Digestion of the Murine Liver for a Flow Cytometric Analysis of Lymphatic Endothelial Cells
Summary
The goal of this protocol is to identify lymphatic endothelial cell populations within the liver using described markers. We utilize collagenase IV and DNase and a gentle mincing of tissue, combined with flow cytometry, to identify a distinct population of lymphatic endothelial cells.
Abstract
Within the liver, lymphatic vessels are found within the portal triad, and their described function is to remove interstitial fluid from the liver to the lymph nodes where cellular debris and antigens can be surveyed. We are very interested in understanding how the lymphatic vasculature might be involved in inflammation and immune cell function within the liver. However, very little has been published establishing digestion protocols for the isolation of lymphatic endothelial cells (LECs) from the liver or specific markers that can be used to evaluate liver LECs on a per cell basis. Therefore, we optimized a method for the digestion and staining of the liver in order to evaluate the LEC population in the liver. We are confident that the method outlined here will be useful for the identification and isolation of LECs from the liver and will strengthen our understanding of how LECs respond to the liver microenvironment.
Introduction
The role of lymphatic vessels and LECs in the liver is not well understood. While lymphatic vessels are found within the portal triad of the liver1 and expand during disease2, very little is understood regarding the function and phenotype of LECs within the liver. With the discovery of markers that are found primarily on LECs3, the importance of these cells within different tissue niches in homeostasis and disease will fill a significant gap in our understanding. LECs have a major role in maintaining peripheral tolerance in the lymph node and in metastatic tumors by interacting directly with T cells4,5,6,7,8,9,10,11,12,13. LECs in the lymph node can promote protective immunity via their interactions with migratory dendritic cells14,15,16. Therefore, there are multiple roles for LECs which may be specific to the tissues and interactions in which they are present. However, very little is understood about how LECs interact with immune cells in the tissue or how LECs function in different organ systems; thus, evaluating LECs on a per cell basis within the liver or other organs may lead to advances in how LECs program tissue-specific immunity. While much of the literature that focuses on LECs in the liver uses microscopy to visualize LECs using one or two markers and morphology17, very little has been done to specifically evaluate LECs on a cell by cell basis using flow cytometry, though one study did evaluate differences between liver sinusoidal endothelial cells (LSECs) and LECs18. Being able to analyze LEC populations in the liver by flow cytometry allows for the in-depth study of LEC phenotype during normal homeostasis or disease.
To evaluate LECs by flow cytometry, multiple surface markers are needed. Typically, LECs are visualized by the expression of prospero-related homeobox 1 (Prox-1), lymphatic vessel endothelial hyaluronan receptor 1 (LYVE1) or vascular endothelial growth factor receptor 3 (VEGFR3) using microscopy. However, in the liver, the expression of these markers is not restricted to LECs. Prox-1 is widely expressed by hepatocytes during liver development, regeneration, and injury19, and LYVE1 and VEGFR3 are expressed by the liver sinusoidal endothelial cells18. In the lymph node, LECs are identified using flow cytometry as clusters of differentiation (CD) CD45-, CD31+, and podoplanin+ (PDPN)16. However, this approach is too minimal to isolate LECs in the liver since CD45- CD31+ cells will capture endothelial cells, and the predominant population of vascular endothelial cells in the liver are LSECs. Thus, other markers are needed to distinguish the rare LEC population from the abundant LSEC population. Both CD16/32 (expressed by mature LSECs18) and CD146 (a common vascular endothelial cell marker that is predominately expressed within the liver sinusoids by liver sinusoidal endothelial cells20 with little to no expression by lymphatic endothelial cells21) were candidate markers.
Therefore, we optimized a method for isolating and visualizing LECs in the liver using the above markers, CD45, CD31, CD146, CD16/32, and PDPN for flow cytometry. We describe the use of collagenase IV, DNase 1, and mechanical separation for liver tissue digestion into a single-cell suspension. We also describe the use of iodixanol density gradient for the isolation of non-parenchymal cells (NPC) and to eliminate cellular debris. Finally, using multiple markers, we determine the optimal flow cytometry gating strategy to identify LECs from the liver with PDPN as the predominant marker.
Protocol
All methods described here have been approved by the Institutional Animal Care and Use Committee (IACUC) of the University of Colorado Anschutz Medical Campus.
1. Preparation of the Materials
- Make a 5 mg/mL solution of DNase I in phosphate-buffered saline (PBS).
- Make a digestion mixture by adding 5,000 U/mL of collagenase IV to Click’s EHAA media.
- Warm the digestion mixture at 37 °C for 30 min prior to use.
- Make an isolation buffer by adding 4.8% bovine serum albumin (BSA) and 2 mM ethylenediaminetetraacetic acid (EDTA) to Hanks’ balanced salt solution (HBSS).
- Make a red blood cell (RBC) lysis buffer by adding 100 mM ammonium chloride, 10 mM KHCO3, and 0.1 mM EDTA to distilled H2O.
2. Preparation of a Single-cell Suspension from a Mouse Liver
- Euthanize the mouse with CO2 and cervical dislocation.
- Spray down the mouse with 70% ethanol to wet its fur. Pin the mouse’s feet to a dissection board.
- Using dissection scissors to cut the skin about 1 cm above the anus, being careful to cut only through the skin (about 1 mm). Pull the skin away from the body with toothed forceps and insert the scissors between the skin and peritoneum. Open the scissors to separate the skin from the peritoneum and, then, cut the skin from the incision to the neck.
- Pin the skin to the dissection board using one pin under each arm and above each leg. Pull the peritoneal sac up and cut upwards toward the neck. Grab the lobes of the liver and cut just below the sternum.
Note: Care should be taken if any of the liver will be used for immunohistochemistry (IHC). - Cut around the liver and remove the liver from the mouse and place it in 4 mL of Click’s EHAA media.
- Using a scalpel, cut the liver in ~1 mm diameter pieces.
- Add 500 µL of the digestion mixture and 500 µL of the DNase I (2 mg/mL) to the liver.
- Incubate the liver for 30 min at 37 °C. After 15 min, mix the liquid using a 5 mL pipette.
- After 30 min of incubation, transfer the digested sample through a 100 µm strainer to a 50 mL conical tube.
- Gently push the remaining pieces through the filter with the plunger of a 1-mL syringe.
- Wash the filter with 5 mL of isolation buffer and gently push the tissue through the strainer with the back of a plunger from a 1 mL syringe. Repeat this until the filter is washed with 25 mL of isolation buffer.
- Centrifuge the cells at 400 x g for 5 min. Carefully aspirate off the supernatant.
- Resuspend the pellet with 4 mL of RBC lysis buffer. Incubate the cells at room temperature for 5 min.
- Wash the cells with 10 mL of isolation buffer and centrifuge at 400 x g for 5 min.
- Count the cells on a hemocytometer to determine the full liver count.
- Resuspend cells in 5 mL of 20% iodixanol and layer them with 1 mL of PBS.
- Centrifuge the cells at 300 x g for 15 min without a brake.
- Remove the layer between the PBS and the iodixanol and place them, through a 100 µm filter, into a new 50 mL conical tube.
- Wash the cells with 10 mL of isolation buffer and centrifuge at 400 x g for 5 min.
- Discard the supernatant and resuspend the cells in 500 µL of PBS with 2% fetal bovine serum (FBS).
3. Flow Cytometric Analysis of Single Cells from the Liver
- Count the cells using a hemocytometer and microscope, using trypan blue exclusion to measure viable cells. Add 10 µL of the cells to 10 µL of trypan blue and immediately place them on a hemocytometer and count the live cells (not blue) under a microscope. Then, calculate the number of cells per microliter.
- Aliquot approximately 5 million of the remaining nonparenchymal cells into a single well of a 96-well plate.
- Centrifuge the cells at 400 x g for 5 min.
- Discard the supernatant and resuspend the cells in 90 µL of PBS with 2% FBS.
- Add anti-CD45 (1:200), anti-CD146 (1:200), anti-CD31 (1:200), and PDPN (1:200) diluted in 10 µL of 10x 2.4G2 or anti-CD16/32 (1:200).
NOTE: No Fc block (2.4G2) was used when anti-CD16/32-labeled antibody was used. - To determine where positive and negative gates should be set, include a fluorescence minus one (FMO) stain for each color and an isotype control antibody.
- To determine live versus dead cells, stain with a viability marker (e.g., ghost red 780). Incubate the cells at 4 °C for 30 min.
- Wash the cells with 100 µL of PBS with 2% FBS.
- Use a small aliquot of cells to adjust the laser and compensation settings on the flow cytometer. Stain the cells with an antibody to each individual fluorophore and one without any antibody.
NOTE: Depending on the flow cytometer being used, a compensation matrix should be established to remove spectral overlap. - Place the sample tube onto the cytometer probe and collect and record all events.
4. Data Analysis
- Looking at side-scatter area vs. forward-scatter area, gate on “live” cells based on size and granularity and viability marker dye.
- Next, using CD45 Brilliant Violet 510 and CD31 PerCp Cy5.5, gate on the CD45- CD31+ cells using the isotype controls and FMO to determine positive and negative populations.
- Lastly, using CD146 v450 or CD16/32 FITC and PDPN APC, take the CD146- PDPN+ or CD16/32- PDPN+ cells, again using isotype controls and FMO, to determine positive and negative populations. These cells are the LECs.
Representative Results
Studies analyzing liver lymphatics have primarily used immunohistochemistry to quantitate the frequency and diameter of lymphatic vessels in the liver. However, this method does not allow for the evaluation of LECs on a cell-by-cell basis or for expression of multiple markers, cytokines, chemokines, or transcription factors. Therefore, we asked whether liver LECs could be isolated from the liver and evaluated using flow cytometry. Previous work isolating lymph node LECs was performed using Liberase DL (collagenase I-II-and-dispase) and DNase 1 combined with the mechanical separation of the lymph node tissue with needles14,16. Therefore, the same digestion protocol on liver tissue was used, or collagenase IV and DNase 1 was used as previously described, for immune cell isolation from the liver22 and followed the digestion with a density gradient separation (iodixanol) step to remove hepatocytes and increase the frequency of other cell types within the liver (Figure 1A). Following liver digestion and centrifugation with the density gradient, the cells were stained with CD45, CD31, PDPN, and CD16/32. CD16/32 has been described to be expressed by mature LSEC populations, but not LEC populations18. Thus, LECs were gated as CD45-, CD31+, CD16/32-, PDPN+ cells, while LSECs were gated as CD45-, CD31+, CD16/32+ and PDPN- (Figure 1A). Gates were set on live cells (ghost red negative) and based on isotype controls and FMO staining (Figure 1A). LYVE-1 APC on the LEC population was also confirmed (Figure 1B). Both methods allowed the visualization of the LEC population within the liver; however, the staining profile of the cells was visually better when using collagenase IV and DNase 1 than collagenase I-II-and-dispase (Figure 1C). Therefore, all future manipulations were performed using collagenase IV and DNase 1, as described in the protocol. On average, we obtained approximately 1,300 LECs per gram of liver tissue or 2,200 LECs per naive liver, using this digestion method.
To optimize the gating strategy and combination of fluorophores and to eliminate contamination of LSECs, both CD16/32 and CD146 were used. CD16/32 is Fc gamma receptors II and III and is expressed on mature LSECs but not on LECs18. CD16/32 could distinguish the PDPN+ CD16/32- cells from the PDPN- CD16/32+ cells, especially when using PDPN conjugated to APC or PE and CD16/32 conjugated to FITC (Figure 1A), but less well when PDPN was conjugated to PE-Cy7 (Figure 1C). The expression of CD16/32 is not found on LECs but is found on LSECs. Fc block uses the CD16/32 antibody to minimize the Fc receptor binding and nonantigen specific binding of immunoglobulins to the Fc receptors. Since CD16/32 was used to visualize LSECs, the non-specific binding of immunoglobulins was higher and CD146 was optimized as an alternative to measure LSECs. Therefore, we tested CD146 conjugated to V450, a vascular endothelial cell marker expressed highly by LSECs and other vascular endothelial cells20 but with low to no expression by LECs23. We first optimized the staining of CD146 using FMO and isotype controls for both CD16/32 and CD146 (Figure 2A). If we evaluated only the CD16/32+ cells or the CD16/32- cells, the CD16/32+ cells were all CD146+ and PDPN- while the CD16/32- cells were primarily CD146- and either PDPN- or PDPN+ (Figure 2B). To confirm this staining, FMO was used. By removing PDPN from the stain, the PDPN+ population disappeared (Figure 2C). Interestingly, there were no CD146+ PDPN+ cells, confirming that CD146 is not or very lowly expressed by PDPN+ LECs. Thus, we are confident that either of these markers can be used to gate out vascular endothelial cells in the liver.
To further validate that PDPN is an appropriate marker for LECs, liver sections from mice were stained with both PDPN (green) and F4/80 (brown) (Figure 3A). Neither the vascular endothelium nor macrophages expressed PDPN in the murine liver. We were also able to distinguish cholangiocytes, which can stain positive for PDPN, from lymphatic vessels, based on the distinct nuclear structures of the bile ducts and by cytokeratin 7 staining (Figure 3B; red: cytokeratin 7, green: PDPN). Since multiple markers are used for flow cytometry, such as CD31, which is not expressed by cholangiocytes24, we are confident that we are removing these cells from the analysis, thereby confirming that cells from the liver that are CD45-, CD31+, CD146lo/neg, CD16/32-, and PDPN+ are LECs. Finally, to provide evidence that the stained cells are LECs, we sorted this population of cells and used qualitative real-time polymerase chain reaction to evaluate the Vegfr3 and prox-1 expressions. Both Vegfr3 and prox-1 were evaluated, as these transcripts are expressed by other cells in the liver—prox1 by hepatocytes and Vegfr3 by LSECS (among others) — but no other cells besides LECs express both. The expression of both these markers was significantly higher in the sorted population than in the cultured murine macrophage cell line (RAW264.7) which does not normally express these markers but is similar in expression to cultured murine LECs (Figure 3C).
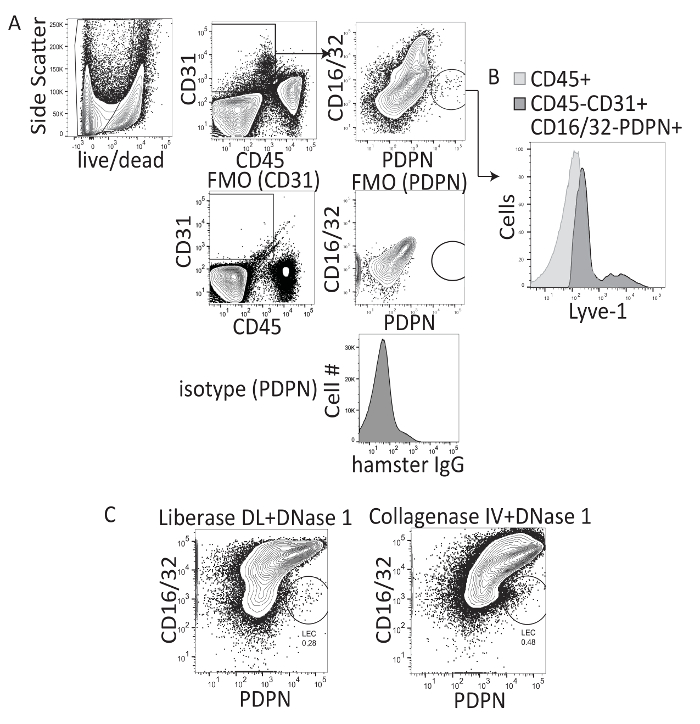
Figure 1: Representative flow cytometry analysis of collagenase I-II-dispase and collagenase IV-digested murine liver tissue. (A) This panel shows the gating strategy, fluorescence minus one staining, and isotype controls for the procedure. (B) This panel shows the LYVE-1 staining of CD16/32- PDPN+ cells. (C) This panel shows the final flow cytometry gate after digesting mouse livers with either collagenase I-II-dispase (e.g., Liberase DL) or collagenase IV and evaluating CD16/32 PerCP X PDPN PE-Cy7 where LECs are CD16/32- and PDPN+. Please click here to view a larger version of this figure.
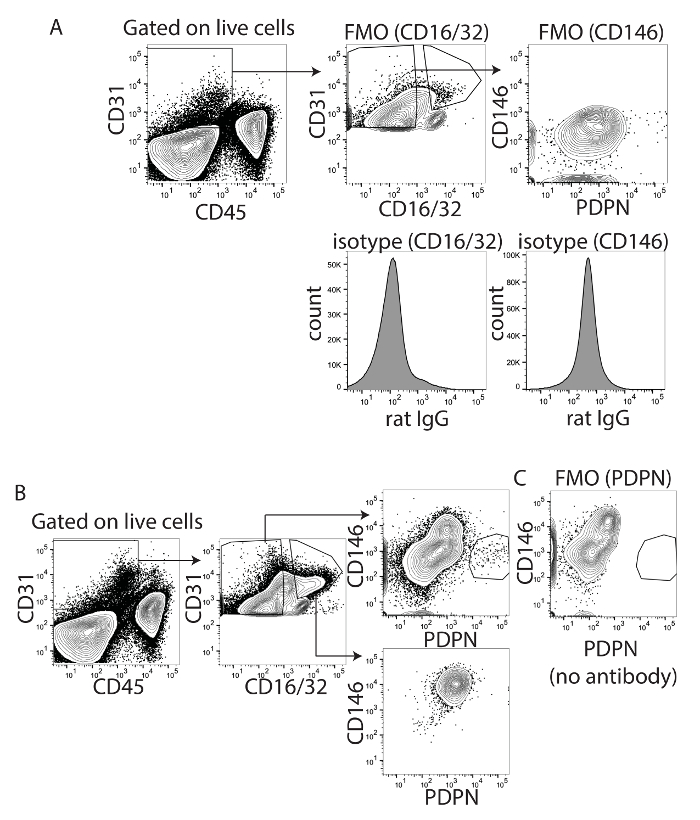
Figure 2: Identification of CD146 and PDPN as appropriate markers for liver lymphatic endothelial cells. (A) This panel shows fluorescence minus one and isotype controls for CD16/32 (left) and CD146 (right). (B) This panel shows gated CD16/32 positive or negative cells determined from panel A. Shown is CD146XPDPN from both populations. (C) This panel shows fluorescence minus one for PDPN to demonstrate that the staining is absent when the antibody is not added. Please click here to view a larger version of this figure.
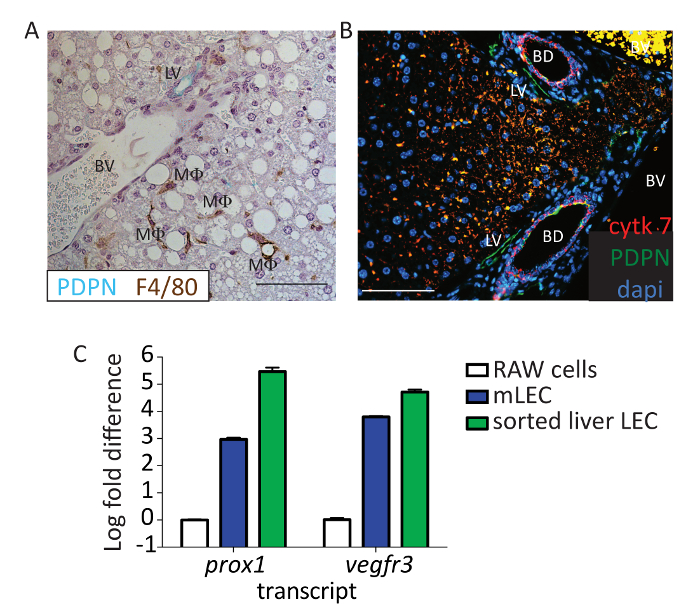
Figure 3: Identification of flow PDPN+ cells as lymphatic endothelial cells. (A) This panel shows representative immunohistochemistry from a mouse liver stained with PDPN (blue/green) and F4/80 (brown). The lymphatic vessel (LV), blood vessel (BV), and macrophage (MF) are labeled. Scale bar = 100 µm. Formalin-fixed paraffin-embedded tissue was deparaffinized for 20 min in xylene. Tissues were hydrated to water through a gradient of ethanol, and antigen retrieval was performed using pH 6 antigen retrieval buffer in a pressure cooker for 15 min. Tissue was blocked using 0.1% BSA and stained using anti-mouse PDPN and anti-mouse F4/80 for 1 h at room temperature. Anti-hamster IgG HRP and anti-rabbit IgG HRP were used as secondary antibodies. 3,3'-Diaminobenzidine (DAB)+ and Vina Green were used to detect the F4/80 and PDPN, respectively. Tissue was counterstained with hematoxylin and imaged on a Microscope. (B) This panel shows the same experiment performed as in panel A, except here, PDPN is shown in green, cytokeratin 7 in red, and 4′,6-diamidino-2-phenylindole (DAPI) in blue. Scale bar = 100 µm. Tissue was blocked using 5% donkey and 5% goat serum and stained using anti-mouse PDPN (8.1.1) 1:100 and anti-mouse cytokeratin 7 1:200 for 1 h at room temperature. Anti-hamster IgG AF647 and anti-rabbit IgG-PE were used as secondary antibodies. Tissue was counterstained with DAPI and imaged. Scale bar = 100 µm. The lymphatic vessel (LV), blood vessel (BV), and bile duct (BD) are labeled. (C) This panel shows a log fold change in Vegfr3 and prox-1 expression from sorted liver LECs based on the staining in the protocol (CD45-, CD31+, CD146lo/neg, and PDPN+) compared to RAW cells or primary murine lymph node LECs. Sorted cells were passed through a biopolymer-shredding column, RNA was extracted using an RNA extraction kit, and cDNA was made using a reverse transcription kit. Transcript abundance was normalized to the housekeeping gene, Gapdh, for every sample. Please click here to view a larger version of this figure.
Discussion
The overall importance of LECs in immune homeostasis and regulation has recently come to light25. Much of the published lymphatic literature focuses on skin and lymph nodes; however, lymphatics are found throughout the body26 and, thus, our understanding of their importance in different organs is needed. Here we show a method in which LECs in the liver can be studied on a cell-by-cell basis to better understand their concurrent expression of different surface markers, cytokines, chemokines, and intracellular proteins such as transcription factors. This method will be useful for future studies to assess the phenotype and function of LECs in the liver during health and disease.
One of the hurdles for the identification of LECs in the liver is their relatively low frequency compared to other cell types. Hepatocytes make up about 80% of the liver, and removing these cells using density gradient (iodixanol) before running the liver on a flow cytometer requires less time and, thus, provides better viability. In order to distinguish the population of LYVE1+ LECs in the liver from LYVE1+ LSECs, we used the markers CD16/32 and CD146 found on LSECs and with low to no expression by LECs. This, coupled with the lack or low expression of PDPN by any other endothelial cell in the liver, allowed the validation by flow cytometry that the population we identified were LECs. Indeed, downstream transcriptional analysis confirmed that this gating strategy produced LECs (Figure 3C).
The isolation of LECs from the lymph node is best done using collagenase I-II-and-dispase; however, we found that, while this method does extract LECs from the liver, a liver digestion protocol using collagenase IV provides a better downstream analysis using flow cytometry. Using a mechanical disruption of the liver allows the collagenase more surface area to better interact with the extracellular matrix-associated cells, like LECs, and using Click's EHAA media without FBS allows for the digestion of the liver to occur in only 30 min. This decreased time maintains LEC viability for downstream assays like flow cytometry or flow sorting. Indeed, we were able to recover enough viable cells to visualize LECs by flow cytometry and flow sorting for downstream transcriptional analysis.
Combined, the separation of hepatocytes from the non-parenchymal cells, the use of collagenase type IV, and the clarification and demonstration of markers specific to LECs in the liver and markers specific to other endothelial cell populations, such as CD16/32 and CD146, allowed the proper identification of LECs in the liver. These methods fill a significant gap in the literature about how LECs in the liver can be identified by flow cytometry, especially since the liver contains a number of other cells that express markers known to be unique to LECs in the lymph node (prox1 and vegfr3). Therefore, these methods will lead to downstream studies regarding liver LEC function. Additionally, this method can be modified for other tissues in order to better evaluate tissue-specific LEC markers and subsets.
Divulgazioni
The authors have nothing to disclose.
Acknowledgements
The authors would like to thank the GI and Liver Innate Immune Programs for monetary support of this project. B.A.J.T. is also funded by R01 AI121209.
Materials
| Clicks/EHAA media | Irvine Scientific | 9195 | |
| Collagenase IV | Worthington Biochemical corporation | LS004188 | |
| DNase I | Worthington Biochemical corporation | LS002145 | Deoxyribonuclease 1 |
| OptiPrep | Sigma Aldrich | D1556 | Density Gradient Medium |
| V450 anti mouse CD146(clone ME-9F1 | BD biosciences | 562232 | |
| FITC anti mouse CD146 (clone ME-9F1 | Biolegend | 134706 | Fluorescein isothiocyanate (FITC) |
| Pacific Blue anti mouse CD31(clone 390) | Biolegend | 102422 | |
| PerCp/Cy5.5 anti mouse CD31( clone 390) | Biolegend | 102420 | Peridinin-chlorophyll proteins-Cyanine 5.5 (PerCP-Cy5.5) |
| APC anti mouse PDPN (clone 8.1.1) | Biolegend | 127410 | Allophycocyanin (APC), podoplanin (PDPN) |
| APC/Cy7 anti mouse CD45 (clone 30-F11) | Biolegend | 103116 | |
| Brilliant Violet 510 anti mouse CD45 (clone 30-F11 | Biolegend | 103138 | |
| FITC anti mouse CD16/32 (clone 93) | Biolegend | 101306 | Fluorescein isothiocyanate (FITC) |
| PerCp/Cy5.5 anti mouse CD16/32( clone 93) | Biolegend | 101324 | Peridinin-chlorophyll proteins-Cyanine 5.5 (PerCP-Cy5.5) |
| ghost red 780 viability dye | TONBO biosceinces | 3-0865-T100 | |
| APC syrian hamster IgG (clone SHG-1) | Biolegened | 402102 | |
| PerCp/Cy5.5 rat IgG2a (clone RTK2758) | Biolegend | 400531 | |
| FITC rat IgG2 (clone eBR2a) | ebioscience | 1-4321-80 | |
| Anti mouse LYVE1 (clone 223322) | R&D systems | FAB2125A | |
| anti-mouse Cytokeratin(clone EPR17078) | abcam | ab181598 | |
| anti-mouse F4/80 (clone Cl:A3-1) | Bio-rad | MCA497 | |
| BSA (fraction V) | Fischer | BP1600-100 | Bovine Serum Albumin (BSA) |
| Goat serum | Jackson Immunoresearch | 017-000-121 | |
| Donkey Serum | Jackson Immunoresearch | 017-000-121 | |
| EDTA | VWR | E177 | Ethylenediaminetetraacetic acid (EDTA) -for RBC lysis buffer |
| Ammonium Chloride | Fischer | A687-500 | for RBC Lysis buffer |
| Potassium Bicarbonate | Fischer | P184-500 | for RBC Lysis buffer |
| Scalpel | Feather | 2975#21 | |
| 100um cell strainer | Fischer | 22363549 | |
| 2.4G2 | in house/ATCC | ATCC HB-197 | FC block to inhibit non-specific binding to Fc gamma + cells -made from hybridoma |
| Phosphate Buffered Saline (PBS) | Corning | 21-040-CV | |
| Hanks Balanced Salt Solution (HBSS) | Gibco | 14185-052 | |
| Fetal Bovine Serum (FBS) | Atlanta biologicals | S11550 | |
| 96 well plate | Corning | 3788 | |
| 6 well plate | Corning | 3506 | |
| 50 ml conical | Truline | TR2004 | |
| 15 ml conical | Falcon | 352196 | |
| 1 ml Pipete tip | USA scientific | 1111-2721 | |
| 200 µl pipete tip | USA scientific | 1110-1700 | |
| 10 µl pipete tip | USA scientific | 1111-3700 | |
| seriological 10ml pipete | greiner bio-one | 607107 | |
| seriological 5ml pipete | greiner bio-one | 606107 | |
| Cell incubator | Fischer | Heracell 160i | |
| BD FacsCanto II flow cytometer | BD biosciences | ||
| Clinical Centrifuge | Beckman coulter | model X-14R |
Riferimenti
- Tanaka, M., Iwakiri, Y. Lymphatics in the liver. Current Opinion in Immunology. 53, 137-142 (2018).
- Vollmar, B., Wolf, B., Siegmund, S., Katsen, A. D., Menger, M. D. Lymph vessel expansion and function in the development of hepatic fibrosis and cirrhosis. The American Journal of Pathology. 151 (1), 169-175 (1997).
- Podgrabinska, S., et al. Molecular characterization of lymphatic endothelial cells. Proceedings of the National Academy of Sciences of the United States of America. 99 (25), 16069-16074 (2002).
- Cohen, J. N., et al. Lymph node-resident lymphatic endothelial cells mediate peripheral tolerance via Aire-independent direct antigen presentation. Journal of Experimental Medicine. 207 (4), 681-688 (2010).
- Cohen, J. N., et al. Tolerogenic properties of lymphatic endothelial cells are controlled by the lymph node microenvironment. PLoS One. 9 (2), e87740 (2014).
- Rouhani, S. J., et al. Roles of lymphatic endothelial cells expressing peripheral tissue antigens in CD4 T-cell tolerance induction. Nature Communications. 6, 6771 (2015).
- Tewalt, E. F., et al. Lymphatic endothelial cells induce tolerance via PD-L1 and lack of costimulation leading to high-level PD-1 expression on CD8 T cells. Blood. 120 (24), 4772-4782 (2012).
- Dubrot, J., et al. Lymph node stromal cells acquire peptide-MHCII complexes from dendritic cells and induce antigen-specific CD4(+) T cell tolerance. Journal of Experimental Medicine. 211 (6), 1153-1166 (2014).
- Hirosue, S., et al. Steady-state antigen scavenging, cross-presentation, and CD8+ T cell priming: a new role for lymphatic endothelial cells. Journal of Immunology. 192 (11), 5002-5011 (2014).
- Lund, A. W., et al. VEGF-C promotes immune tolerance in B16 melanomas and cross-presentation of tumor antigen by lymph node lymphatics. Cell Reports. 1 (3), 191-199 (2012).
- Lund, A. W., et al. Lymphatic vessels regulate immune microenvironments in human and murine melanoma. Journal of Clinical Investigation. 126 (9), 3389-3402 (2016).
- Swartz, M. A. Immunomodulatory roles of lymphatic vessels in cancer progression. Cancer Immunology Research. 2 (8), 701-707 (2014).
- Dietrich, T., et al. Cutting edge: lymphatic vessels, not blood vessels, primarily mediate immune rejections after transplantation. Journal of Immunology. 184 (2), 535-539 (2010).
- Kedl, R., et al. Migratory Dendritic Cells acquire archived antigen from Lymphatic Endothelial Cells for antigen presentation during lymph node contraction. Nature Communications. 8, 2034 (2017).
- Kedl, R. M., Tamburini, B. A. Antigen archiving by lymph node stroma: A novel function for the lymphatic endothelium. European Journal of Immunology. 45 (10), 2721-2729 (2015).
- Tamburini, B. A., Burchill, M. A., Kedl, R. M. Antigen capture and archiving by lymphatic endothelial cells following vaccination or viral infection. Nature Communications. 5, 3989 (2014).
- Yokomori, H., et al. Lymphatic marker podoplanin/D2-40 in human advanced cirrhotic liver–re-evaluations of microlymphatic abnormalities. BMC Gastroenterology. 10, 131 (2010).
- Nonaka, H., Tanaka, M., Suzuki, K., Miyajima, A. Development of murine hepatic sinusoidal endothelial cells characterized by the expression of hyaluronan receptors. Developmental Dynamics. 236 (8), 2258-2267 (2007).
- Dudas, J., et al. Prospero-related homeobox 1 (Prox1) is a stable hepatocyte marker during liver development, injury and regeneration, and is absent from “oval cells”. Histochemistry and Cell Biology. 126 (5), 549-562 (2006).
- Schrage, A., et al. Murine CD146 is widely expressed on endothelial cells and is recognized by the monoclonal antibody ME-9F1. Histochemistry and Cell Biology. 129 (4), 441-451 (2008).
- Amatschek, S., et al. Blood and lymphatic endothelial cell-specific differentiation programs are stringently controlled by the tissue environment. Blood. 109 (11), 4777-4785 (2007).
- Huang, L., Soldevila, G., Leeker, M., Flavell, R., Crispe, I. N. The liver eliminates T cells undergoing antigen-triggered apoptosis in vivo. Immunity. 1 (9), 741-749 (1994).
- Shay, T., Kang, J. Immunological Genome Project and systems immunology. Trends in Immunology. 34 (12), 602-609 (2013).
- Li, B., et al. Adult Mouse Liver Contains Two Distinct Populations of Cholangiocytes. Stem Cell Reports. 9 (2), 478-489 (2017).
- Randolph, G. J., Ivanov, S., Zinselmeyer, B. H., Scallan, J. P. The Lymphatic System: Integral Roles in Immunity. Annual Review of Immunology. 35, 31-52 (2016).
- Olszewski, W. L. The lymphatic system in body homeostasis: physiological conditions. Lymphatic Research and Biology. 1 (1), 11-21 (2003).

