seen in Figure 2, with the increase of [Ag(NH3)2]+ concentration, the size of AgNPs increased from ~24 nm to ~28 nm, and the number of NPs raised from 97 to 117, corresponding to the [Ag(NH3)2]+ concentration of 0.4 to 0.6 mg/mL, respectively. This phenomenon can also be characterized by UV-vis spectroscopy. The absorbance peak of nanoparticles gradually appeared as the concentration of [Ag(NH3)2]+ increased (Figure 4). For example, the maximum absorbance of nanoparticles, formed by reducing 0.4 and 0.6 mg/mL of [Ag(NH3)2]+, is 410 and 430 nm, which corresponds to AgNPs with the diameters of ∼20 and ∼30 nm, respectively. This is consistent with TEM observation17.
The diameter of reduced AgNPs follows the first order linear differential equation (5), which is similar to the seeded growth synthesis of AuNPs28, where the S is the surface area of PDA-NDs, C2 is the initial concentrations of Ag[(NH3)2]+, t is the reaction time, r is the radius of AgNPs, k2 is a constant, p2 is the density of Ag, V2 is the reaction volume, N2 is the number of AgNPs, and equals to S · n, in which n is the average number of active catechol groups that can reduce Ag[(NH3)2]+. The AgNPs are treated as spheres:
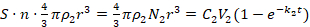 (5)
(5)
In the equation, the number of AgNPs was assumed to be directly proportional to the surface area of PDA, which depended on the thicknesses of the PDA layers. On the surface of the PDA layers, the AgNPs grew with the continuous reduction of Ag[(NH3)2]+, while the metal (0) bonds at the O-site of the PDA served as the seed precursor of AgNPs. The number of AgNPs is proportional to the O-site on PDA, which is directly proportional to the surface area23,29,30,31. On the other hand, the reduced AgNPs are distributed evenly on the PDA surface because the Ag[(NH3)2]+ was reduced by the uniformed catechol groups on the PDA layers. Experimental results showed that the higher the initial concentrations of Ag[(NH3)2]+ were, the larger the AgNPs were, but with a similar number of NPs on each ND. The ratio of initial concentrations of [Ag(NH3)2]+ (C2) ratio (0.6 mg/mL : 0.4 mg/mL=1.5) were consistent with the average radius cubed [(14/12)3=1.588]. Therefore, if a higher density of particles is desired on the PDA-NDs, a thicker layer of PDA-NDs should be selected but, if larger sizes of NPs are needed, a longer reduction duration would meet the requirement.
To remove the unreacted [Ag(NH3)2]+ during the purification process, a high centrifugation speed is recommended because of the low density of NDs. The higher the centrifugation speed is, the shorter the purification duration will be, which will provide better control of the sizes of AgNPs. In addition, sonication is an indispensable approach for obtaining uniform AgNPs. Samples should be sonicated for several minutes initially before Ag[(NH3)2]+ solutions are added.
We have demonstrated a facile method for the surface modification of NDs with self-polymerized PDA. Compared with the Click Chemistry method, this strategy, not only enhances ND dispersity and stability, but also provides a reactive platform (PDA layer) for potential post-modification by reducing the metal nanoparticles or linking with amino/thiol attached species. The thickness of a PDA layer and the size of the nanoparticles on ND surfaces can be changed by varying PDA and Ag[(NH3)2]+ concentrations. They can also be used to reduce AuNPs or other noble metal NPs. By combining the diversity of PDA chemistry and the unique properties of NDs, this method will open the door for extending ND's applications in the catalyst, energy, and biomedical areas.
Divulgazioni
The authors have nothing to disclose.
Acknowledgements
This research was supported by National Science Foundation (CCF 1814797) and University of Missouri Research Board, Material Research Center, and the College of Arts and Science at Missouri University of Science and Technology
Materials
| Nanodiamond | FND Biotech, Inc. | brFND-100 | dispersed in water, and used without further purification |
| Dopamine hydrochloride | Sigma | H8502-25G | prepare freshly |
| Silver Nitrate | Fisher | S181-25 | |
| Ammonium Hydroxide | Fisher | A669S-500 | highly toxic |
| Tris Hydrochloride | Fisher | BP153-500 | |
| TEM grid carbon film | Ted Pella | 01843-F | 300 mesh copper |
Riferimenti
- Mochalin, V. N., Shenderova, O., Ho, D., Gogotsi, Y. The properties and applications of nanodiamonds. Nature Nanotechnology. 7 (1), 11-23 (2011).
- Kucsko, G., et al. Nanometre-scale thermometry in a living cell. Nature. 500 (7460), 54-58 (2013).
- Liu, J., et al. Origin of the Robust Catalytic Performance of Nanodiamond-Graphene-Supported Pt Nanoparticles Used in the Propane Dehydrogenation Reaction. ACS Catalysis. 7 (5), 3349-3355 (2017).
- Chang, B. -. M., et al. Highly Fluorescent Nanodiamonds Protein-Functionalized for Cell Labeling and Targeting. Advanced Functional Materials. 23 (46), 5737-5745 (2013).
- Ho, D., Wang, C. H., Chow, E. K. Nanodiamonds: The intersection of nanotechnology, drug development, and personalized medicine. Science Advances. 1 (7), 1500439 (2015).
- Hsu, M. H., et al. Directly thiolated modification onto the surface of detonation nanodiamonds. ACS Applied Materials and Interfaces. 6 (10), 7198-7203 (2014).
- Krueger, A. Diamond Nanoparticles: Jewels for Chemistry and Physics. Advanced Materials. 20 (12), 2445-2449 (2008).
- Turcheniuk, K., Trecazzi, C., Deeleepojananan, C., Mochalin, V. N. Salt-assisted ultrasonic deaggregation of nanodiamond. ACS Applied Materials and Interfaces. 8 (38), 25461-25468 (2016).
- Akiel, R. D., Zhang, X., Abeywardana, C., Stepanov, V., Qin, P. Z., Takahashi, S. Investigating Functional DNA Grafted on Nanodiamond Surface Using Site-Directed Spin Labeling and Electron Paramagnetic Resonance Spectroscopy. Journal of Physical Chemistry B. 120 (17), 4003-4008 (2016).
- Gaillard, C., et al. Peptide nucleic acid-nanodiamonds: covalent and stable conjugates for DNA targeting. RSC Advances. 4 (7), 3566-3572 (2014).
- Zhang, T., et al. DNA-based self-assembly of fluorescent nanodiamonds. Journal of the American Chemical Society. 137 (31), 9776-9779 (2015).
- Liu, W., et al. Fluorescent Nanodiamond-Gold Hybrid Particles for Multimodal Optical and Electron Microscopy Cellular Imaging. Nano Letters. 16 (10), 6236-6244 (2016).
- Lee, H., Dellatore, S. M., Miller, W. M., Messersmith, P. B. Mussel-inspired surface chemistry for multifunctional coatings. Science. 318 (5849), 426-430 (2007).
- Wang, C., Zhou, J., Wang, P., He, W., Duan, H. Robust Nanoparticle-DNA Conjugates Based on Mussel-Inspired Polydopamine Coating for Cell Imaging and Tailored Self-Assembly. Bioconjugate Chemistry. 27 (3), 815-823 (2016).
- Liu, R., Guo, Y., Odusote, G., Qu, F., Priestley, R. D. Core-shell Fe3O4 polydopamine nanoparticles serve multipurpose as drug carrier, catalyst support and carbon adsorbent. ACS Applied Materials and Interfaces. 5 (18), 9167-9171 (2013).
- Liu, R., et al. Dopamine as a Carbon Source: The Controlled Synthesis of Hollow Carbon Spheres and Yolk-Structured Carbon Nanocomposites. Angewandte Chemie International Edition. 50 (30), 6799-6802 (2011).
- Zeng, Y., Liu, W., Wang, Z., Singamaneni, S., Wang, R. Multifunctional surface modification of nanodiamonds based on dopamine polymerization. Langmuir. 34 (13), 4036-4042 (2018).
- Qin, S., et al. Dopamine@Nanodiamond as novel reinforcing nanofillers for polyimide with enhanced thermal, mechanical and wear resistance performance. RSC Advances. 8 (7), 3694-3704 (2018).
- Barras, A., Lyskawa, J., Szunerits, S., Woisel, P., Boukherroub, R. Direct functionalization of nanodiamond particles using dopamine derivatives. Langmuir. 27 (20), 12451-12557 (2011).
- Khanal, M., et al. Toward Multifunctional “Clickable” Diamond Nanoparticles. Langmuir. 31 (13), 3926-3933 (2015).
- Rad, M. H., Zamanian, A., Hadavi, S. M. M., Khanlarkhani, A. A Two-Stage Kinetics Model for Polydopamine Layer Growth. A Two-Stage Kinetics Model for Polydopamine Layer Growth. Macromolecular Chemistry and Physics. , 1700505 (2018).
- Ball, V., Frari, D. D., Toniazzo, V., Ruch, D. Kinetics of polydopamine film deposition as a function of pH and dopamine concentration: Insights in the polydopamine deposition mechanism. Journal of Colloid and Interface Science. 386 (1), 366-372 (2012).
- Liu, Y., Ai, K., Lu, L. Polydopamine and its derivative materials: synthesis and promising applications in energy, environmental, and biomedical fields. Chemical Reviews. 114 (9), 5057-5115 (2014).
- Hu, J., Wu, S., Cao, Q., Zhang, W. Synthesis of core-shell structured alumina/Cu microspheres using activation by silver nanoparticles deposited on polydopamine-coated surfaces. RSC Advances. 6 (85), 81767-81773 (2016).
- Orishchin, N., et al. Rapid Deposition of Uniform Polydopamine Coatings on Nanoparticle Surfaces with Controllable Thickness. Langmuir. 33, 6046-6053 (2017).
- González, A. L., Noguez, C., Beránek, J., Barnard, A. S. Size, Shape, Stability, and Color of Plasmonic Silver Nanoparticles. Journal of Physical Chemistry C. 118 (17), 9128-9136 (2014).
- Muthuchamy, N., Gopalan, A., Lee, K. -. P. A new facile strategy for higher loading of silver nanoparticles onto silica for efficient catalytic reduction of 4-nitrophenol. RSC Advances. 5 (93), 76170-76181 (2015).
- Bastus, N. G., Comenge, J., Puntes, V. Kinetically Controlled Seeded Growth Synthesis of Citrate-Stabilized Gold Nanoparticles of up to 200 nm: Size Focusing versus Ostwald Ripening. Langmuir. 27 (17), 11098-11105 (2011).
- Jana, J., Gauri, S. S., Ganguly, M., Dey, S., Pal, T. Silver nanoparticle anchored carbon dots for improved sensing, catalytic and intriguing antimicrobial activity. Dalton Transactions. 44 (47), 20692-20707 (2015).
- Zamudio, A., et al. Efficient anchoring of silver nanoparticles on N-doped carbon nanotubes. Small. 2 (3), 346-350 (2006).
- Chen, K., Li, T. Modification of membranes with polydopamine and silver nanoparticles formed in situ to mitigate biofouling. U.S. Patent Application. , (2016).

