Pattern-Triggered Oxidative Burst and Seedling Growth Inhibition Assays in Arabidopsis thaliana
Summary
This paper describes two methods for quantifying defense responses in Arabidopsis thaliana following exposure to immune elicitors: the transient oxidative burst, and the inhibition of seedling growth.
Abstract
Plants have evolved a robust immune system to perceive pathogens and protect against disease. This paper describes two assays that can be used to measure the strength of immune activation in Arabidopsis thaliana following treatment with elicitor molecules. Presented first is a method for capturing the rapidly-induced and dynamic oxidative burst, which can be monitored using a luminol-based assay. Presented second is a method describing how to measure immune-induced inhibition of seedling growth. These protocols are fast and reliable, do not require specialized training or equipment, and are widely used to understand the genetic basis of plant immunity.
Introduction
To perceive and defend against pathogens, plants have evolved membrane-bound pattern recognition receptors (PRRs) that detect conserved microbial molecules outside the cell known as microbe-associated molecular patterns (MAMPs)1. The binding of MAMPs to their cognate PRRs initiates protein kinase-mediated immune signaling resulting in broad-spectrum disease resistance2. One of the earliest responses following PRR activation is the phosphorylation and activation of integral plasma membrane RESPIRATORY BURST OXIDASE HOMOLOG (RBOH) proteins that catalyze the production of extracellular reactive oxygen species (ROS)3,4. ROS play an important role in establishing disease resistance, acting both as secondary messengers to propagate immune signaling as well as direct antimicrobial agents5. The first observation of an immune-elicited oxidative burst was described using potato tubers of cv. Rishiri following Phytophthora infestans inoculation6. ROS production has been evaluated in several plant species using leaf discs7, cell suspension cultures8, and protoplasts6. Described here is a simple method for assaying pattern-triggered ROS production in leaf discs of Arabidopsis thaliana (Arabidopsis).
As a response to MAMP perception, activated RBOH proteins catalyze the production of superoxide radicals (O2–), hydroxyl radicals (•OH), and singlet oxygen (1O2) that are converted into hydrogen peroxide (H2O2) in the extracellular space9. H2O2 can be quantified by luminol-based chemiluminescence in the presence of the oxidizing agent horseradish peroxidase (HRP)10. HRP oxidizes H2O2 generating a hydroxide ion (OH−) and oxygen gas (O2) which react with luminol to produce an unstable intermediate that releases a photon of light10. Photon emission can be quantified as relative light units (RLUs) using a microplate reader or imager capable of detecting luminescence, which have become standard pieces of equipment in most molecular laboratories. By measuring the light produced over a 40-60-minute interval, a transient oxidative burst can be detected as early as 2-5 minutes after the elicitor treatment, peaking at 10-20 minutes, and returning to basal levels after ~60 minutes11. The cumulative light produced over this time course can be used as a measure of immune strength corresponding to the activation of RBOH proteins12. Conveniently, this assay does not require specialized equipment or cumbersome sample preparation.
Peaking shortly after MAMP detection, the oxidative burst is considered an early immune response, along with MAPK activation and ethylene production5. Later immune responses include transcriptional reprogramming, stomatal closure, and callose deposition2,5. Prolonged exposure to MAMPs continually activates energetically-costly immune signaling resulting in the inhibition of plant growth, indicative of a trade-off between development and immunity13. Pattern-triggered seedling growth inhibition (SGI) is widely used to assess immune output in Arabidopsis and has been integral to the identification of several key components of immune signaling including PRRs14,15,16. Therefore, this paper additionally presents an assay for pattern-triggered SGI in Arabidopsis, whereby seedlings are grown in multi-well plates containing standard media or media supplemented with an immune elicitor for 8-12 days and then weighed using an analytical scale.
To demonstrate how ROS and SGI assays can be used to monitor PRR-mediated signaling, three genotypes that represent varying immune outputs were chosen: (1) the wild type Arabidopsis accession Columbia (Col-0), (2) the dominant-negative bak1-5 mutant in which the multi-functional PRR co-receptor BRASSINOSTEROID INSENSITIVE 1-ASSOCIATED KINASE 1 (BAK1) is non-functional in immune signaling17,18, and (3) the recessive cpk28-1 mutant, which lacks the regulatory protein CALCIUM-DEPENDENT PROTEIN KINASE 28 (CPK28) and displays heightened immune-triggered responses19,20. ROS and SGI assays are presented in response to a synthetically-produced elf18 peptide epitope of bacterial Elongation Factor Tu (EF-Tu), recognized in Arabidopsis by the PRR EF-Tu RECEPTOR (EFR)15. These protocols can be used with other immune elicitors such as the bacterial motility protein flagellin14 or endogenous Plant Elicitor Proteins (AtPeps)16, however, it should be noted that plant responsiveness differs depending on the elicitor21. Together, ROS and SGI assays can be used for the quick and quantitative assessment of early and late PRR-mediated responses.
Protocol
1. Detection of ROS burst in Arabidopsis leaf discs following immune elicitation
- Plant growth and maintenance.
- To synchronize germination, stratify Arabidopsis seeds by suspending approximately 50 seeds in 1 mL of sterile 0.1% agar [w/v] and store at 4 °C (no light) for 3-4 days.
NOTE: Stratify a wild type background control (for example, Col-0) and genotypes with high and low immune outputs (for example, cpk28-1 and bak1-5, respectively) to serve as internal controls. - Sow seeds on soil and germinate under standard short-day conditions (22 °C, 10 h light, 150 μE/m2/s light intensity, and 65-70% relative humidity).
- After approximately 7 days, use forceps to transplant individual seedlings to new pots, separated by at least 4 cm to permit full rosette development. Transplant 6-12 seedlings per genotype and return to standard short-day conditions. Regularly water and fertilize plants (1 g/L of 20-20-20 fertilizer every 2 weeks).
- To synchronize germination, stratify Arabidopsis seeds by suspending approximately 50 seeds in 1 mL of sterile 0.1% agar [w/v] and store at 4 °C (no light) for 3-4 days.
- Collect leaf discs in 96-well plates for overnight recovery.
- At 4-5 weeks post-germination (Figure 1A), use a sharp 4 mm biopsy punch to remove one leaf disc per plant, avoiding the mid-vein and being careful to limit wounding (Figure 1B). Place leaf discs in an unused 96-well luminometer plate containing 100 μL of ddH2O with the adaxial side facing upwards to prevent desiccation22 (Figure 1C). If assessing multiple elicitors, remove 1 leaf disc from the same leaf for each elicitor treatment.
NOTE: Sample leaves that are fully expanded, third to fifth from the top of the rosette, and approximately the same size and age to limit variability. If possible, cut leaf discs in half using a razor blade prior to overnight recovery, as this increases the surface area exposed to elicitor solution23. - Recover overnight at room temperature to prevent the interference of ROS produced by wounding during leaf disc collection. Cover plates with a lid to prevent evaporation.
- At 4-5 weeks post-germination (Figure 1A), use a sharp 4 mm biopsy punch to remove one leaf disc per plant, avoiding the mid-vein and being careful to limit wounding (Figure 1B). Place leaf discs in an unused 96-well luminometer plate containing 100 μL of ddH2O with the adaxial side facing upwards to prevent desiccation22 (Figure 1C). If assessing multiple elicitors, remove 1 leaf disc from the same leaf for each elicitor treatment.
- Perform the elicitor treatment and measure ROS production.
- Program the plate reader prior to adding the reaction solution, as this will reduce the time between ROS burst initiation and the first measurements recorded. Use a commercial software that is appropriate for the plate reader. In our case (see Indice), click Settings, and select the LUM96 Cartridge and the Kinetic Read Type. Click the Read Area category and drag to select a subset of wells or the entire plate to be read. Under the PMT and Optics tab, set the integration time to 1,000 ms. Under the Timing tab, set the Total Run Time to 40-60 min and the Interval to 2 min.
- Remove water from each well using a multi-channel pipette.
NOTE: Do not puncture leaf discs, as this may cause wounding stress and result in more variable ROS outputs. - Prepare a reaction solution containing 100 μM luminol, 10 μg/mL HRP, and the desired concentration of elicitor (for example: elf18 at 1 nM, 10 nM, 100 nM, or 1,000 nM) in sterile ddH2O using 10 mL of solution for one 96-well plate.
NOTE: Dissolve lyophilized peptides in sterile H2O in low-binding tubes to make a 10 mM stock, flash-freeze in liquid N2, and store at -80 °C. When ready to use, dilute stocks in sterile H2O to generate a 100 μM working stock and store at -20 °C. - Use a multi-channel pipette to dispense 100 μL of the reaction mixture to each well, adding the solution to all leaf discs of the same treatment at the same time22. Include a control reaction (no elicitor) for each genotype to assess basal ROS levels in the absence of elicitation. Immediately measure light emission for all wavelengths in the visible spectrum using a microplate reader.
NOTE: Prepare and apply the reaction solution under low light, as luminol and HRP are light-sensitive reagents. Keep reagents on ice or at -20°C unless in use. - Measure light emission with a 1,000 ms integration time in 2 min intervals over a 40-60 min period in a microplate reader in order to capture the dynamic oxidative burst (Figure 1D).
NOTE: Use a longer integration time to improve assay sensitivity11, while ensuring that all samples in a 96-well plate can be measured within one interval.
- Data interpretation.
- For each genotype, use a spreadsheet application to calculate the average photon count and standard error at each time point, and display ROS production as a function of time using a scatter plot.
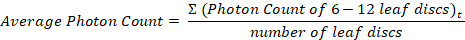
Where t = each time point - Alternatively, sum the photon counts for each leaf disc and present the average of these values for each genotype using a bar graph with standard error bars or a box and whisker plot.
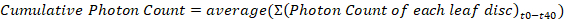
- For each genotype, use a spreadsheet application to calculate the average photon count and standard error at each time point, and display ROS production as a function of time using a scatter plot.
2. Seedling Growth Inhibition Assay
- Sterilize seeds and sow on Murashige and Skoog (MS) plates.
- Prepare sterile half-strength (0.5x) MS medium (2.16 g/L) containing 0.8% agar [w/v]. Pour media into plates (90 x 15 mm) in a laminar flow hood.
- Sterilize approximately 100 seeds in a microcentrifuge tube by washing with 1 mL of 70% ethanol for 2 min and remove by aspiration. Add 1 mL of 40% bleach and gently rock for 17 mins at room temperature. Remove bleach by aspiration and wash three times in 1 mL of sterile water for 5 min. Resuspend in 1 mL of sterile 0.1% agar.
NOTE: Alternatively, use a chlorine gas seed sterilization protocol24. - Sow approximately 100 seeds per genotype on MS agar plates using a pipette and seal with micropore tape in a laminar flow hood.
NOTE: Avoid placing seeds in close proximity as this will make transplanting seedlings difficult later. - Stratify seeds by placing plates at 4 °C (no light) for 3-4 days to synchronize germination (Figure 2A).
- Transplant seedlings into 48-well plates containing immune elicitor peptides.
- After stratification, move plates to light under short day conditions (22 °C, 10 h light, 150 μE/m2/s light intensity, and 65-70% relative humidity) for 3-4 days to allow germination.
- In a laminar flow hood, prepare elicitor peptide dilutions (0 nM, 1 nM, 10 nM, 100 nM, and 1,000 nM) in sterile 0.5x MS liquid media containing 1% sucrose [w/v], using 25 mL of MS for each 48-well plate. Prepare plates by pipetting 500 µL of MS liquid medium or MS medium containing peptides per well. If available, use a repeat pipettor for plate preparation to expedite the process.
NOTE: For each genotype, grow seedlings in MS without elicitor to account for any inherent differences in seedling growth. - Using sterile forceps carefully transplant one seedling to each well of the same size and age, ensuring that there is no damage to the seedling or breakage to the root and that the root is submerged in media. For each genotype, transplant a minimum of 6-8 seedlings into each peptide dilution (Figure 2B).
NOTE: Transplant seedlings at 4 days post-germination, as short roots are easier to manipulate, and older seedlings may yield less optimal results. - Seal plates with micropore tape and move back to light under standard short-day conditions (22 °C, 10 h light, 150 μE/m2/s light intensity, and 65-70% relative humidity). Allow seedlings to grow for 8-12 days.
- Determine percent growth inhibition.
- Carefully remove seedlings from 48-well plates and dry by dabbing on paper towel. Weigh seedlings on an analytical scale and record values. If available, use an analytical scale equipped with USB output to record fresh weight values on a spreadsheet. Before weighing seedlings, take a photo to visually display growth inhibition (Figure 2C).
- Determine percent growth inhibition of elicitor-treated seedlings compared to seedlings grown in MS only (Figure 2D) as follows:
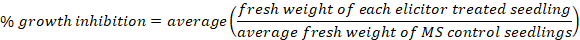
- Plot data using a bar graph with standard error bars or using a box and whisker plot to better display inter-experimental variance.
Representative Results
Mutant cpk28-119,25 and bak1-517,18 plants were used to demonstrate expected outcomes for genotypes with high and low immune responses, respectively, in oxidative burst and SGI assays relative to a wild-type background control (Col-0). To assess dose-dependent effects, a 10-fold peptide dilution series (1-1,000 nM) of elf18 was used. As expected, cpk28-1 loss-of-function lines had a higher cumulative (Figure 3A) and average (Figure 3B) ROS burst compared to Col-0, whereas bak1-5 displayed reduced ROS production at concentrations between 10 nM and 1,000 nM (Figure 3). Expected differences in SGI could be discerned between all genotypes grown in 100 nM and 1,000 nM elf18 (Figure 4A), which could also be visually observed in the 1,000 nM elf18 treatment (Figure 4B). Characteristic of high immune signaling, cpk28-1 mutants were markedly smaller than Col-0 when grown in 1,000 nM elf18, while bak1-5 mutants displayed weak growth inhibition relative to Col-0 due to disrupted MAMP detection.
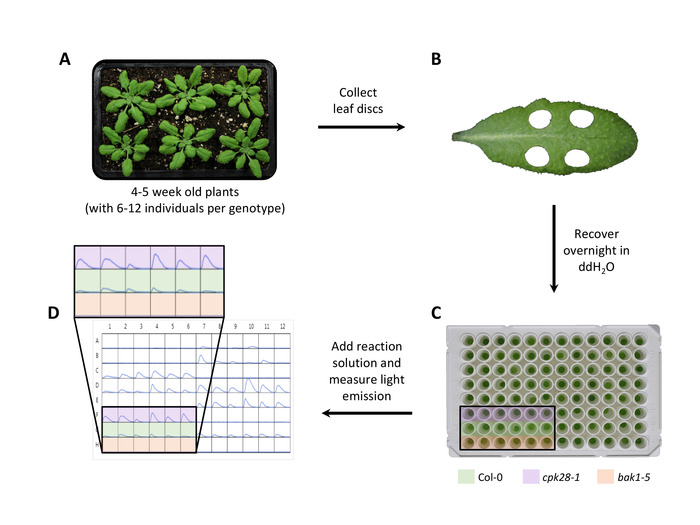
Figure 1. Luminol-based oxidative burst assay following immune induction in Arabidopsis. (A) Grow plants on soil in short day conditions for 4-5 weeks. (B) Use a 4 mm biopsy punch to collect leaf discs from each plant and recover overnight in ddH2O. (C) Add reaction solution (100 μM luminol, 10 μg/mL HRP, and the desired concentration of elicitor) and measure light emission over 40-60 min, in 2 min intervals with an integration time of 1,000 ms. (D) Determine average photon counts for each genotype relative to Col-0 (shown in green), a high ROS control such as cpk28-1 (shown in purple), and a low ROS control such as bak1-5 (shown in orange). Please click here to view a larger version of this figure.
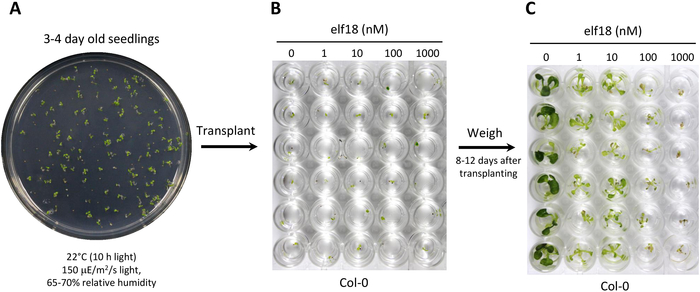
Figure 2. Elicitor-induced seedling growth inhibition assay in Arabidopsis. (A) Sow seedlings on MS agar and grow for 3-4 days under standard short-day conditions. (B) Transplant seedlings to 48-well plates containing MS medium or MS containing different concentrations of elf18. (C) After 8-12 days, visually assess the seedling size and then measure fresh weight using an analytical scale to determine percent growth inhibition. Please click here to view a larger version of this figure.
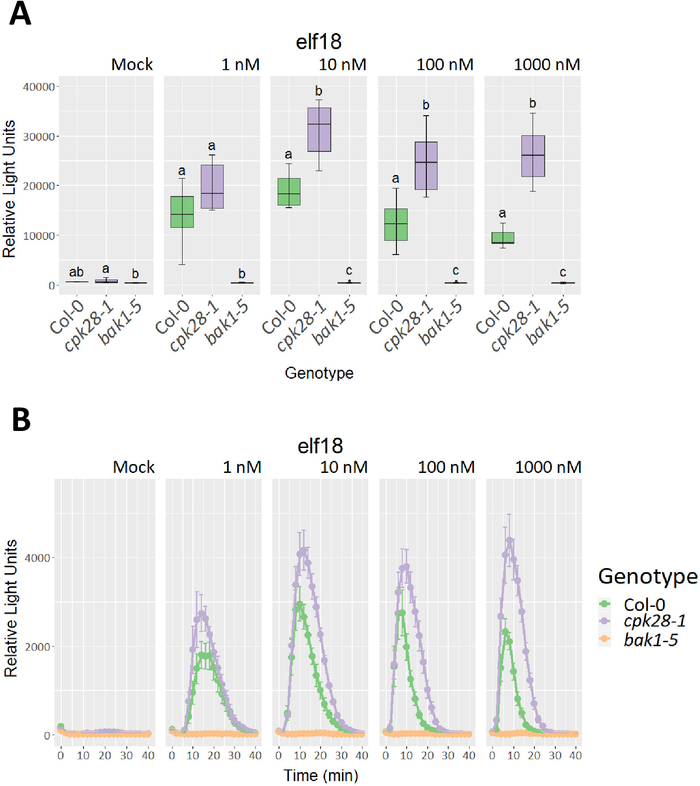
Figure 3. Representative elf18-induced oxidative burst in three Arabidopsis genotypes. Four-week-old plants were treated with a dilution series of elf18 (0 nM 'mock', 1 nM, 10 nM, 100 nM, and 1,000 nM). Col-0 represents the wild-type background control, with cpk28-1 and bak1-5 representing high and low ROS phenotypes, respectively. (A) Total photon count represented as relative light units following elf18 treatment (n=6 leaf discs from independent plants). Statistical differences are represented by lower-case letters and were calculated using a one-way ANOVA with a post-hoc Tukey's Honest Significant Difference test (p<0.05). (B) Average photon count, represented as relative light units, over 40 min following elf18 treatment (n=6 leaf discs from independent plants). Error bars represent standard error of the mean. Similar results were obtained in two of three experiments. Please click here to view a larger version of this figure.
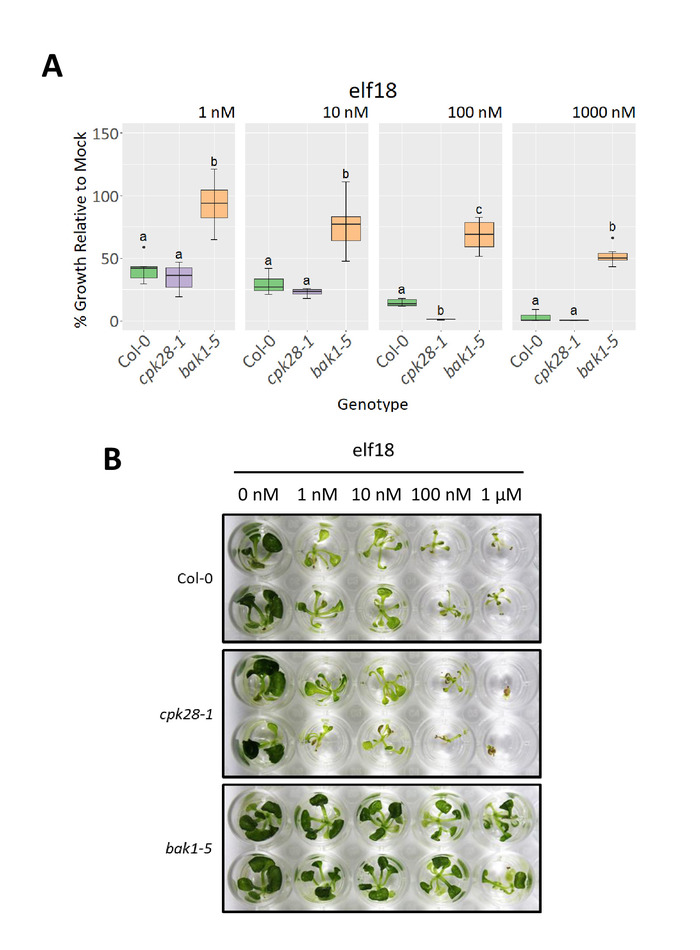
Figure 4. Representative elf18-induced seedling growth inhibition in three Arabidopsis genotypes. Wild-type (Col-0), cpk28-1, and bak1-5 seedlings were grown in a 10-fold dilution series (0-1,000 nM) of elf18. (A) Percent growth inhibition was calculated by comparing the weight of individual seedlings grown in elf18 (n=6 individual seedlings) to the average weight of seedlings of the same genotype grown in MS only ('mock') (n=6 individual seedlings) over a 10-day period. Statistical differences are represented by lower-case letters and were calculated using a one-way ANOVA with a post-hoc Tukey's Honest Significant Differences test (p<0.05). (B) Visual demonstration of SGI in two representative seedlings of Col-0, cpk28-1, and bak1-5 in response to increasing concentrations of elf18. Similar results were obtained in three of four experiments. Please click here to view a larger version of this figure.
Discussion
This paper describes two methods for assaying pattern-triggered immune responses in Arabidopsis, offering quantitative approaches to evaluating immune output without the use of specialized equipment. In combination, pattern-triggered ROS and SGI can be used to assess early and late responses to microbe perception, respectively.
The major limitation of the oxidative burst assay is variability. For reasons that are not completely understood, absolute RLUs often differ by an order of magnitude between experiments. Because between-experiment variability is high, it is advisable to include internal reference controls with high (e.g., cpk28-1) and low (e.g., bak1-5) oxidative bursts in addition to a wild-type control (e.g., Col-0). However, measures can be taken to increase reproducibility between experiments. Environmental conditions such as temperature, humidity, photoperiod, and light intensity should be identical between replicates. The age and health of plants should also be considered when sampling. Leaf discs can be collected from plants that are between 4 and 7 weeks of age grown under short day conditions (6-10 hours of light). Anecdotally, the most consistent results were found with plants older than 6 weeks post germination but not yet flowering or senescing. It is important to grow plants in clean environmental chambers so that they are not exposed to common glasshouse pests such as powdery mildew or chewing insects. Since seedlings for SGI are grown in sterile MS media, and for a shorter time, environmental fluctuations are largely not a concern. However, variation can occur if seedlings become damaged while transplanting, if seedlings selected for elicitor treatments are different sizes, or if the growth media becomes contaminated. It is recommended to include the internal reference control genotypes described above in SGI experiments as well.
Elicitor concentration is another important consideration when conducting immune assays. Elicitors are perceived by PRRs resulting in a rapid and robust ROS burst peaking 10-20 minutes after elicitor treatment (Figure 2B). However, the magnitude of the burst is dependent on both the elicitor as well as the plant genotype. Therefore, an elicitor dose series, as presented in Figure 3 and Figure 4, is recommended in order to identify a suitable elicitor concentration. Pattern-triggered ROS can also be assayed by inoculating leaf discs with live microbes23,26 or microbial extracts26,27,28. For example, transient and dose-dependent ROS bursts can be detected in Arabidopsis in response to virulent pathovars of Pseudomonas syringae, peaking at 35-40 minutes and reaching basal levels approximately 70 mins after elicitation23,29. In response to avirulent bacteria29 or the fungal pathogen P. infestans30,31, a second more pronounced accumulation of ROS is produced that can be monitored 6-10 hours after inoculation. For weaker elicitors, such as fungal chitin32 or chitosan33, more sensitive luminescent indicators can be used, such as the luminol derivative L-012, however, the background signal produced is often higher32,34. Importantly, the plant ecotype will also dictate its responsiveness to specific elicitors35,36. For example, while bacterial flagellin is capable of eliciting immune responses in several Arabidopsis ecotypes including Col-0, the Wassilewskija (Ws-0) ecotype expresses a non-functional variant of the PRR FLAGELLIN-SENSING 2 (FLS2) and is therefore insensitive to flagellin14,37.
Immune-induced ROS can also be observed in leaf discs of other dicotyledonous plants including Brassica napus38, tomato39, Nicotiana benthamiana22,40,41, and several other Solanaceous species41. Additional luminol-based ROS detection assays have been developed for plants using tissue extracts10, cell suspension cultures8,42, and protoplasts43, and are particularly useful in systems where leaf disc protocols are not effective42. For example, elicitor-induced ROS bursts have been described using cell suspension cultures in rice42,44 and wheat45,46, as well as the gymnosperm Araucaria angustifolia47 and the moss Physcomitrella patens48. SGI has not been used as broadly to measure immune signaling. However, growth inhibition in response to elicitors has been demonstrated in N. benthamiana41 and B. napus38,49. Rapid growth inhibition has also been demonstrated in P. patens in response to fungal chitin occurring within 2 mins of exposure, which can be observed using time-lapse photography48.
With modification, both SGI and oxidative burst assays can be used for high-throughput screens to identify immune regulators. For example, mutagenized populations of Arabidopsis can be grown on MS medium and flooded with elicitors to identify insensitive mutants15,50,51,52,53. Alternatively, mutagenized populations can be assessed for elicitor-triggered ROS, which has been successfully executed with both leaf-discs54 and whole seedlings grown on MS plates19. Another useful screening method is the transient expression of proteins in N. benthamiana for ROS analysis prior to the development of transgenic overexpression lines22,40,55. However, intra-experimental variation is higher than in stable Arabidopsis lines due to differential protein expression in N. benthamiana leaves22, although this can be partially mitigated by infiltrating reference controls on the same leaf as experimental samples.
In summary, immune-induced oxidative burst and SGI assays are quick and reliable methods for assessing PRR-mediated signaling in Arabidopsis. These methods can be extended to other systems and used for large-scale screens to uncover novel immune regulators.
Divulgazioni
The authors have nothing to disclose.
Acknowledgements
Work in our lab is funded through the Natural Resources and Engineering Research Council of Canada (NSERC) Discovery Program, the Canadian Foundation for Innovation John R. Evans Leader's Fund, and Queen's University. KS and IS are supported by tandem Ontario Graduate Scholarships and NSERC Canada Graduate Scholarships for master's students (CGS-M).
Materials
| 20-20-20 Fertilizer | Plant Prod | 10529 | Mix 1g/L in water and apply to plants every 2 weeks for optimal growth. |
| 4 mm Biopsy Punch | Medical Mart | 232-33-34-P | A cork borer set with a 0.125 cm^2 surface area can also be used. |
| 48-Well Sterile Plates with Lid | Sigma-Aldrich | CLS3548 | |
| Analytical Scale with Draft Sheid | VWR | VWR-225AC | Any standard analytical scale can be used for growth inibition assays, however, a direct computer output is optimal. |
| BioHit mLine Mechanical 12 Multichannel Pipette (30-300 uL) | Sartorius | 725240 | Any multichannel pipette can be used, as can a single pipetter if necessary. |
| elf18 (Ac-SKEKFERTKPHVNVGTIG) | EZ Biolab | cp7211 | Store 10 mM stock peptide at -80C in low protein binding tubes. When thawed, store 100 uM working stock at -20C. |
| Forceps | Fisher Scientific | 22-327379 | |
| Horseradish Peroxidase | Sigma-Aldrich | P6782 | Dissolve in pure water. Store at -20C and away from light. |
| Luminol | Sigma-Aldrich | A8511 | Dissolve in DMSO. Store at -20C and away from light. |
| Murisage and Skoog Basal Salts | Cedarlane Labs | MSP09-100LT | Store at 4C. |
| Soil | SunGrow Horticulture | Sunshine Mix #1 | Other soil types can also be used to grow Arabidopsis. Mix with water when filling pots. |
| SpectraMax Paradigm Multi Mode Microplate Reader with LUM Module | Molecular Devices | Must request a quote | Any plate reader capable of detecting luminescence can be used for these assays. |
| Sucrose | Sigma-Aldrich | S0389-1KG | Store at room temperature. |
| White Polystyrene 96-Well Plates | Fisher Scientific | 07-200-589 |
Riferimenti
- Couto, D. E., Zipfel, C. Regulation of pattern recognition receptor signalling in plants. Nature Reviews Immunology. 16, 537-552 (2016).
- Boller, T., Felix, G. A Renaissance of Elicitors: Perception of Microbe-Associated Molecular Patterns and Danger Signals by Pattern-Recognition Receptors. Annual Review of Plant Biology. 60, 379-406 (2009).
- Marino, D., Dunand, C., Puppo, A., Pauly, N. A burst of plant NADPH oxidases. Trends in Plant Science. 56 (8), 1472-1480 (2012).
- Kadota, Y., Shirasu, K., Zipfel, C. Regulation of the NADPH Oxidase RBOHD during Plant Immunity. Plant and Cell Physiology. 56 (8), 1472-1480 (2015).
- Yu, X., Feng, B., He, P., Shan, L. From chaos to harmony: responses and signaling upon microbial pattern recognition. Annual Review of Phytopathology. 55, 109-137 (2017).
- Doke, N. Involvement of superoxide anion generation in the hypersensitive response of potato tuber tissues to infection with an incompatible race of Phytophthora infestans and to the hyphal wall components. Physiological Plant Pathology. 23 (3), 345-357 (1983).
- Bindschedler, L. V., et al. Peroxidase-dependent apoplastic oxidative burst in Arabidopsis required for pathogen resistance. The Plant Journal. 47 (6), 851-863 (2006).
- Keppler, L. D. Active Oxygen Production During a Bacteria-Induced Hypersensitive Reaction in Tobacco Suspension Cells. Phytopathology. 110 (3), 759-763 (1989).
- Wrzaczek, M., Brosché, M., Kangasjärvi, J. ROS signaling loops – production, perception, regulation. Current Opinion in Plant Biology. 16 (5), 575-582 (2013).
- Warm, E., Laties, G. G. Quantification of hydrogen peroxide in plant extracts by the chemiluminescence reaction with luminol. Phytochemistry. 21 (4), 827-831 (1982).
- Trujillo, M. Analysis of the lmmunity-Related Oxidative Bursts by a Luminol-Based Assay. Methods in Molecular Biology. 1398, 323-329 (2016).
- Nühse, T. S., Bottrill, A. R., Jones, A. M. E., Peck, S. C. Quantitative phosphoproteomic analysis of plasma membrane proteins reveals regulatory mechanisms of plant innate immune responses. The Plant Journal. 51 (5), 931-940 (2007).
- Belkhadir, Y., Yang, L., Hetzel, J., Dangl, J. L., Chory, J. The growth-defense pivot: Crisis management in plants mediated by LRR-RK surface receptors. Trends in Biochemical Sciences. 39 (10), 447-456 (2014).
- Gómez-Gómez, L., Felix, G., Boller, T. A single locus determines sensitivity to bacterial flagellin in Arabidopsis thaliana. The Plant Journal. 18 (3), 277-284 (1999).
- Zipfel, C., et al. Perception of the Bacterial PAMP EF-Tu by the Receptor EFR Restricts Agrobacterium-Mediated Transformation. Cell. 125 (4), 749-760 (2006).
- Krol, E., et al. Perception of the Arabidopsis danger signal peptide 1 involves the pattern recognition receptor AtPEPR1 and its close homologue AtPEPR2. Journal of Biological Chemistry. 285 (18), 13471-13479 (2010).
- Schwessinger, B., et al. Phosphorylation-dependent differential regulation of plant growth, cell death, and innate immunity by the regulatory receptor-like kinase BAK1. PLoS Genetics. 7 (4), e1002046 (2011).
- Roux, M., et al. The Arabidopsis Leucine-Rich Repeat Receptor-Like Kinases BAK1/SERK3 and BKK1/SERK4 Are Required for Innate Immunity to Hemibiotrophic and Biotrophic Pathogens. The Plant Cell. 23 (6), 2440-2455 (2011).
- Monaghan, J., et al. The calcium-dependent protein kinase CPK28 buffers plant immunity and regulates BIK1 turnover. Cell Host and Microbe. 16 (5), 605-615 (2014).
- Wang, J., et al. A Regulatory Module Controlling Homeostasis of a Plant Immune Kinase. Molecular Cell. 69 (3), 493-504 (2018).
- Mott, G. A., et al. Genomic screens identify a new phytobacterial microbe-associated molecular pattern and the cognate Arabidopsis receptor-like kinase that mediates its immune elicitation. Genome Biology. 17, 98 (2016).
- Sang, Y., Macho, A. P. Analysis of PAMP-Triggered ROS Burst in Plant Immunity. Methods in Molecular Biology. 1578, 143-153 (2017).
- Smith, J. M., Heese, A. Rapid bioassay to measure early reactive oxygen species production in Arabidopsis leave tissue in response to living Pseudomonas syringae. Plant Methods. 10 (1), 6 (2014).
- Lindsey, B. E., Rivero, L., Calhoun, C. S., Grotewold, E., Brkljacic, J. Standardized Method for High-throughput Sterilization of Arabidopsis Seeds. Journal of Visualized Experiments. 128, (2017).
- Matschi, S., Werner, S., Schulze, W. X., Legen, J., Hilger, H. H., Romeis, T. Function of calcium-dependent protein kinase CPK28 of Arabidopsis thaliana in plant stem elongation and vascular development. The Plant Journal. 73 (6), 883-896 (2013).
- Felix, G., Duran, J. D., Volko, S., Boller, T. Plants have a sensitive perception system for the most conserved domain of bacterial flagellin. The Plant Journal. 18 (3), 265-276 (2002).
- Kunze, G., Zipfel, C., Robatzek, S., Niehaus, K., Boller, T., Felix, G. The N Terminus of Bacterial Elongation Factor Tu Elicits Innate Immunity in Arabidopsis Plants. The Plant Cell. 16 (12), 3496-3507 (2004).
- Zipfel, C., et al. Bacterial disease resistance in Arabidopsis through flagellin perception. Nature. 428 (6984), 764-767 (2004).
- Mur, L. A. J., Kenton, P., Draper, J. In planta measurements of oxidative bursts elicited by avirulent and virulent bacterial pathogens suggests that H2O2 is insufficient to elicit cell death in tobacco. Plant, Cell and Environment. 28 (4), 548-561 (2005).
- Kobayashi, M., et al. Calcium-dependent protein kinases regulate the production of reactive oxygen species by potato NADPH oxidase. The Plant Cell. 19 (3), 1065-1080 (2007).
- Yoshioka, H., et al. Induction of Plant gp91 phox Homolog by Fungal Cell Wall, Arachidonic Acid, and Salicylic Acid in Potato. Molecular Plant-Microbe Interactions. 14 (6), 725-736 (2001).
- Klauser, D., Flury, P., Boller, T., Bartels, S. Several MAMPs, including chitin fragments, enhance AtPep-triggered oxidative burst independently of wounding. Plant Signaling and Behavior. 8 (9), e25346 (2013).
- El Gueddari, N. E., Rauchhaus, U., Moerschbacher, B. M., Deising, H. B. Developmentally regulated conversion of surface-exposed chitin to chitosan in cell walls of plant pathogenic fungi. New Phytologist. 156 (1), 103-112 (2002).
- Daiber, A., et al. Detection of superoxide and peroxynitrite in model systems and mitochondria by the luminol analogue L-012. Free Radical Research. 38 (3), 259-269 (2004).
- Bauer, Z., Gómez-Gómez, L., Boller, T., Felix, G. Sensitivity of Different Ecotypes and Mutants of Arabidopsis thaliana toward the Bacterial Elicitor Flagellin Correlates with the Presence of Receptor-binding Sites. Journal of Biological Chemistry. 276 (49), 45669-45676 (2001).
- Vetter, M. M., et al. Flagellin perception varies quantitatively in arabidopsis thaliana and its relatives. Molecular Biology and Evolution. 29 (6), 1655-1667 (2012).
- Chinchilla, D. The Arabidopsis Receptor Kinase FLS2 Binds flg22 and Determines the Specificity of Flagellin Perception. The Plant Cell. 18 (2), 465-476 (2006).
- Lloyd, S. R., Schoonbeek, H., Trick, M., Zipfel, C., Ridout, C. J. Methods to Study PAMP-Triggered Immunity in Brassica Species. Molecular Plant-Microbe Interactions. 27 (3), 286-295 (2014).
- Clarke, C., Vinatzer, B. Characterizing the Immune-Eliciting Activity of Putative Microbe-Associated Molecular Patterns in Tomato. Methods in Molecular Biology. 1578, 249-261 (2017).
- Gimenez-Ibanez, S., Hann, D. R., Chang, J. H., Segonzac, C., Boller, T., Rathjen, J. P. Differential Suppression of Nicotiana benthamiana Innate Immune Responses by Transiently Expressed Pseudomonas syringae Type III Effectors. Frontiers in Plant Science. 9, 688 (2018).
- Wei, Y., et al. The Ralstonia solanacearum csp22 peptide, but not flagellin-derived peptides, is perceived by plants from the Solanaceae family. Plant Biotechnology Journal. 16 (7), 1349-1362 (2018).
- Melcher, R. L. J., Moerschbacher, B. M. An improved microtiter plate assay to monitor the oxidative burst in monocot and dicot plant cell suspension cultures. Plant Methods. 12, 5 (2016).
- Perraki, A., et al. Phosphocode-dependent functional dichotomy of a common co-receptor in plant signalling. Nature. 561 (7722), 248-252 (2018).
- Yamaguchi, K., Kawasaki, T. Chitin-Triggered MAPK Activation and ROS Generation in Rice Suspension-Cultured Cells. Methods in Molecular Biology. 1578, 309-316 (2017).
- Ortmann, I., Conrath, U., Moerschbacher, B. M. Exopolysaccharides of Pantoea agglomerans have different priming and eliciting activities in suspension-cultured cells of monocots and dicots. FEBS Letters. 580 (18), 4491-4494 (2006).
- Ortmann, I., Sumowski, G., Bauknecht, H., Moerschbacher, B. M. Establishment of a reliable protocol for the quantification of an oxidative burst in suspension-cultured wheat cells upon elicitation. Physiological and Molecular Plant Pathology. 64 (5), 227-232 (2004).
- Dos Santos, A. L. W., El Gueddari, N. E., Trombotto, S., Moerschbacher, B. M. Partially acetylated chitosan oligo- and polymers induce an oxidative burst in suspension cultured cells of the gymnosperm Araucaria angustifolia. Biomacromolecules. 9 (12), 3411-3415 (2008).
- Bressendorff, S., Rasmussen, M., Petersen, M., Mundy, J. Chitin-Induced Responses in the Moss Physcomitrella patens. Methods in Molecular Biology. , 317-324 (2017).
- Lloyd, S. R., Ridout, C. J., Schoonbeek, H. Methods to Quantify PAMP-Triggered Oxidative Burst, MAP Kinase Phosphorylation, Gene Expression, and Lignification in Brassicas. Methods in Molecular Biology. 1578, 325-335 (2017).
- Gómez-Gómez, L., Boller, T. FLS2: An LRR Receptor-like Kinase involved in the perception of the bacterial elicitor flagellin in Arabidopsis. Molecular Cell. 5 (6), 1003-1011 (2000).
- Li, J., et al. Specific ER quality control components required for biogenesis of the plant innate immune receptor EFR. Proceedings of the National Academy of Sciences of the United States of America. 106 (37), 15973-15978 (2009).
- Lu, X., et al. Uncoupling of sustained MAMP receptor signaling from early outputs in an Arabidopsis endoplasmic reticulum glucosidase II allele. Proceedings of the National Academy of Sciences of the United States of America. 106 (52), 22522-22527 (2009).
- Nekrasov, V., et al. Control of the pattern-recognition receptor EFR by an ER protein complex in plant immunity. EMBO Journal. 28 (21), 3428-3438 (2009).
- Boutrot, F., et al. Direct transcriptional control of the Arabidopsis immune receptor FLS2 by the ethylene-dependent transcription factors EIN3 and EIL1. Proceedings of the National Academy of Sciences of the United States of America. 107 (32), 14502-14507 (2010).
- Kadota, Y., et al. Direct Regulation of the NADPH Oxidase RBOHD by the PRR-Associated Kinase BIK1 during Plant Immunity. Molecular Cell. 54 (1), 43-55 (2014).

