Bacterial Cell Culture at the Single-cell Level Inside Giant Vesicles
Summary
We demonstrate single-cell culture of bacteria inside giant vesicles (GVs). GVs containing bacterial cells were prepared by the droplet transfer method and were immobilized on a supported membrane on a glass substrate for direct observation of bacterial growth. This approach may also be adaptable to other cells.
Abstract
We developed a method for culturing bacterial cells at the single-cell level inside giant vesicles (GVs). Bacterial cell culture is important for understanding the function of bacterial cells in the natural environment. Because of technological advances, various bacterial cell functions can be revealed at the single-cell level inside a confined space. GVs are spherical micro-sized compartments composed of amphiphilic lipid molecules and can hold various materials, including cells. In this study, a single bacterial cell was encapsulated into 10–30 μm GVs by the droplet transfer method and the GVs containing bacterial cells were immobilized on a supported membrane on a glass substrate. Our method is useful for observing the real-time growth of single bacteria inside GVs. We cultured Escherichia coli (E. coli) cells as a model inside GVs, but this method can be adapted to other cell types. Our method can be used in the science and industrial fields of microbiology, biology, biotechnology, and synthetic biology.
Introduction
The culture of bacterial cells at the single-cell level has received increasing attention. Culturing bacterial cells at the single-cell level inside a confined space can elucidate bacterial functions such as phenotypic variability1,2,3,4, cell behavior5,6,7,8,9, and antibiotic resistance10,11. Because of recent advances in culture techniques, the culture of single bacteria can be achieved inside a confined space, such as in a well-chip4,7,8, gel droplet12,13, and water-in-oil (W/O) droplet5,11. To promote understanding or utilization of single bacterial cells, further technical developments of cultivation techniques are needed.
Vesicles that mimic the biological cell membrane are spherical compartments consisting of amphiphilic molecules and can hold various materials. Vesicles are classified according to size and include small vesicles (SVs, diameter < 100 nm), large vesicles (LVs, <1 μm), and giant vesicles (GVs, >1 μm). SVs or LVs are commonly used as drug carriers because of their affinity to the biological cell membrane14. GVs have also been used as a reactor system for the construction of protocells15 or artificial-cells16. Encapsulation of biological cells into GVs has been reported17,18, and thus GVs show potential as a cell culture system when combined with the reactor system.
Here, along with a video of experimental procedures, we describe how GVs can be used as novel cell-culture vessels19. GVs containing bacteria were made by the droplet transfer method20 and were then immobilized on a supported membrane on a cover glass. We used this system to observe bacterial growth at the single-cell level inside GVs in real-time.
Protocol
1. Preparation of GVs Containing Bacterial Cells by the Droplet Transfer Method
- Prepare lipid stock solutions of 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine (POPC, 10 mM, 1 mL) and 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[biotinyl(polyethyleneglycol)-2000] (biotin-PEG-DSPE, 0.1 mM, 1 mL) in chloroform/methanol solution (2/1, v/v) and store the stock at -20 °C.
- Preparation of a lipid-containing oil solution
- Pour 20 μL of the POPC solution and 4 μL of the biotin-PEG-DSPE solution into a glass tube (Figure 1b (i)).
- Evaporate the organic solvent by air flow to form a lipid film and place the film in a desiccator for 1 h to completely evaporate the organic solvent (Figure 1b (ii)).
NOTE: It is necessary to evaporate the organic solvent in a fume hood. - Add 200 μL of mineral oil (0.84 g/mL, Table of Materials) to the glass vial (Figure 1b (iii)).
- Wrap the opening part of the glass vial with film and sonicate it in an ultrasonic bath (120 W) for at least 1 h (Figure 1b (iii)). The final concentrations of POPC and biotin-PEG-DSPE are 1 mM and 0.002 mM, respectively.
- Pre-culture of bacterial cells
- Inoculate E. coli into 1x LB medium (1 g yeast extract, 2 g bacto tryptone, and 2 g sodium chloride in 200 mL of deionized water) from an LB plate and incubate at 37 °C for 12–14 h (overnight).
- After incubation, collect 20 μL of the culture solution and transfer to 1.98 mL of fresh 1x LB medium, and culture the cells again for 2 h.
- Check the optical density at 600 nm (OD600) value of the pre-culture solution (prepared in step 1.3.2). A pre-culture solution of OD600 = 1.0–1.5 should be used.
- Preparation of the outer and inner aqueous solutions of GVs
- Dissolve glucose in 1x LB medium to prepare an outer aqueous solution of GVs. Prepare 20 mL of a stock glucose solution (500 mM).
- Dilute the stock glucose solution with 1x LB medium to 200 mM (Table 1).
- Dissolve sucrose in 1x LB medium to prepare an inner aqueous solution of GVs. Prepare 20 mL of a stock sucrose solution (500 mM).
- Mix the pre-culture solution (OD600 = 1.0–1.5), sucrose solution (500 mM), and 1x LB medium (Table 1). The final OD600 value of the culture solution should be 0.01–0.015 and the final sucrose concentration should be 200 mM.
NOTE: Take care to avoid osmotic pressure. It is necessary to balance the concentration between the inner and outer aqueous solution.
- Preparation of water-in-oil (W/O) droplets containing bacterial cells
- Add 2 μL of the inner aqueous solution of GVs (prepared in step 1.4.4) to 50 μL of the oil solution containing lipids (mineral oil with POPC and biotin-PEG-DSPE) in a 0.6 mL lidded plastic tube (Figure 1b (iv)).
- Emulsify the two components in the plastic tube by tapping the tube by hand (Figure 1b (v)).
- Formation of GVs containing bacterial cells
- Add 50 μL of the outer aqueous solution of GVs (prepared in step 1.4.2) in a 1.5 mL lidded plastic tube (Figure 1b (vi)) and gently layer 150 μL of the oil solution containing lipids (mineral oil with POPC and biotin-PEG-DSPE) on the surface of the outer aqueous solution (Figure 1b (vii)). Incubate this sample at room temperature (RT, 25 °C) for 10–15 min. Check to ensure that the interface of the oil and aqueous solutions is flat.
- Add 50 μL of the W/O droplet solution (prepared in step 1.5.2) on the interface of the oil and aqueous solution using a pipette (Figure 1b (viii)).
- Centrifuge the 1.5 mL lidded plastic tube (from step 1.6.2) for 10 min at 1,600 x g at RT in a desktop centrifuge (Figure 1b (ix)). After centrifugation, aspirate the oil (top layer) from the 1.5-mL lidded plastic tube using a pipette, and collect the GVs containing bacterial cells (Figure 1b (x)).
2. Preparation of a GV Observation System (Bacterial Cell Culture System)
- Preparation of small vesicles (SVs) for constructing a supported bilayer membrane
- Pour 20 μL of the POPC solution and 4 μL of the biotin-PEG-DSPE solution into a glass tube (using the same lipid composition as used for GV preparation in step 1.1).
- Evaporate the organic solvent by air flow to form a lipid film and place this sample in a desiccator for 1 h to completely evaporate the organic solvent.
- Add 200 μL of 200 mM glucose in 1x LB medium (the outer aqueous solution of GVs) to the glass vial.
- Wrap the opening part of the glass vial with film and sonicate it in an ultrasonic bath (120 W) for at least 1 h.
- Prepare SVs by the extrusion method21 using a mini-extruder and polycarbonate membrane with 100 nm pore size.
- Preparation of a handmade chamber
- Drill a 7 mm hole with a hollow punch on a double-faced seal (10 mm x 10 mm x 1 mm).
- Paste the double-faced seal with the hole on a cover glass (30 mm x 40 mm, thickness 0.25–0.35 mm).
- Preparation of a supported bilayer membrane on the cover glass in the hole of the chamber
- Add 30 μL of the SV solution to the hole of the chamber (prepared in section 2.2) and incubate at RT for 30 min.
- Gently wash the hole twice with 20 μL of 1x LB medium containing 200 mM glucose (the outer aqueous solution of GVs) by pipetting.
- Immobilization of GVs on the supported bilayer membrane on the cover glass in the hole of the chamber
- Introduce 10 μL of neutravidin with the outer aqueous solution of GVs (1 mg/mL) into the hole and incubate at RT for 15 min.
- Gently wash the hole twice with 20 μL of 1x LB medium containing 200 mM glucose (the outer aqueous solution of GVs) by pipetting.
- Add all solution containing GVs (prepared in step 1.6.3) into the hole of the chamber and seal with a cover glass (18 mm x 18 mm, thickness 0.13–0.17 mm) (Figure 2b).
- Microscopic observation of bacterial cell growth inside GVs
- Set a microscopic heating stage system with an inverted microscope equipped with a 40x/0.6 numerical aperture (NA) objective lens with a long working distance (Figure 2b).
- Place the chamber on the microscopic heating stage system (Figure 2b). Incubate the GVs containing bacterial cells in the chamber at a static condition for 6 h at 37 °C.
- Capture and record microscope images of bacterial cell growth inside GVs every 30 min by using a scientific complementary metal oxide semiconductor (sCMOS) camera.
Representative Results
We present a simple method for generating GVs containing single bacterial cells using the droplet transfer method (Figure 1). Figure 1a shows a schematic image of the precipitation of GVs containing bacteria. W/O droplets containing bacteria are transferred across the oil-water (lipid monolayer) interface by centrifugation to form GVs. The difference in density between sucrose (inner aqueous solution) and glucose (outer aqueous solution) also assists the crossing of the oil-water interface of the W/O droplets. It is necessary to monitor the osmotic pressure in inner and outer aqueous solution because a slight difference in concentration between these solutions can induce deformation and collapse of GVs. A flow chart for preparing GVs containing bacterial cells by the droplet transfer method is shown in Figure 1b. By following this procedure, GVs containing single bacterial cells can be easily obtained.
To observe bacterial cell growth within GVs, an original culture system was constructed for microscopic observation (Figure 2). GVs containing bacteria were immobilized on a supported membrane surface coated with neutravidin on a cover glass (Figure 2a). This immobilization technique has enabled prolonged observation of GVs.
Typical phase-contrast microscopy images of the different sized GVs containing single bacterial cells are shown in Figure 3. In this experiment, we also obtained GVs containing bacterial cells with sizes ranging from 10 μm to 30 μm. Figure 3 shows bacterial growth at the single-cell level inside GVs with different sizes of 10.7 μm (Figure 3a) and 28 μm (Figure 3b). For both sizes of GVs, E. coli cells underwent elongation and division processes, with one or two E. coli cells growing to a very large number of cells over 6 h. Thus, E. coli cells grew stably inside the GVs.
The relative frequency of GVs containing a given number of bacterial cells is shown in Figure 4. In our experimental condition (OD600 = 0.01–0.015), bacterial cells were encapsulated at the single cell level in approximately 10% of the obtained GVs (empty GVs were approximately 80%). The GVs encapsulated at the single cell level were approximately 50% of the GVs containing bacterial cells, as estimated from the inset of Figure 4.
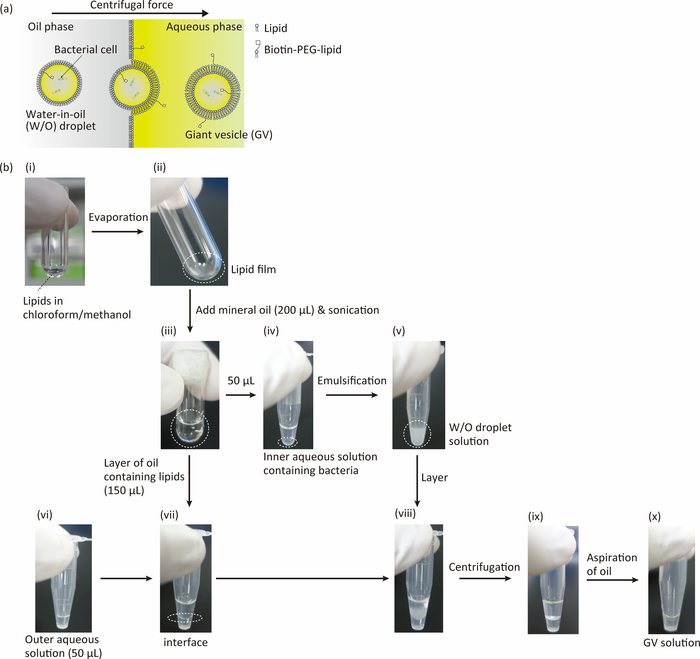
Figure 1: Experimental procedures of GVs containing bacteria. (a) Scheme of GVs containing bacteria prepared by a droplet-transfer method. W/O microdroplets containing bacteria pass through a lipid monolayer interface by centrifugal force and then form a lipid bilayer membrane. (b) Flow of the synthesis of GVs containing bacteria. (i) Organic solvent containing lipids (POPC and biotin-PEG-DSPE, 100:0.2 molar ratio). (ii) Lipid film at the bottom of the glass vial. (iii) Oil solution containing lipids. (iv) Mixture of 50 μL of oil solution and 2 μL of inner aqueous solution (200 mM sucrose and 1x LB medium) containing bacterial cells. (v) Emulsification by hand tapping (over 50 times). (vi) 50 μL of the outer aqueous solution (200 mM glucose in 1x LB medium). (vii) Layering of 150 μL of oil solution on the outer aqueous solution. (viii) Layering of the W/O droplet solution. (ix) Centrifugation of the tube. (x) Precipitated GVs containing bacterial cells after aspiration of the oil. Please click here to view a larger version of this figure.
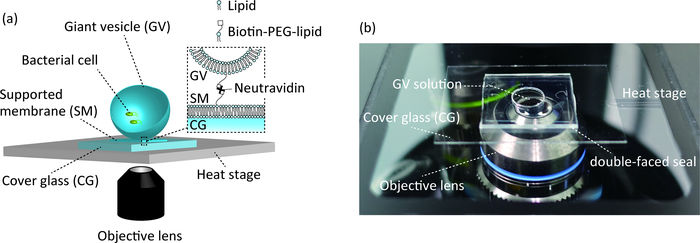
Figure 2: The observation system of bacterial cell culture inside GVs. (a) GVs are immobilized on a supported membrane through biotin–neutravidin binding on the cover glass. GVs are incubated by a heating system. (b) Picture of the observation system including a handmade chamber. Please click here to view a larger version of this figure.
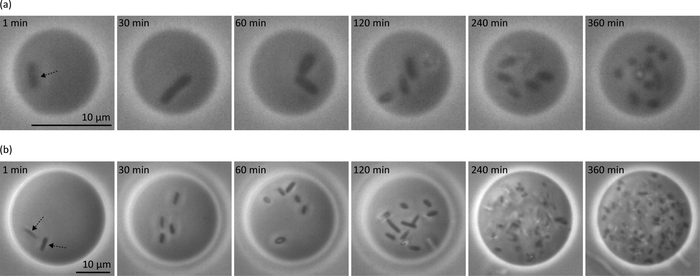
Figure 3: Phase-contrast microscope images of GVs containing single bacteria cells (indicated by the black arrows). Snap-shots of bacterial cell growth inside different sized GVs. (a) Vesicle size = 10.7 μm. (b) Vesicle size = 28 μm. Please click here to view a larger version of this figure.
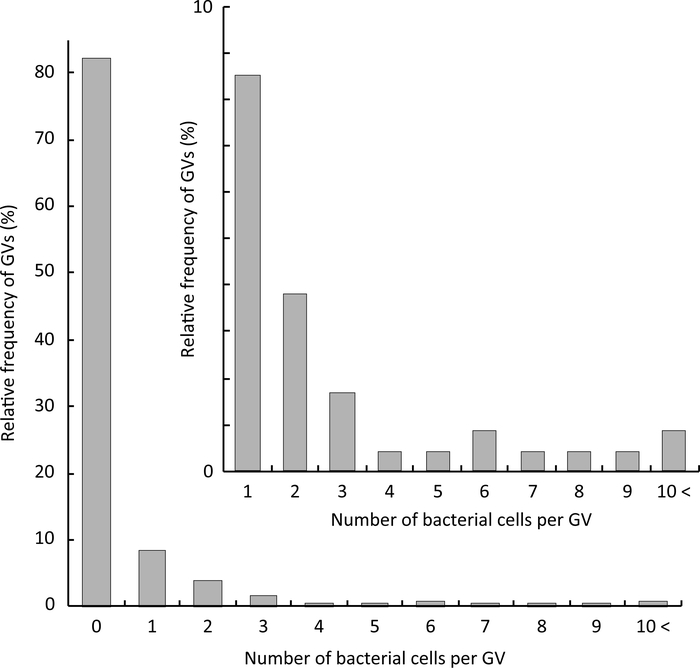
Figure 4: Statistical analysis of the number of encapsulated bacterial cells per GV. The relative frequencies of GVs were plotted as histograms. Inset: Magnification of the relative frequencies of GVs containing bacterial cells from single to 10< cells. A total of 235 GVs were analyzed. Please click here to view a larger version of this figure.
| Outer aqueous solution | Inner aqueous solution | Final | |
| 500 mM Glucose with LB medium | 200 μL | – | 200 mM |
| 500 mM Sucrose with LB medium | – | 200 μL | 200 mM |
| 1x LB medium | 300 μL | 295 μL | – |
| Pre-culture solution (OD600 = 1.0–1.5) | – | 5 μL | OD600 = 0.01–0.015 |
| Total volume | 500 μL | 500 μL |
Table 1: The composition and volumes of the outer and inner aqueous solutions of GVs.
Discussion
Here, we describe a method for culturing bacterial cells at the single-cell level inside GVs. This simple method involves forming GVs containing bacterial cells at the single-cell level by using the droplet transfer method. Compared with other approaches for obtaining GVs containing bacterial cells, this method has two advantages: (i) it is easy to develop, and (ii) a small volume (2 μL) of the sample solution is required to prepare the GVs. The droplet transfer method20 for preparing GVs containing bacterial cells is simpler than the classical hydration22 and microfluidics methods17. For example, the classical hydration method22 is a simple and easy method for preparing GVs, but the encapsulation efficiency of materials into GVs is quite low and at least a few hundred microliters of sample is required. The recently developed cellulose paper-abetted hydration23 and gel-assisted hydration24 methods for making GVs have a high encapsulation efficiency of biomolecules compared with the classical hydration method22. Their encapsulation efficiency is as high as that of the droplet transfer technique, and it is expected that these two methods may allow the encapsulation of cells inside GVs. Moreover, the microfluidics method17 accurately encapsulates single cells inside GVs and shows a very high encapsulation efficiency of materials into GVs but requires complicated handling and techniques for fabricating microdevices and a large sample volume (at least a few milliliters) to flow the tube.
In this protocol, the stability of the oil-water interface is important for obtaining GVs containing bacterial cells (Figure 1b (vii)). To obtain many GVs, it is essential to flatten the oil-water interface. Therefore, proper preparation of the oil phase is necessary. We sonicated the oil phase for at least 1 h in a high-power ultrasonic bath (120 W) to completely dissolve the lipid molecules. It is important to layer the oil phase on the outer aqueous solution immediately after sonication (Figure 1b (vii)).
The method described here has two limitations. First, GVs often break and bacterial cells leak into the outer aqueous solution. This is because during GV formation, some W/O droplets cannot transfer through the oil-water interface and become ruptured. This is unavoidable when using the droplet transfer method20. Additionally, GVs may break during observation. The stability of GVs must be improved, such as by using an artificial cytoskeleton that stabilizes GVs25. Second, the number of encapsulated bacterial cells cannot be perfectly controlled. Figure 4 shows that a large number of bacterial cells were encapsulated in the GVs, and therefore, it is difficult to control the number of bacterial cells in GVs using the droplet transfer method. To control the cell number, microfluidics technology can be used.
GVs may control their inner aqueous solution more effectively than other materials (gel droplets12,13 or W/O droplets5,11). For example, the aqueous conditions of the inner and outer solutions of GVs are altered by natural membrane permeability26 or permeability facilitated by a membrane pore16 or transporter27. In the present method, oil molecules (mineral oil in this case) remained in the membrane20. The influence of the oil remaining in the membrane on the permeability of nutrients or oxygen for bacterial growth is unknown. Although we do not know the natural membrane permeability of nutrients or oxygen, we consider that the amount of nutrients or oxygen in the growth medium was sufficient for bacterial growth in the present study. The natural membrane permeability of nutrients or oxygen is very important for bacterial cell growth and is an important topic for future study. The technique for controlling permeability cannot be conducted using the culture method with gel droplets12,13 or W/O droplets5,11. GVs will thus become the first choice for bacterial culture applications in a confined space.
Our bacterial culture method is a potentially new concept and tool in microbiology19 to culture unknown environmental bacteria for obtaining or analyzing their metabolic products. Moreover, our bacterial cell-containing GVs are a hybrid system of an artificial cell model (GVs) and a living cell (bacterial cells) to make a new tool for biotechnology28 and bottom-up synthetic biology29.
Divulgazioni
The authors have nothing to disclose.
Acknowledgements
This work was supported by a Leading Initiative for Excellent Young Researchers (LEADER, No. 16812285) from the Ministry of Education, Culture, Sports, Science and Technology (MEXT) of Japan, a Grant-in-Aid for Young Scientist Research (No. 18K18157, 16K21034) from Japan Society for the Promotion of Science (JSPS) to M.M., and Grant-in-Aid from MEXT to K.K. (No. 17H06417, 17H06413).
Materials
| Bactotryptone | BD Biosciences | 211705 | |
| Chloroform | Wako Pure Chemicals | 032-21921 | |
| Cover glass (18 × 18 mm) | Matsunami Glass Ind. | C018181 | thickness 0.13–0.17 mm |
| Cover glass (30 × 40 mm) | Matsunami Glass Ind. | custom-order | thickness 0.25–0.35 mm |
| Desktop centrifuge | Hi-Tech Co. | ATT101 | swing rotor type |
| Double-faced seal (10 × 10 × 1 mm) | Nitoms | T4613 | |
| Glass vial | AS ONE | 6-306-01 | Durham fermentation tube |
| Glucose | Wako Pure Chemicals | 049-31165 | |
| Inverted microscope | Olympus | IX-73 | |
| Methanol | Wako Pure Chemicals | 133-16771 | |
| Microscopic heating stage system | TOKAI HIT | TP-110R-100 | |
| Mineral oil | Nacalai Tesque | 23334-85 | |
| Mini-extruder | Avanti Polar Lipids | 610000 | |
| Neutravidin | Thermo Fisher Scientific | 31000 | |
| Objective lens | Olympus | LUCPLFLN 40×/0.6 NA | |
| Polycarbonate membranes | Avanti Polar Lipids | 610005 | pore size 100 nm |
| sCMOS camera | Andor | Zyla 4.2 plus | |
| Sodium chloride | Wako Pure Chemicals | 191-01665 | |
| Sucrose | Wako Pure Chemicals | 196-00015 | |
| Ultrasonic bath | AS ONE | ASU-3D | |
| Yeast extract | BD Biosciences | 212750 | |
| 0.6 mL lidded plastic tube | Watson | 130-806C | |
| 1.5 mL lidded plastic tube | Sumitomo Bakelite Co. | MS4265-M | |
| 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocoline | Avanti Polar Lipids | 850457P | POPC |
| 1,2-distearoyl-snglycero-3-phosphoethanolamine-N-[biotinyl(polyethyleneglycol)-2000] | Avanti Polar Lipids | 880129P | Biotin-PEG-DSPE |
Riferimenti
- Ozbudak, E. M., Thattai, M., Kurtser, I., Grossman, A. D., van Oudenaarden, A. Regulation of noise in the expression of a single gene. Nature Genetics. 31, 69-73 (2002).
- Rosenfeld, N., Young, J. W., Alon, U., Swain, P. S., Elowitz, M. B. Gene regulation at the single-cell level. Science. 307, 1962-1965 (2005).
- Eldar, A., Elowitz, M. B. Functional roles for noise in genetic circuits. Nature. 467, 167-173 (2010).
- Hashimoto, M., et al. Noise-driven growth rate gain in clonal cellular populations. Proceedings of the National Academy of Sciences of the United States of America. 113 (12), 3251-3256 (2016).
- Boedicker, J. Q., Vincent, M. E., Ismagilov, R. F. Microfluidic confinement of single cells of bacteria in small volumes initiates high-density behavior of quorum sensing and growth and reveals its variability. Angewandte Chemie International Edition. 48, 5908-5911 (2009).
- Christopher, M., Waters, B. L. B. Quorum sensing: cell-to-cell communication in bacteria. Annual Review of Cell and Developmental Biology. 21, 319-346 (2005).
- Inoue, I., Wakamoto, Y., Moriguchi, H., Okano, K., Yasuda, K. On-chip culture system for observation of isolated individual cells. Lab on a Chip. 1, 50-55 (2001).
- Wang, P., et al. Robust growth of Escherichia coli. Current Biology. 20, 1099-1103 (2010).
- Reshes, G., Vanounou, S., Fishov, I., Feingold, M. Cell shape dynamics in Escherichia coli. Biophysical Journal. 94, 251-264 (2008).
- Balaban, N. Q., Merrin, J., Chait, R., Kowalik, L., Leibler, S. Bacterial Persistence as a Phenotypic Switch. Science. 305, 1622-1625 (2004).
- Brouzes, E., et al. Droplet microfluidic technology for single-cell high-throughput screening. Proceedings of the National Academy of Sciences of the United States of America. 106 (34), 14195-14200 (2009).
- Zengler, K., et al. Cultivating the uncultured. Proceedings of the National Academy of Sciences of the United States of America. 99 (24), 15681-15686 (2002).
- Eun, Y., Utada, A. S., Copeland, M. F., Takeuchi, S., Weibel, D. B. Encapsulating bacteria in agarose microparticles using microfluidics for high-throughput cell analysis and isolation. ACS Chemical Biology. 6, 260-266 (2011).
- Allen, T. M., Cullis, P. R. Liposomal drug delivery systems: From concept to clinical applications. Advanced Drug Delivery Reviews. 65, 36-48 (2013).
- Szostak, J. W., Bartel, D. P., Luisi, P. L. Synthesizing life. Nature. 409, 387-390 (2001).
- Noireaux, V., Libchaber, A. A vesicle bioreactor as a step toward an artificial cell assembly. Proceedings of the National Academy of Sciences of the United States of America. 101 (51), 17669-17674 (2004).
- Tan, Y. C., Hettiarachchi, K., Siu, M., Pan, Y. R., Lee, A. P. Controlled microfluidic encapsulation of cells, proteins, and microbeads in lipid vesicles. Journal of the American Chemical Society. 128 (17), 5656-5658 (2006).
- Chowdhuri, S., Cole, C. M., Devaraj, N. K. Encapsulation of Living Cells within Giant Phospholipid Liposomes Formed by the Inverse-Emulsion Technique. ChemBioChem. 17, 886-889 (2016).
- Morita, M., Katoh, K., Noda, N. Direct observation of bacterial growth in giant unilamellar vesicles: a novel tool for bacterial cultures. ChemistryOpen. 7, 845-849 (2018).
- Pautot, S., Frisken, B. J., Weitz, D. A. Production of Unilamellar Vesicles Using an Inverted Emulsion. Langmuir. 19 (7), 2870-2879 (2003).
- Hope, M. J., Bally, M. B., Webb, G., Cullis, P. R. Production of large unilamellar vesicles by a rapid extrusion procedure. Characterization of size distribution, trapped volume and ability to maintain a membrane potential. Biochimica et Biophysica Acta (BBA) – Biomembranes. 812, 55-65 (1985).
- Tsumoto, K., Matsuo, H., Tomita, M., Yoshimura, T. Efficient formation of giant liposomes through the gentle hydration of phosphatidylcholine films doped with sugar. Colloids and Surfaces B: Biointerfaces. 68, 98-105 (2009).
- Li, A., Pazzi, J., Xu, M., Subramaniam, A. B. Cellulose abetted assembly and temporally decoupled loading of cargo into vesicles synthesized from functionally diverse lamellar phase forming amphiphiles. Biomacromolecules. 19, 849-859 (2018).
- Weinberger, A., et al. Gel-assisted formation of giant unilamellar vesicles. Biophysical Journal. 105, 154-164 (2013).
- Kurokawa, C., et al. DNA cytoskeleton for stabilizing artificial cells. Proceedings of the National Academy of Sciences of the United States of America. 114 (28), 7228-7233 (2017).
- Nourian, Z., Roelofsen, W., Danelon, C. Triggered gene expression in fed-vesicle microreactors with a multifunctional membrane. Angewandte Chemie International Edition. 51, 3114-3118 (2012).
- Dezi, M., Di Cicco, A., Bassereau, P., Levy, D. Detergent-mediated incorporation of transmembrane proteins in giant unilamellar vesicles with controlled physiological contents. Proceedings of the National Academy of Sciences of the United States of America. 110 (18), 7276-7281 (2013).
- Trantidou, T., Dekker, L., Polizzi, K., Ces, O., Elani, Y. Functionalizing cell-mimetic giant vesicles with encapsulated bacterial biosensors. Interface Focus. 8, 20180024 (2018).
- Elani, Y., et al. Constructing vesicle-based artificial cells with embedded living cells as organelle-like modules. Scientific Reports. 8, 4564 (2018).

