Effect of Fluorescent Proteins on Fusion Partners Using Polyglutamine Toxicity Assays in Yeast
Summary
This article describes protocols to assess the effect of fluorescent proteins on the aggregation and toxicity of misfolded polyglutamine expansion for the rapid evaluation of a newly uncharacterized fluorescent protein in the context of fluorescent reporters.
Abstract
For the investigation of protein localization and trafficking using live cell imaging, researchers often rely on fusing their protein of interest to a fluorescent reporter. The constantly evolving list of genetically encoded fluorescent proteins (FPs) presents users with several alternatives when it comes to fluorescent fusion design. Each FP has specific optical and biophysical properties that can affect the biochemical, cellular, and functional properties of the resulting fluorescent fusions. For instance, several FPs tend to form nonspecific oligomers that are susceptible to impede on the function of the fusion partner. Unfortunately, only a few methods exist to test the impact of FPs on the behavior of the fluorescent reporter. Here, we describe a simple method that enables the rapid assessment of the impact of FPs using polyglutamine (polyQ) toxicity assays in the budding yeast Saccharomyces cerevisiae. PolyQ-expanded huntingtin proteins are associated with the onset of Huntington's disease (HD), where the expanded huntingtin aggregates into toxic oligomers and inclusion bodies. The aggregation and toxicity of polyQ expansions in yeast are highly dependent on the sequences flanking the polyQ region, including the presence of fluorescent tags, thus providing an ideal experimental platform to study the impact of FPs on the behavior of their fusion partner.
Introduction
Since the initial characterization of the green fluorescent protein (GFP) from Aequorea victoria1, a wide palette of genetically encoded FPs have been developed, allowing cell biologists to simultaneously localize and track multiple cellular events/proteins in living cells2,3. FPs are derived from multiple organisms, from jellyfish to coral, and therefore, display specific biophysical properties that divert extensively beyond their respective fluorescent spectrum. These properties include brightness, photostability, and a tendency to oligomerize among others2,4. Selecting monomeric FPs is an important aspect in the selection of a suitable tag when designing a fluorescent reporter, in order to minimize inappropriate interactions and alterations of the fusion partner's function and maximize the reporter efficiency for a given cellular compartment4,5,6. While GFP has, over time, been evolved to minimize the effect of adding the fluorescent tag to the fusion partner5,7,8, how new FP variants perform compared to GFP remains difficult to assess.
Few methods exist to characterize the behavior of FPs. Most of them involve testing biophysical properties of FPs using biochemical approaches, such as ultracentrifugation and gel filtration protocols9,10,11,12. Such methods have the caveat of using purified FPs in solution, offering little insight into their behavior in intact cells. The development of the organized smooth endoplasmic reticulum (OSER) assay offers a quantifiable assessment of FPs' tendency to oligomerize in living cells13 by testing the ability of overexpressed FPs to reorganize endoplasmic reticulum tubules into OSER whorls14. This technique can successfully detect changes between monomeric and oligomeric variants of GFP and other FPs. However, it relies mostly on overexpression in transiently transfected cells, and the quantitation and image analysis can be time-consuming unless the technique is adopted as an automated data collection and analysis workflow.
In order to complement these approaches, we established an assay that takes advantage of the effect of fluorescent tags on the toxicity and aggregation of polyQ expansions in yeast15,16. The expansion of the polyQ stretch with more than 36 repeats within the first exon of the gene encoding the huntingtin protein (Htt) is associated with Huntington's disease17,18. The expression of expanded Httex1 in yeast results in a strong aggregation of the misfolded Htt protein coupled to a severe growth defect. Interestingly, these phenotypes are strongly influenced by the sequences flanking the polyQ stretch, including FPs15,16. It was rationalized that the different properties of FPs can differentially affect polyQ toxicity in yeast. Indeed, compared to GFP-like FPs, red fluorescent proteins and their evolved forms have shown a reduced toxicity and aggregation16. This manuscript provides a detailed protocol to assess the effect of the next generation of FPs on polyQ toxicity and aggregation in yeast. This assay allows for a rapid and potentially high-content analysis of FP variants that can be used in parallel with previously characterized techniques for the optimal characterization of new FPs and can assess how they perform compared to GFP.
Protocol
1. Generation of New Fluorescently Tagged Httex1 Reporters for an Expression in Yeast
Note: This section has been modified from the protocol by Jiang et al.16 and Albakri et al.19.
- Design primers to amplify the sequence encoding the fluorescent protein or interest by PCR. The forward primer should include a leader sequence to assist the restriction enzyme during digestion (GATC), followed by a SpeI restriction site (ACTAGT) and 20 bases downstream of the ATG (excluding ATG) of the fluorescent protein gene of interest. The reverse primer should include the leader sequence (GATC), followed by a SalI restriction site (GTCGAC) and the reverse complement of 20 bases upstream of the stop codon of the FP sequence (including stop).
- Using the primers designed in step 1.1, perform the PCR reaction using a thermocycler with the following settings: heat to 95 °C for 1 min and cycle at 95 °C for 30 s, 60 °C for 30 s, and 72 °C for 2 min per kB of PCR product. Performing 18 cycles is ample.
- Run the PCR reaction on an agarose gel (0.5% in Tris-acetate-EDTA). There should a single band corresponding to the expected product size. Isolate the fragment using a gel purification kit.
- The protocol employs an Httex1 vector carrying 25 (nontoxic) and 72 (HD-associated, displaying a strong aggregation) polyQ repeats. Digest both the PCR fragments and the vector with SpeI and SalI restriction enzymes for 3 h at 37 °C.
- Purify the digested vector by running it on an agarose gel as in step 1.3.
- Purify the digested PCR fragment using a PCR purification kit.
- Ligate the resulting digested PCR fragment and p415-GAL1–FLAG-25/72QpolyQ plasmids1 using T4 ligase (1 h at room temperature). Use a 10 µL reaction (1 µL of T4 enzyme, 1 µL of 10X buffer, 6 µL of PCR fragment, and 2 µL of vector).
- Transform 2 µL of the ligation reaction into 50 µL of Escherichia coli-competent cells and incubate them on ice for 30 min. Then, heat-shock the cells at 42 °C for 30 s. Add 1 mL of SOC outgrowth media and incubate at 37 °C for 1 h in a shaker. Plate 200 µL of the reaction on an LB-agar plate containing 100 µg/mL ampicillin. Incubate the plate at 37 °C overnight.
- Select three individual bacterial colonies, grow them overnight in 3 mL of LB-broth containing 100 µg/mL ampicillin at 37 °C in a shaker and extract the plasmid DNA using a plasmid purification kit.
- Check the plasmid by digesting 500 ng of DNA using SpeI and SalI restriction enzymes for 1 h at 37 °C and run the reaction on an agarose gel (0.5% in Tris-acetate-EDTA). There should be two bands at the right sizes of the vector (~7 kb) and the insert (size varies according to the gene of interest). Then, verify the plasmid by sequencing.
- Transform the p415-GAL1–FLAG-polyQ-FP plasmids into the yeast strain W303 following a standard yeast transformation protocol2.
2. Spotting Assay
- Streak the yeast clones carrying 25Q/72Q tagged with the FP of interest on an agar plate containing yeast selection media (synthetic complete-SC without leucine) with glucose as the carbon source. At the same time, also streak 25Q/72Q-ymsfGFP to serve as a positive control.
Note: 25Q/72Q constructs that do not contain a fluorescent tag are not toxic and can serve as negative control. - Incubate the plates at 30 °C for 2 – 3 d.
- Select up to three single colonies from the plate.
- Inoculate 5 mL of SC supplemented with 2% glucose as the carbon source.
- Pellet 200 µL of each overnight culture and wash it 3x with sterile distilled water.
- Resuspend the cells in SC media containing 2% galactose as the carbon source to induce the expression of polyQ fusions. Incubate the galactose media overnight at 30 °C in a tube rotator. As a control, repeat this step by using glucose-containing media.
- The next morning, equalize the cell densities to optical density at 600 nm (OD600) of 0.2 in 100 µL of SC media in a sterile 96-well plate.
- Prepare four fivefold dilutions of each sample with sterile water by pipetting 20 µL of the sample from the previous well into 80 µL of media in the next well.
- Use a yeast pinning tool to spot the cells onto selective plates (containing glucose or galactose) and incubate at 30 °C for 2 d.
- Image the plates with an image documentation device.
3. Quantification of Cell Growth in Liquid Culture
- Prepare the cell cultures, following steps 2.1 – 2.5 of this protocol.
- Measure the OD600 using a spectrophotometer.
- Dilute the cells to an OD600 of 0.1 in 300 µL of media in a 96-well plate.
- Run each sample in triplicate.
- Incubate the plate in a plate reader/incubator with shaking capabilities. Set the number of samples, the temperature at 30 °C, the absorbance at 600 nm, the length of the experiments to 24 h, and the measurement intervals to 15 min, and select the continuous shaking mode.
- Create the growth curve and quantify the area under the curve using scientific graphing software. The GraphPad Prism 7 is recommended. Paste the data into an XY table with three replicate values. The growth curve will be shown under the Graphs folder at the left side. To quantify the area under the curve, select Analyze at the top left and click Area under curve di XY analyses.
4. Fluorescent Microscopy
- Prepare the cell cultures, following steps 2.1 – 2.5 of this protocol.
- Dilute the cells 10x in growth media and transfer 200 µL of each sample to 8-well imaging chambers.
- Image the cells using a confocal microscope equipped with a 63X Plan Aprochromoat objective (1.4 NA) at room temperature.
Note: The usage of a confocal microscope is optional. A standard wide-field fluorescent microscope can also be employed. - Adjust the pinhole and laser power for optimal image acquisition. Since the 72Q aggregates are much brighter than the diffuse 25Q signal, it is often required to use a different acquisition setting between the different plasmids in order to avoid saturation of the fluorescent signal.
- Process the images using ImageJ20 or another image-processing software. At this step, the percentage of cells that display aggregate can be calculated manually is desired.
5. Dot Blot
Note: In this protocol, dot blot is used to examine the protein expression levels. Prepare the cell cultures, following steps 2.1 – 2.5 of this protocol.
- Generate protein lysates using glass beads in lysis buffer (100 mM Tris, pH 7.5; 200 mM NaCl; 1 mM EDTA; 5% glycerol, 1 mM dithiothreitol [DTT]). Add protease inhibitors, 4 mM phenylmethylsulfonyl fluoride (PSMF) and protease inhibitor cocktail, directly before use. Pellet 5 mL of the overnight culture and resuspend it in 200 µL of glass beads and 200 µL of lysis buffer. Vortex 30 s for 12 rounds. Centrifuge at 12,000 x g at 4 °C for 10 min and collect the supernatant.
- Spot an equal amount of total proteins on a nitrocellulose membrane using a microfiltration apparatus. Prewet the membrane with PBS and assemble the apparatus. Connect to a vacuum source and make sure the screws are tightened. Turn on the vacuum and let the sample filter through the membrane by gravity.
- Block the membrane in PBS- 0.05% Tween/5% fat-free milk.
- Incubate the membrane with primary anti-FLAG antibody overnight at 4 °C. The monoclonal anti-FLAG M1 is recommended.
- Wash the membrane 3x for 10 min each with PSB- 0.05% Tween.
- Incubate the membrane with a fluorescently labeled secondary antibody (anti-mouse IgG) for 1 h at room temperature in PBS- 0.05% Tween/5% fat-free milk.
- Wash membrane 3x 10 min with PSB- 0.05% Tween.
- Image-blot using an immunoblot documentation system.
Representative Results
FPs have different biophysical properties, including their tendency to oligomerize, that can affect the behavior of their fusion partners in the context of fluorescent reporters. This protocol describes a simple method where multiple FPs can be fused to toxic polyQ expansions. Since polyQ toxicity is highly dependent on the sequences flanking the polyQ stretch15, this assay allows a rapid and direct comparison of fluorescent polyQ fusion reporters (Figure 1). A non-HD-associated polyQ length (25Q) is used as a negative control and does not display significant toxicity or aggregation15,16,21,22. 72Q is employed to obtain the HD-like phenotypes, including strong growth inhibition and polyQ aggregation. Importantly, the Httex1 coding sequence employed lack the proline-rich domain that follows the polyQ stretch. In the presence of the proline-rich domain, Httex1 is not toxic in yeast15. In this assay, an Httex1 fused to a yeast-optimized monomeric variant of superfolder GFP12 (ymsfGFP)16 is used as a positive control as previously described16. The constructs also contain a FLAG epitope tag at the N-terminus of Httex1. This allows detection of the different fusions with the same antibody (anti-FLAG) for biochemical analysis. As a proof-of-principle, 72Q Httex1 fused to yeast-optimized TagBFP2 (yomTagBFP2)23 does not result in slow growth measured by either spot assays on agar plates or growth in liquid media (Figure 2), indicating that the nature of the fluorescent tag can indeed impede polyQ expansion behavior in cells.
Aggregation of the fluorescent polyQ fusions can be assessed using fluorescent microscopy. 72Q-ymsfGFP displays significant aggregation compared to 25Q. However, the 72-yomTagBFP fluorescent signal remains diffused throughout the cytoplasm (Figure 3). In most of the cases, it is not recommended to use the same image acquisition settings (laser power, exposure time) to acquire both 25Q and 72Q images. The aggregates in the 72Q-expressing cells are much brighter than the diffused 25Q signal. Therefore, under imaging conditions used to acquire 72Q images, the diffused 25Q signal may appear very weak or not be visible at all. Appropriate acquisition settings should also be applied to minimize the saturation during the imaging of the 72Q-expressing cells.
Expression levels of the various polyQ fusions could affect toxicity. Detergent-insoluble amyloids, such as polyQ aggregates, are notoriously difficult to study biochemically and are not suitable for an analysis by standerd SDS-PAGE. Therefore, dot blots can be performed to assess protein levels. The inclusion of the FLAG tag at the amino terminus end of Httex1 allows detection of all the fluorescent fusions simultaneously, despite the presence of FPs (Figure 4). Alternatively, semi-denaturing detergent agarose gel electrophoresis (SDD-AGE) can be performed to assess the formation of polyQ oligomers16. A detailed protocol and video are available in Halfmann and Lindquist24.
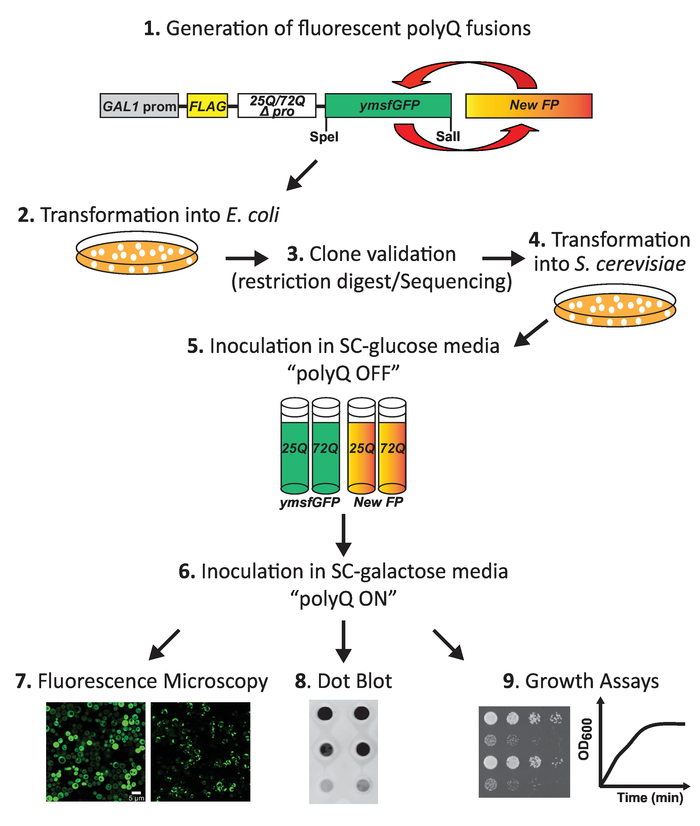
Figure 1: Workflow diagram for the analysis of the effect of fluorescent protein tag on the aggregation and toxicity of polyQ expansion proteins in yeast. First, FPs are cloned into yeast expression vectors encoding a galactose-inducible version of FLAG-tagged Httex1 harboring either 25Q (nontoxic) or 72Q (HD-associated, aggregating and toxic) repeats. Clones are selected and verified by sequencing and, subsequently, transformed in yeast. Following the induction of polyQ fusion expression by incubation in galactose-containing media, either spotting assays on agar plates or growth liquid media can assess the polyQ toxicity. PolyQ aggregation is analyzed by fluorescent microscopy. A relative expression of the different constructs is assessed using dot blot. Please click here to view a larger version of this figure.
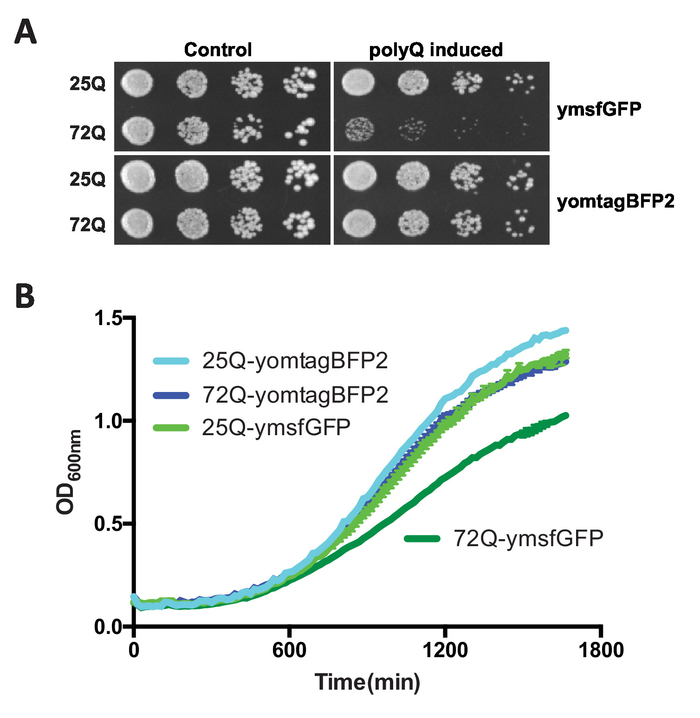
Figure 2: Representative growth assay results following the expression of Httex1 fluorescent fusions in yeast. Yeast expressing either 25Q or 72Q Httex1 fused to ymsfGFP or yomTagBFP was cultured in glucose (control) or galactose media (polyQ-induced) overnight and either (A) spotted on agar plates or (B) incubated further in liquid media to assess growth under the different conditions. While 72Q-ymsfGFP induces a significant growth defect, 72Q-yomTagBFP displays a growth phenotype similar to the nontoxic 25Q counterparts. Please click here to view a larger version of this figure.
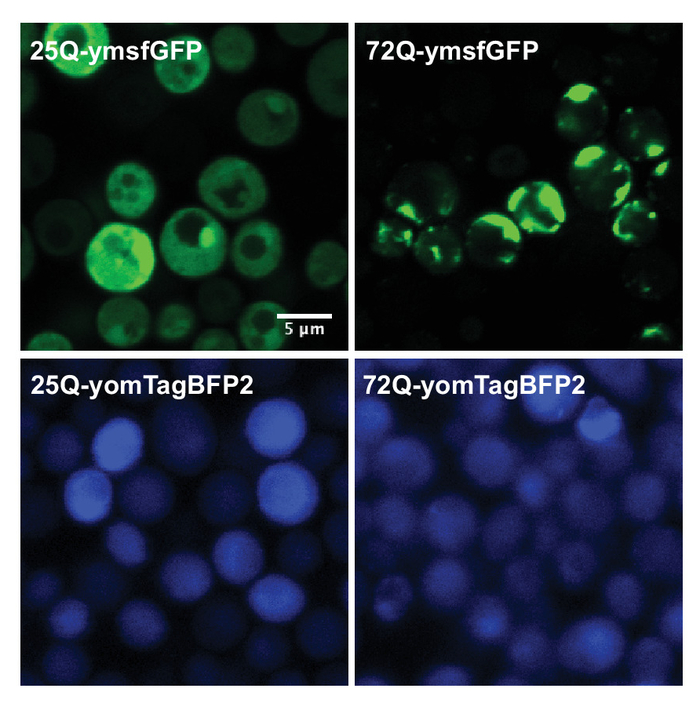
Figure 3: Representative fluorescent images of Httex1 fluorescent fusions in yeast. Yeast expressing either 25Q or 72Q Httex1 fused to ymsfGFP or yomTagBFP was cultured in glucose (control) or galactose media (polyQ-induced) overnight and imaged with a confocal microscope. While the 72Q-ymsfGFP expression results in a strong polyQ protein aggregation, 72Q-yomTagBFP displays a diffused cytoplasmic signal similar to the nontoxic 25Q counterparts. Please click here to view a larger version of this figure.
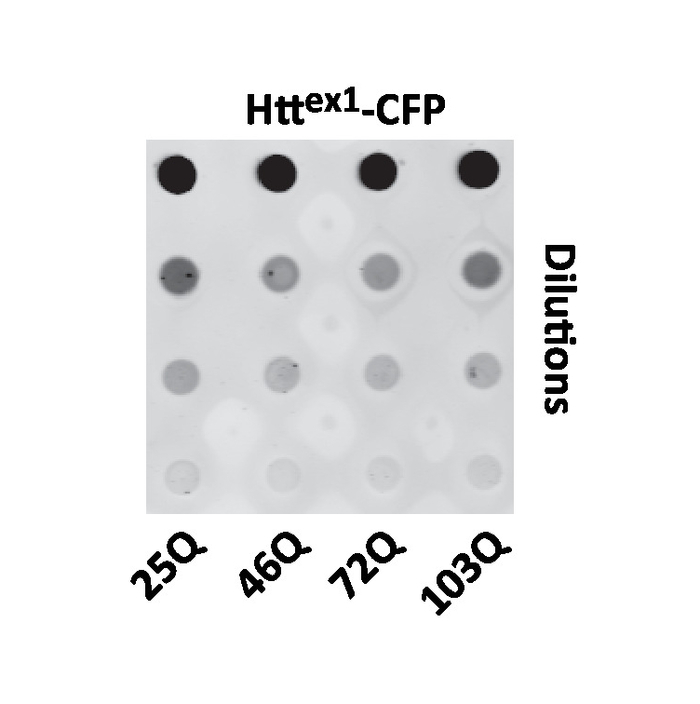
Figure 4: Representative dot blot analysis of Httex1 fluorescent fusion expression in yeast. Yeast expressing 25Q, 46Q, 72Q, or 103Q Httex1 fused to CFP was cultured in galactose media (polyQ-induced) overnight and processed for dot blot analysis. Fivefold dilutions of the cell lysates are shown. Please click here to view a larger version of this figure.
Discussion
In this article, various assays to measure the aggregation of Httex1 polyQ expansions and their effect on yeast growth were employed as a model to study how different fluorescent proteins alter their fusion partners in the context of fluorescent reporters. Using a GFP variant (ymsfGFP) as a positive control, we showed that this detects significant changes in polyQ toxicity and aggregation between different fluorescent tags and allows for a direct and rapid comparison of the polyQ-FP fusion performance against GFP-tagged constructs16,19.
While the present protocol focuses on fluorescent proteins, various parts of the protocol could be readily adapted to test the effects of other protein tags. In addition, the present protocol employs low-copy yeast centromeric vectors that can vary in terms of copy numbers (generally one to two copies) present in cells25. Using integrative vectors to ensure a uniform expression across experimental conditions could circumvent this problem. While this protocol has been optimized for use in the W303 background, other S. cerevisiae strains can be employed. However, susceptibility to polyQ toxicity should be determined using the ymsfGFP-tagged vectors prior to designing new constructs. In certain cases, it may be appropriate to employ high-copy (2µ) vectors to generate a significant growth defect. It is also suggested to test multiple isolates following the yeast transformation with polyQ vectors to avoid selecting spontaneous suppressors showing a reduced polyQ toxicity. Of note, the W303 yeast strain26 is usually used as it is more sensitive to polyQ toxicity than other S288C derivatives, such as BY4741/BY474227, thus allowing for a wider range of growth phenotypes. Importantly, strains employed for this assay need to carry the Rnq1 prion protein since rnq1Δ cells do not display polyQ toxicity and aggregation28. It is also important to use Httex1 constructs carrying the amino-terminal FLAG tag and lacking the proline-rich domain. Other variations of the fusion design may alter toxic phenotypes15. Finally, the induction of the polyQ fusion expression in galactose-containing media is a critical step of the protocol21. When transferring cells from glucose- to galactose-containing media, it is important to wash the cells at least three times with sterile water to eliminate all traces of glucose that could contribute to repressing the induction of the Gal1 promoter29. When performing spotting assays, culturing the cells overnight in galactose media to induce the expression of the fusion can exacerbate the toxic phenotype of the 72Q fusion and help discriminate changes in growth across different fusions16.
As a limitation, previous studies did not observe differential effects between a nonmonomeric version of CFP (a GFP derivative) and ymsfGFP16. Thus, at least for GFP variants, this assay may not be sensitive enough to discriminate between monomeric and oligomeric variants, highlighting the need to complement the polyQ toxicity assays with other standard methods, such as the OSER assay13 and biochemical analysis9,10,11,12 that can directly assess oligomerization. Also, it should be noted that FPs can behave differently in yeast compared to in vitro assays or their expression in other organisms23.
Collectively, these methods allow researchers to rapidly characterize new FPs and measure their effect on their fusion partner. In the future, this protocol will enable the quick screening of new derivatives of previously characterized FPs to identify mutants that behave similarly to GFP variants, which are still the gold standard measure for FP reporters. While this protocol focuses on fluorescent proteins, it can easily be adapted to screen for the effects of other genetically encoded tags, such as SNAP-tag30 and SunTag31.
In conclusion, this protocol provides a rapid and easily scalable assay to enable further characterization of the new generation of FPs and other genetically encoded tags to guide research in fusion protein design.
Divulgazioni
The authors have nothing to disclose.
Acknowledgements
This study is supported by an operating grant from the Canadian Institutes for Health Research to M.L.D. and P.L. The work presented here is supported by a John R. Evans Leader Fund award from the Canadian Foundation for Innovation and a matching fund from the Ontario Research Fund to P.L. Y.J. is supported by an MSc to PhD transfer scholarship from the Dean of the Schulich School of Medicine and Dentistry at The University of Western Ontario. S.D.G. is supported by a PhD Scholarship from ALS Canada.
Materials
| 5-alpha Competent E. coli (High efficiency) | New Englanfd Biolab | C2987 | |
| SpeI-HF | New Englanfd Biolab | R3133 | High fidelity enzymes are preferred |
| SalI-HF | New Englanfd Biolab | R0138 | High fidelity enzymes are preferred |
| Agarose | Fisher Scientific | BP160 | |
| LB-Agar | Fisher Scientific | BP1425 | |
| LB-Broth | Fisher Scientific | BP1426 | |
| Ampicilin | Fisher Scientific | BP1760 | |
| PfuUltra High-fidelity DNA Polymerase | Agilent Technologies | 600382 | |
| EPOCH2 microplate spectrophotometer | BioTek Instruments inc | EPOCH2TC | |
| Yeast Pin Replicator | V&P Scientific inc. | VP407AH | |
| SPI imager | S&P Robotics inc. | spImager-M | |
| Zeiss LSM 800 confocal with AryScan | Carl Zeiss Microscopy | LSM 800 | |
| 8 well Lab-Tek imaging chambers | Fisher Scientific | 12565470 | |
| Bio-Dot apparatus | Bio-Rad | 1706545 | |
| Chemi Doc XRS+ | Bio-Rad | 1708265 | |
| anti-FLAG M1 antibody | Sigma-Aldrich | F3040 | |
| Goat anti-mouse IgG alexa 555 secondary antibody | Thermo | A32727 | |
| Plasmid MiniPrep Kit | Fisher Scientific | K0503 | |
| Plasmid Gel extraction Kit | Fisher Scientific | K0831 | |
| PCR Purification Kit | Fisher Scientific | K0702 | |
| Prizm | GraphPad | N/A | |
| TAE (Tris-Acetate-EDTA) | Fisher Scientific | BP13354 |
Riferimenti
- Chalfie, M., Tu, Y., Euskirchen, G., Ward, W. W., Prasher, D. C. Green fluorescent protein as a marker for gene expression. Science. 263 (5148), 802-805 (1994).
- Thorn, K. Genetically encoded fluorescent tags. Molecular Biology of the Cell. 28 (7), 848-857 (2017).
- Shcherbakova, D. M., Subach, O. M., Verkhusha, V. V. Red fluorescent proteins: advanced imaging applications and future design. Angewandte Chemie. 51 (43), 10724-10738 (2012).
- Snapp, E. L. Fluorescent proteins: a cell biologist’s user guide. Trends in Cell Biology. 19 (11), 649-655 (2009).
- Costantini, L. M., et al. A palette of fluorescent proteins optimized for diverse cellular environments. Nature Communications. 6, 7670 (2015).
- Costantini, L. M., Snapp, E. L. Fluorescent proteins in cellular organelles: serious pitfalls and some solutions. DNA and Cell Biology. 32 (11), 622-627 (2013).
- Yang, F., Moss, L. G., Phillips, G. N. The molecular structure of green fluorescent protein. Nature Biotechnology. 14 (10), 1246-1251 (1996).
- Zacharias, D. A., Violin, J. D., Newton, A. C., Tsien, R. Y. Partitioning of lipid-modified monomeric GFPs into membrane microdomains of live cells. Science. 296 (5569), 913-916 (2002).
- Pédelacq, J. -. D., et al. Engineering soluble proteins for structural genomics. Nature Biotechnology. 20 (9), 927-932 (2002).
- Baird, G. S., Zacharias, D. A., Tsien, R. Y. Biochemistry, mutagenesis, and oligomerization of DsRed, a red fluorescent protein from coral. Proceedings of the National Academy of Sciences of the United States of America. 97 (22), 11984-11989 (2000).
- Laue, T. M., Stafford, W. F. Modern applications of analytical ultracentrifugation. Annual Review of Biophysics and Biomolecular Structure. 28, 75-100 (1999).
- Pédelacq, J. -. D., Cabantous, S., Tran, T., Terwilliger, T. C., Waldo, G. S. Engineering and characterization of a superfolder green fluorescent protein. Nature Biotechnology. 24 (1), 79-88 (2006).
- Costantini, L. M., Fossati, M., Francolini, M., Snapp, E. L. Assessing the tendency of fluorescent proteins to oligomerize under physiologic conditions. Traffic. 13 (5), 643-649 (2012).
- Snapp, E. L., et al. Formation of stacked ER cisternae by low affinity protein interactions. The Journal of Cell Biology. 163 (2), 257-269 (2003).
- Duennwald, M. L., Jagadish, S., Muchowski, P. J., Lindquist, S. Flanking sequences profoundly alter polyglutamine toxicity in yeast. Proceedings of the National Academy of Sciences of the United States of America. 103 (29), 11045-11050 (2006).
- Jiang, Y., Di Gregorio, S. E., Duennwald, M. L., Lajoie, P. Polyglutamine toxicity in yeast uncovers phenotypic variations between different fluorescent protein fusions. Traffic. 18 (1), 58-70 (2017).
- Penney, J. B., Vonsattel, J. P., MacDonald, M. E., Gusella, J. F., Myers, R. H. CAG repeat number governs the development rate of pathology in Huntington’s disease. Annals of Neurology. 41 (5), 689-692 (1997).
- Gusella, J. F., MacDonald, M. E. Huntington’s disease: seeing the pathogenic process through a genetic lens. Trends in Biochemical Sciences. 31 (9), 533-540 (2006).
- Albakri, M. B., Jiang, Y., Lajoie, P. Polyglutamine toxicity assays highlight the advantages of mScarlet for imaging in Saccharomyces cerevisiae [version 1; referees: 1 approved, 1 approved with reservations]. F1000Research. 7, 1242 (2018).
- Schindelin, J., Rueden, C. T., Hiner, M. C., Eliceiri, K. W. The ImageJ ecosystem: An open platform for biomedical image analysis. Molecular Reproduction and Development. 82 (7-8), 518-529 (2015).
- Duennwald, M. L. Yeast as a platform to explore polyglutamine toxicity and aggregation. Methods in Molecular Biology. 1017, 153-161 (2013).
- Duennwald, M. L., Jagadish, S., Giorgini, F., Muchowski, P. J., Lindquist, S. A network of protein interactions determines polyglutamine toxicity. Proceedings of the National Academy of Sciences of the United States of America. 103 (29), 11051-11056 (2006).
- Lee, S., Lim, W. A., Thorn, K. S. Improved blue, green, and red fluorescent protein tagging vectors for S. cerevisiae. Plos One. 8 (7), e67902 (2013).
- Halfmann, R., Lindquist, S. Screening for amyloid aggregation by Semi-Denaturing Detergent-Agarose Gel Electrophoresis. Journal of Visualized Experiments. (17), e838 (2008).
- Sikorski, R. S., Hieter, P. A system of shuttle vectors and yeast host strains designed for efficient manipulation of DNA in Saccharomyces cerevisiae. Genetica. 122 (1), 19-27 (1989).
- Thomas, B. J., Rothstein, R. Elevated recombination rates in transcriptionally active DNA. Cell. 56 (4), 619-630 (1989).
- Brachmann, C. B., et al. Designer deletion strains derived from Saccharomyces cerevisiae S288C: a useful set of strains and plasmids for PCR-mediated gene disruption and other applications. Yeast. 14 (2), 115-132 (1998).
- Meriin, A. B., et al. toxicity in yeast model depends on polyglutamine aggregation mediated by a prion-like protein Rnq1. The Journal of Cell Biology. 157 (6), 997-1004 (2002).
- Mumberg, D., Müller, R., Funk, M. Yeast vectors for the controlled expression of heterologous proteins in different genetic backgrounds. Gene. 156 (1), 119-122 (1995).
- Keppler, A., et al. A general method for the covalent labeling of fusion proteins with small molecules in vivo. Nature Biotechnology. 21 (1), 86-89 (2003).
- Tanenbaum, M. E., Gilbert, L. A., Qi, L. S., Weissman, J. S., Vale, R. D. A protein-tagging system for signal amplification in gene expression and fluorescence imaging. Cell. 159 (3), 635-646 (2014).

