Assessing Therapeutic Angiogenesis in a Murine Model of Hindlimb Ischemia
Summary
Here, a critical hindlimb ischemia experimental model is presented followed by a battery of functional, histologic and molecular tests to assess the effectiveness of angiogenic therapies.
Abstract
Critical limb ischemia (CLI) is a serious condition that entails a high risk of lower limb amputation. Despite revascularization being the gold-standard therapy, a considerable number of CLI patients are not suited for either surgical or endovascular revascularization. Angiogenic therapies are emerging as an option for these patients but are currently still under investigation. Before application in humans, those therapies must be tested in animal models and its mechanisms must be clearly understood. An animal model of hindlimb ischemia (HLI) has been developed by the ligation and excision of the distal external iliac and femoral arteries and veins in mice. A comprehensive panel of tests was assembled to assess the effects of ischemia and putative angiogenic therapies at functional, histologic and molecular levels. Laser Doppler was used for the flow measurement and functional assessment of perfusion. Tissue response was evaluated by the analysis of capillary density after staining with the anti-CD31 antibody on histological sections of gastrocnemius muscle and by measurement of collateral vessel density after diaphonization. Expression of angiogenic genes was quantified by RT-PCR targeting selected angiogenic factors exclusively in endothelial cells (ECs) after laser capture microdissection from mice gastrocnemius muscles. These methods were sensitive in identifying differences between ischemic and non-ischemic limbs and between treated and non-treated limbs. This protocol provides a reproducible model of CLI and a framework for testing angiogenic therapies.
Introduction
Peripheral arterial disease (PAD) affects predominantly the lower limbs. PAD is caused by atherosclerosis, an artery obstruction that can cause severe restriction to the blood flow in the lower limbs1. Intermittent claudication is the first manifestation of PAD and refers to muscle pain when walking. CLI is the most severe stage of PAD, being diagnosed in patients that show ischemic rest pain, ulcers or gangrene2. Patients with CLI have a high risk of amputation, especially if untreated3. Lower limb revascularization (either by open surgery or an endovascular procedure) is currently the only way to achieve limb salvage. However, around 30% of CLI patients are not suited for these procedures, for reasons that include the location of the lesions, the pattern of arterial occlusion and extensive comorbidity4,5. Therefore, new therapies are needed for these otherwise untreatable patients, with the promotion of angiogenesis being the strategy under more intense investigation.
Before testing in humans, the effectiveness and safety of new therapies in vivo must be considered in animal models. Several models have been developed for the study of CLI, mostly by inducing hindlimb ischemia (HLI) in mice6,7,8,9,10. However, these models differ in several aspects including the nature of the arteries that are ligated and/or excised and whether the veins and nerves surrounding are dissected as well6,7,8,9,10. Taken together, these aspects will affect the severity of the ischemia-reperfusion injury in each animal, making the results difficult to be compared. Therefore, it is critical to develop an effective protocol in which the procedure to induce ischemia and the evaluation of different targets should be standardized to assess whether a given angiogenic therapy will be effective. An experimental protocol designed to cover all these aspects would provide a comprehensive understanding of the mechanisms by which angiogenic therapies exert their effects and a measure of their efficacy at each of their outcomes. Two distinct works recently published by our team are a good example11,12, in which different approaches to induce therapeutic angiogenesis were assessed using the same protocol that will be described with more detail in this protocol.
The overall goal of this protocol is to describe a reproducible experimental model that can mimic the effects of CLI and lay the experimental foundation for a comprehensive assessment of the functional, histologic and molecular effects of putative angiogenic agents.
Protocol
All animal procedures are in accordance with Directive 2010/63/EU and have been approved by the Institutional Animal Welfare Body and licensed by DGAV, the Portuguese competent authority for animal protection (license number 023861/2013)
CAUTION: Several of the chemicals used in the protocols are toxic and harmful. Please use all appropriate safety practices and personal protective equipment (gloves, lab coat, full-length pants, and closed-toe shoes)
1. The murine model of hindlimb ischemia
NOTE: All experiments are performed on 22-week-old C57BL/6 female mice.
- Preparation of solutions
- Preparation of the anesthetic solution
Note: This anesthetic combination (medetomidine and ketamine) provides up to 30 min immobilization and a surgical window of 15 min. The effect may be reverted by the administration of atipamezole.- With a clean, 1cc syringe, withdraw 1 mL of medetomidine (1 mg/Kg body weight), eliminate air bubbles for accurate measurements, and add it to a 15 mL falcon tube.
- With a clean, 1 cc syringe, withdraw 0.75 mL of Ketamine (75 mg/Kg body weight), eliminate air bubbles for accurate measurements and add it to a 15 mL falcon tube containing the medetomidine.
- Add 8.25 mL of sterile saline (0.9% NaCl), in order to obtain 10 mL of anesthetic solution.
- Identify the tube with the name of the compounds, the date mixed and the expiration date.
- Store in the dark at 4 °C.
- Preparation of the anti-sedative solution
- With a clean, 1 cc syringe, withdraw 1 mL of atipamezole (5 mg/Kg body weight), eliminate air bubbles for accurate measurements, and add it to a 15 mL falcon tube.
- Add 9 mL of sterile saline (0.9% NaCl), in order to obtain 10 mL of reversion solution.
- Identify the tube with the name of the compounds, the date mixed and the expiration date.
- Store in the dark at 4 °C.
- Preparation of the post-operative analgesia solution
- With a clean, 1 cc syringe, withdraw 0.5 mL of buprenorphine (100 µL/15-30 g body weight), eliminate air bubbles for accurate measurements, and add it to a 15 mL falcon tube.
- Add 9.5 mL of sterile saline (0.9% NaCl), in order to obtain 10 mL of analgesia solution.
- Identify the tube with the name of the compounds, the date mixed and the expiration date.
- Store in the dark at 4 °C.
- Preparation of the anesthetic solution
- Surgical induction of hindlimb ischemia
- Surgical tools needed for this intervention: pointed forceps, spring scissors, ophthalmic needle holder, surgical scissors and a needle holder. Sterilize all surgical tools in an autoclave before use. Use cotton swabs for the dissection of subcutaneous fat.
- Load the syringe with the anesthesia solution before holding the mouse.
- Restrain the animal by applying force behind its ears against the cage’s grid, holding as much skin as possible to keep the mouse immobile.
- Keep the animal tilted with its head lowered and perform an intraperitoneal injection of the anesthesia solution, keeping the syringe at a 45° angle with the mouse’s body.
- Check for the absence of pedal withdrawal reflexes, to evaluate the depth of anesthesia.
- Remove the hair from the right hindlimb using an electric shaver. Use a chlorhexidine disinfectant soap scrub for skin preparation. Use a clean paper towel to remove the suds. Apply a chlorhexidine surgical scrub and protect the skin area with a sterile drape before transporting the mice to the surgical sterile suite.
- Place the animal in dorsal decubitus and restrain it with the four limbs spread apart and secured with tape.
CAUTION: Perform the procedure on a heated pad to keep the animal’s body temperature stable; the surgical procedure is performed using a dissection microscope at 10x or 20x magnification. - Apply a chlorhexidine antiseptic solution using sterile gauze to finalize skin preparation. Using a number 11 surgical scalpel blade perform a 1-cm skin incision overlying the thigh of the right hindlimb.
- Blunt dissect subcutaneous fat exposing the neurovascular bundle. Using the pointed forceps, the spring scissors and the ophthalmic needle holder, open the membranous ilio-femoral sheath to expose the distal external iliac and femoral vessels.
- Dissect and separate the vessels (artery and vein) from the nerve. Using a 7.0 non-absorbable polypropylene suture ligate the distal external iliac artery and vein and the distal femoral artery and vein.
- Transect the segment of the ilio-femoral artery and veins between the distal and proximal knots with the spring scissors.
- Close the incision with an absorbable suture (e.g., 5.0 Vicryl suture).
- Load the syringe with the anti-sedative solution and use the same method detailed in 1.2.3 and 1.2.4 to perform an intraperitoneal injection of the anti-sedative solution.
- Post-operative monitoring
NOTE: Animals are carefully observed every 15-20 min to make sure all animals recover well from the anti-sedative; anesthetized animals are never left unattended.- Assess the recovery from anesthesia: 1) monitor the rate and depth of respiration; 2) assess animal reflexes (pedal and eye position)
- Perform a subcutaneous injection of the post-operative analgesia every 8-12 h.
- Monitor the animals daily after the surgery recording a brief description of the animal’s health status and surgery site appearance, ensure all sutures are closed.
- Re-suture the incision, if dehiscence occurs. If unsuccessful, apply a skin barrier film on the incision to protect from urine, fecal excretions, friction and shear of the skin and to help the healing process.
2. Assessment of the angiogenic effect
NOTE: After ischemia induction, apply the therapeutic agent in the study and achieve the following procedures. Steps undertaken at other time points are detailed under the corresponding section.
- Laser Doppler perfusion imaging
- Remove the hair from both hindlimbs one day before the analysis using an electrical shaver followed by depilatory cream.
- Anesthetize the mice using the anesthetic solution.
- Place the animal on a 37 °C heating pad for 5 min to ensure that temperature will not change during the procedure
- Place the animal in the supine position with legs secured in an extended position. Measure the distance between right and left legs and feet for each animal since the positioning of the animal should be similar whenever the procedure must be repeated in order to follow changes in hindlimb perfusion over time.
- Start acquiring data by using a laser Doppler perfusion imager.
- After the acquisition is complete, revert the anesthesia with the anti-sedative solution.
- Open the image analysis software and draw the region of interest (ROI) around the ischemic hindlimb and a comparable ROI around the non-ischemic hindlimb.
- Observe the flux values and determine the perfusion as a ratio of the perfusion in the ischemic limb to that in the non-ischemic limb. Changes in the perfusion ratio are measured over time.
- Immunohistochemistry and capillary density analysis
- After anesthetizing, sacrifice the mice by cervical dislocation.
- To harvest the gastrocnemius muscles of both legs, first, remove the skin from both hindlimbs.
- Identify the Achilles tendon and separate the tendon from the bone using an 11-blade scalpel.
- Hold the Achilles tendon and separate the muscle from the tibia and fibula up to the knee joint with spring scissors.
- Separate the biceps femoris from the gastrocnemius muscle.
- Use spring scissors to cut the insertions of the gastrocnemius muscles at the knee joint level.
- Place the harvested gastrocnemius muscles in a transverse orientation on a small cork disk with the help of 10% tragacanth.
- Snap freeze the specimens in liquid nitrogen-cooled isopentane and store them at -80 °C until sectioning.
- Cut 7 µm sections of the muscle specimens on a cryostat and mount on glass slides.
NOTE: The protocol can be paused here. - Fix the glass slides in a bottle containing cooled pure acetone (approximately 50 mL) for 10 min, at -20 °C.
- Add hydrogen peroxidase 0.3% diluted in methanol (50 mL) for 30 min at room temperature.
- Wash twice in phosphate-buffered saline (PBS) (50 mL) for a total of 10 min.
- Use a hydrophobic pen around the sections.
NOTE: The use of a hydrophobic pen allows reduce the amount of solutions spent during the protocol. For all incubations use 100 µL of all solutions, per section. - Apply a blocking solution of 5% rabbit serum in PBS for 30 min at room temperature.
- Incubate the specimens for 1 h at room temperature with anti-CD31 rat monoclonal antibody diluted at 1:500 in 1 % bovine serum albumin (BSA) in PBS.
- Wash three times in PBS for a total of 30 min.
- Add a secondary biotinylated rabbit anti-rat IgG antibody at 1:200 in 1% BSA in PBS and 5% rabbit serum for 30 min at room temperature.
- Wash in PBS as before and add labeled avidin-conjugated peroxidase complex for 30 min at room temperature.
- Rinse 3 times in PBS for 5 min and add diaminobenzidine (DAB) peroxidase substrate kit for another 5 min.
- Counterstain the sections with hematoxylin for 10 s before mounting.
- Measure the capillary densities by using an upright brightfield microscope (i.e., number of capillaries per number of myocytes) in 2 different sections of 4 distinct anatomic areas in each specimen.
- Contrast agent perfusion
- Anesthetize the mice using the anesthetic solution.
- Perform a median laparotomy using a number 15 scalpel blade.
- Expose the abdominal aorta and the caudal vena cava, after retracting laterally the small bowel and using spring scissors.
- Insert one 26-gauge catheter cranially into the abdominal aorta and another into the caudal vena cava in the same direction.
- Secure catheters in place using a 5/0 silk suture to perform a stay suture.
- Wash the vascular system with a 37 °C saline solution containing heparin (500 units/100 mL) pushed into the abdominal aorta using a 10 mL syringe. Continue gentle perfusion until a clear solution is seen coming out of the catheter placed inside the caudal vena cava.
NOTE: Usually 30 to 40 mL of a heparinized saline solution are required to rinse the vascular system of an adult rat. - Administer a mixture of adenosine (1 mg/L) and papaverine (4 mg/L) for 2 min.
- Inject into the aortic catheter 10 mL of a mixture of 50% barium sulfate and 5% gelatine.
NOTE: The solution must be kept at 60 °C to avoid thickening. - Leave the mice at - 4 °C for at least 4 h, in order to allow the vascular cast to solidify.
- Remove the skin from the lower body of each mouse.
- Diaphonization – Spalteholz technique
- Fix the mice by immersion in a 10% formaldehyde solution for at least 72 h.
- Bleach the mice by immersion in a 30% hydrogen peroxide solution for 24 h.
- Dehydrate the mice using the freeze substitution technique. This methodology consists of submitting the mice to successive passages (on average four) of pure acetone at - 20 °C for 24 h each.
- Clarify the mice by immersing it in a solution containing a mixture of 100% benzyl benzoate and 100% methyl salicylate in a 3:5 proportion (this mixture is also known as Spalteholz solution) 13.
- Leave the mice immersed in the Spalteholz solution inside in a vacuum chamber until it becomes diaphanous, which usually takes 5 to 7 days.
- Replace the solution, if it becomes dark before the specimen becomes clear.
- Collateral vessels quantification
- Observe the mice suspended in the Spalteholz solution under transillumination using a stereotaxic microscope. Use microsurgery forceps to gently grasp and move it.
- Photograph the entire limbs using a digital camera connected to the microscope.
- Obtain entire limb photographs by using the appropriate software.
- Perform a manual segmentation of the collateral vessels by highlighting them using the appropriate software.
NOTE: Collateral vessels are defined according to Longland’s definition, a defined stem, mid-zone, and re-entrant, with a diameter between 20 and 300 µm, excluding femoral, saphenous and popliteal arteries and all venous structures. - Define ROIs in every mouse in the same anatomic region in the adductor, surrounding and below the surgical occlusion.
- Quantify the collateral vessel density (CVD) in equivalent ROIs corresponding to 20% of the total limb area. The CVD is calculated as the ratio between the vascular area and the ROIs areas. All density measurements are performed using ImageJ software.
NOTE: The CVD value of the non-ischemic limb for each mouse is assumed to correspond to 100% to exclude variations in the anatomy, perfusion or diaphonization procedures. The CVD percentage in the ischemic limb was, therefore, calculated relative to the non-ischemic one. - Determine the percentage of CVD increase as the difference between the CVD percentage among the ischemic and nonischemic limbs.
- Laser capture microdissection of capillaries
- After anesthetizing, sacrifice the mice by cervical dislocation.
- To harvest the gastrocnemius muscles of both legs, remove the skin from mice both hindlimbs.
- Identify the Achilles tendon and separate the tendon from the bone using an 11-blade scalpel.
- Hold the Achilles tendon and separate the muscle from the tibia and fibula up to the knee joint with spring scissors.
- Separate the biceps femoris from the gastrocnemius muscle.
- Use spring scissors to cut the insertions of the gastrocnemius muscles at the knee joint level.
- Place the harvested gastrocnemius muscles in a transverse orientation on a small cork disk with the help of 10 % tragacanth.
- Snap freeze the specimens in liquid nitrogen-cooled isopentane and store them at -80 °C until sectioning.
- Cut on a cryostat 12 µm sections of the muscle specimens and mount on glass slides.
NOTE: For RNA protection take a precooled slide and touch the back-side of the slide with your finger (gloves) to warm only the region for placing the section. Transfer section from the cryostat knife by touching with the warmed area and dry at -20 °C in the cryostat for 2-3 min. The protocol can be paused here.- Fix the glass slides in a bottle containing cooled pure acetone (approximately 50 mL) for 5 min at -20 °C and air-dry them.
- Use a hydrophobic pen around the sections.
NOTE: The use of the hydrophobic pen helps in reduction of the amount of solutions spent during the protocol. For all incubations use 100 µL of all solutions, per section. - Rehydrate with 2 M NaCl/PBS at 4 °C and incubate the specimens overnight at 4 °C with anti-CD31 rat monoclonal antibody at 1:500 in 2M NaCl/PBS.
- Wash twice in 2M NaCl/PBS for a total of 6 min.
- Add a secondary biotinylated rabbit anti-rat IgG antibody at 1:200 in 2M NaCl/PBS and 5% rabbit serum for 30 min at 4 °C.
- Wash in 2 M NaCl/PBS as before and add labeled avidin-conjugated peroxidase complex for 30 min at 4 °C.
- Rinse and add DAB peroxidase substrate kit for 5 min.
- Dehydrate the sections in ice-cold 90 % ethanol followed by 100 % ethanol and leave them to dry.
- Dissect 10 000 capillaries per mice using a laser microdissection system equipped with a pulsed solid-state 355 nm laser and catapult the dissected capillaries into a microfuge tube adhesive-cap.
- RNA extraction, cDNA synthesis, pre-amplification, and RT-PCR
- Isolate total RNA from the microdissected capillaries using an appropriate kit, the total RNA obtained is between 1.5-3 ng/µL.
- Use a cDNA synthesis kit for synthesis and two rounds of pre-amplification, using the primers described in Table 1.
- Perform RT-PCR for the same targets described in the previous step. Use a program consisting of an initial denaturation step at 95 °C for 10 min, followed by 50 cycles at 95 °C for 15 s and at 60 °C for 1 min.
Representative Results
Using the described protocol, umbilical cord mesenchymal stem cells and low-dose ionizing radiation (LDIR) were tested as putative angiogenic therapies 11,12. Laser Doppler perfusion readings were obtained before ischemia induction and at pre-specified timepoints ranging from immediately after ischemia induction to 45 days post-ischemia. Tissue perfusion readings by laser Doppler were recorded as color-coded images, with no perfusion displayed as dark blue and the highest perfusion level displayed as red. As shown in Figures 2A and 3A and quantified in Figures 2B and 3B, a complete loss of hindlimb perfusion was observed in all mice immediately after induction of hindlimb ischemia, ensuring the reproducibility of the technique for inducing hindlimb ischemia. Importantly, the severity of ischemia is similar in all animals. An ROI was drawn around the ischemic hindlimb and a comparable ROI was drawn around the non-ischemic hindlimb to perform the quantification of perfusion. Perfusion is expressed as a ratio of the perfusion in the ischemic limb to that in the non-ischemic limb. Changes in the perfusion ratio are measured over time.
Immunohistochemistry was performed between 15 and 90-days post-ischemia. Diaphonization was undertaken between 19- and 90-days post-ischemia and capillary microdissection between 45- and 70-days post-ischemia induction ensuring that the different pro-angiogenic therapies induced a sustained and prolonged proangiogenic effect.
Quantification of CD31-positive capillaries in histological sections of gastrocnemius muscle assessed the capillary density and this was expressed as the number of capillaries per number of muscle fibers. As shown in Figure 4, capillary density was greater in the ischemic versus the non-ischemic limb.
In order to evaluate collateral vessel density, mice were diaphonized and an equivalent ROI, corresponding to 20 % of the limb area, was selected for quantification. Collateral vessel density consistently increased in the ischemic limb, so data pertaining to treated and control limbs were expressed as the percentage of collateral vessel density increase in the ischemic limb relatively to the non-ischemic one (Figure 5).
Expression of pro-angiogenic genes by ECs was analyzed by quantitative RT-PCR of CD31-positive cells. Transcripts for Vegfr2, Vegfr1, Fgf2, Angpt2, Pdgfc, Tgfb2, Hgf, and Met showed a clear variation in expression between ischemic and non-ischemic limbs, exclusively in mice exposed to the pro-angiogenic stimulus (Figure 6).
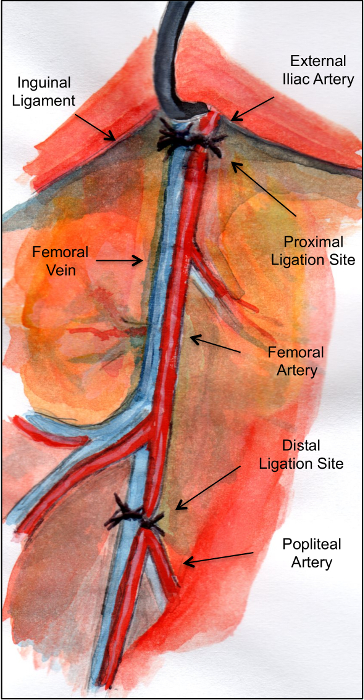
Figure 1: Schematic illustration of the anatomy of the mouse hindlimb vasculature showing the ligation sites. Please click here to view a larger version of this figure.
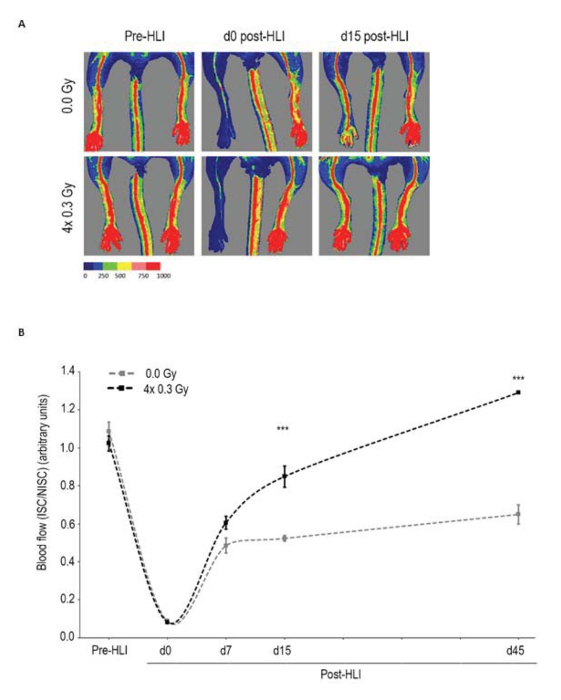
Figure 2: LDIR increases perfusion recovery. After surgical induction of unilateral HLI, both hindlimbs of C57BL/6 mice were sham irradiated or irradiated with four daily fractions of 0.3Gy, in consecutive days and allowed to recover. (A) Representative laser Doppler flow images pre-HLI, and at days 0 (d0) and 15 (d15) post-HLI induction. (B) Quantitative evaluation of blood flow expressed as a ratio of ISC to NISC limb demonstrated significantly enhanced limb blood perfusion in irradiated mice vs. sham-irradiated ones both at days 15 (d15) and 45 (d45) post-HLI. Between-group changes were assessed by two-way repeated measurements ANOVA followed by Bonferroni post-hoc test (n = 12 mice per group). Means ± SEM are shown. *** P < 0.001; ns, nonsignificant. HLI, hindlimb ischemia; ISC, ischemic; NISC, non-ischemic; Pre-HLI, before hindlimb ischemia. Adapted from 11. Please click here to view a larger version of this figure.
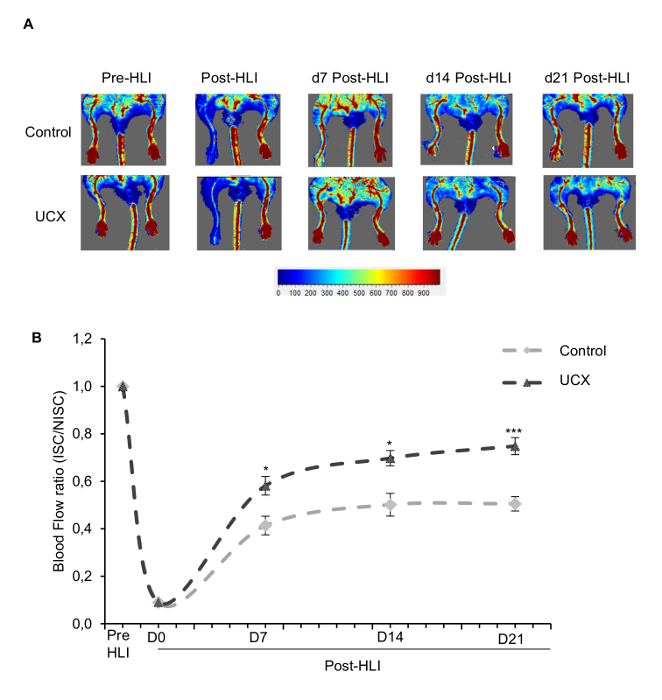
Figure 3: Umbilical cord mesenchymal stem cells (UCX) increase perfusion recovery. UCX or their vehicle (as a control) were administered in the ischemic gastrocnemius muscle 5 hours after HLI induction. (A) Representative laser doppler flow images before (PRE-HLI), immediately after (d0 POST-HLI) and at 7, 14 and 21 days post-HLI induction (d7 POST-HLI, d14 POST-HLI, d21 POST-HLI). (B) Quantitative evaluation of blood flow expressed as a ratio of ISC to NISC limb demonstrated significantly enhanced limb blood perfusion in umbilical cord mesenchymal stem cells -treated mice at 7, 14 and 21 days post-HLI. (n=16 for each experimental group; D7: t (22.69) = 4.26; ***P ˂ 0.001; effect size was 1.51 and power 0.98; D14: t (30) = 4.7; ***P ˂ 0.001; effect size was 1.66 and power 0.99; D21: t (30) = 7.22; ***P ˂ 0.001; effect size was 2.56 and power 0.99). HLI, hindlimb ischemia; ISC, ischemic; NISC, non-ischemic. Adapted from11. Please click here to view a larger version of this figure.
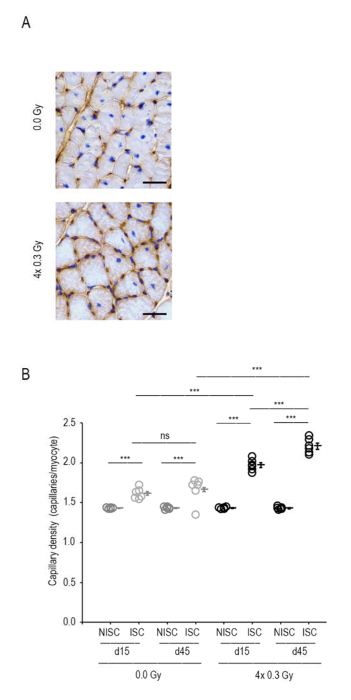
Figure 4: LDIR increases capillary density. (A) Representative sections from sham-irradiated and irradiated ischemic gastrocnemius muscles at day 45 post-HLI. Capillaries and myocytes were identified by CD31 immunohistochemistry and hematoxylin, respectively. Scale bar, 150 μm. (B) Quantitative analysis revealed increased capillary density (capillaries/myocyte) in irradiated ischemic gastrocnemius muscles compared to sham-irradiated ischemic ones at days 15 and 45 post-HLI. Mixed ANOVA followed by Bonferroni post-hoc test was conducted with a within-subject factor of ISC and between-subject factors of day and irradiation (n=6 mice per group). Individual data and means ± SEM are shown. ***P<0.001; ns, nonsignificant. ISC, ischemic; NISC, non-ischemic. Adapted from11. Please click here to view a larger version of this figure.
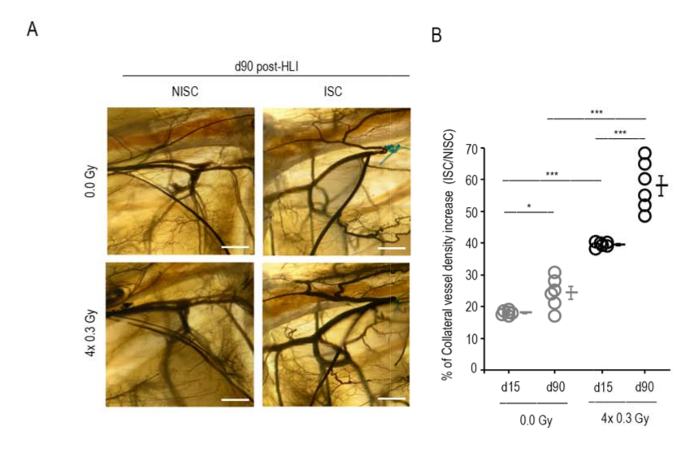
Figure 5: LDIR increases collateral density. (A) Illustrative images of selected ROIs for sham-irradiated and irradiated mice. ISC and NISC limbs at day 90 post-HLI are shown. Scale bar, 300 μm. (B) Data are represented as the percentage of CVD increase of the ISC limb relatively to the NISC one. At days 15 and 90 post-HLI, irradiated mice presented a significantly higher CVD increase (%) vs. sham-irradiated mice. Two-way ANOVA was conducted followed by Bonferroni post-hoc test with a between subject factors of day and irradiation (n=6 mice per group). Individual data and means ± SEM are shown. *P<0.05; ***P<0.001; ns, nonsignificant. HLI, hindlimb ischemia; ISC, ischemic; NISC, non-ischemic. Adapted from11. Please click here to view a larger version of this figure.
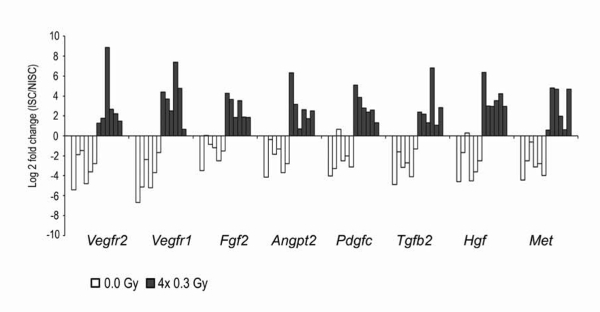
Figure 6: LDIR upregulates the expression of angiogenic genes in ECs isolated from irradiated ischemic gastrocnemius muscles. After surgical induction of unilateral HLI, both hindlimbs of C57BL/6 mice were sham-irradiated or irradiated with four daily fractions of 0.3Gy, in consecutive days and allowed to recover. At day 45 post-HLI, the expression of pro-angiogenic factors and their receptors was evaluated by qRT-PCR exclusively on ECs. Gastrocnemius muscle sections were stained for CD31. Individual endothelial CD31+ cells were visualized, dissected, and isolated using a laser microdissection system. Each bar represents the relative gene expression in one animal. White and gray bars represent sham-irradiated and irradiated mice, respectively. Values were normalized to 18S to obtain relative expression levels. Results expressed as log2 fold changes between ischemic and non-ischemic samples demonstrated the relative abundance of the transcripts in irradiated mice; in contrast, a down-regulation is observed in sham-irradiated mice. Adapted from11. Please click here to view a larger version of this figure.
| Vegfr1_F (5’-TTGAGGAGCTTTCACCGAACTCCA-3’); |
| Vegfr1_R (5’-TATCTTCATGGAGGCCTTGGGCTT-3’); |
| Vegfr2_F (5’-AGGCCCATTGAGTCCAACTACACA-3’); |
| Vegfr2_R (5’-AGACCATGTGGCTCTGTTTCTCCA-3’); |
| Pdgf-c_F (5’-ATGCCACAAGTCACAGAAACCACG-3’); |
| Pdgf-c_R (5’-AAGGCAGTCACAGCATTGTTGAGC-3’); |
| Met_F (5’-ACGTTGAAATGCACAGTTGGTCCC-3’); |
| Met_R (5’-TTGCGTCGTCTCTCGACTGTTTGA-3’); |
| Fgf2_F (5’-ACTCCAGTTGGTATGTGGCACTGA-3’); |
| Fgf2_R (5’-AACAGTATGGCCTTCTGTCCAGGT-3’); |
| Tgfb2_F (5’-GCTTTGGATGCGGCCTATTGCTTT-3’); |
| Tgfb2_R (5’-CTCCAGCACAGAAGTTGGCATTGT-3’); |
| Ang2_F (5’-ATCCAACACCGAGAAGATGGCAGT-3’); |
| Ang2_R (5’-AACTCATTGCCCAGCCAGTACTCT-3’); |
| Hgf_F (5’-GCATTCAAGGCCAAGGAGAAGGTT-3’); |
| Hgf_R (5’-TCATGCTTGTGAGGGTACTGCGAA-3’); |
| 18s_F (5’-GCCCTATCAACTTTCGATTGGTAGT-3’); |
| 18s_R (5’-CCGGAATCGAACCCTGATT-3’). |
Table 1: Primers used for the study.
Discussion
Murine models of CLI have mostly consisted in ligation of the femoral artery just distal to the origin of the profunda femoris 4,5,6,7,8,9. This has shown to leave most of the collateral circulation intact, which restores blood flow to the limb within 7 days 9. Removal of the collateral bed can be achieved by excision of the femoral artery. However, up to a third of the original blood flow can be restored as soon as 7 days after the procedure, given that most collateral vessels arise from the internal iliac artery 7. Lejay et al have proposed sequential ligations with a second ligation performed on the common iliac artery, effectively reducing the collateral perfusion provided by the internal iliac artery 4. Critical steps within this protocol include not only the identification of the external iliac artery just above the inguinal ligament, but also the ligation and excision of the distal external iliac and femoral arteries and veins, which serves a double purpose: to increase the severity of ischemia as well as to improve the technical reproducibility of the model, as exposure of the femoral artery alone often results in tearing of the vein.
One limitation of this technique is the acute interruption of the blood flow to the limb, which differs from the protracted process associated with the build-up of atherosclerotic plaque leading to arterial stenosis and occlusion. Still, reduction of blood flow was present well after 2 weeks, the established timepoint for the diagnosis of chronic ischemia. Also, collateralization was increased with the ischemic insult alone, which mimics the process of collateral formation observed in chronically ischemic limbs.
Neovascularization of ischemic limbs involves a complex interplay of biological events, namely, angiogenesis and arteriogenesis. This protocol includes a variety of assays that assessed the interference of putative angiogenic therapies on angiogenic sprouting (capillary vessel density) and collateral vessel development (arteriogenesis), the most important mechanism in human tolerance to lower limb ischemia. Simultaneously, laser Doppler is used for unveiling the function of these new or enlarged vessels, and for the first-time laser microdissection of capillaries is used to collect ECs disclosing the molecular mechanisms that underlie functional recovery. This protocol yields a reproducible animal model that can mimic the effects of CLI a standard of testing when the animals are treated with possible angiogenic therapies that could be applied in a clinical context in the future.
Divulgazioni
The authors have nothing to disclose.
Acknowledgements
We thank José Rino and Tânia Carvalho, heads of the Bioimaging Facility and Histology and Comparative Pathology Laboratory of Instituto de Medicina Molecular João Lobo Antunes, respectively. We also thank Vyacheslav Sushchyk from the Department of Anatomy of Nova Medical School/Faculdade de Ciências Médicas, Universidade Nova de Lisboa.
Funding reference: project funded by UID/IC/0306/2016 Fundação para a Ciência e a Tecnologia. Paula de Oliveira is supported by a fellowship (SFRH/BD/80483/2011) from Fundação para a Ciência e Tecnologia.
Materials
| 7500 Fast Real-Time PCR | Applied Biosystems | Instrument | |
| Acetone | Merk | 1000141000 | Reagent; Caution – highly flammable |
| Adenosine | Valdepharm | Reagent | |
| Atipamezole | OrionPharma | Reagent | |
| Barium sulphate (Micropaque) | Guebert | 8671404 (ref. Infarmed) | Reagent |
| Buprenorphine | RichterPharma | Reagent | |
| Carl Zeiss Opmi-1 FC Surgical Microscope | Carl Zeiss Microscopy, Germany | Instrument | |
| cDNA RT2 PreAMP cDNA Synthesis kit | Qiagen | 7335730 | Reagent |
| Cryostat Leica CM | Leica Microsystems | 3050S | Instrument |
| DAB peroxidase substrate kit | DAKO;Vector Laboratories | K3468 | Reagent |
| hydrogen peroxidase | Merk | 1072090250 | Reagent; Caution – nocif |
| hydrophobic pen | Dako | 411121 | Reagent; Caution – toxic |
| Ketamidor | Richterpharma | CN:580393,7 630/01/12 Dfvf | Reagent |
| Laser Doppler perfusion imager moorLDI2-HIR | MoorLDI-V6.0, Moor Instruments Ltd, Axminster, UK | 5710 | Instrument |
| Leica DM2500 upright brightfield microscope | Leica Microsystems | Instrument | |
| Medetor | Virbac | 037/01/07RFVPT | Reagent |
| methanol | VWR | UN1230 | Reagent; Caution – toxic and highly flammable |
| Papaverine | Labesfal | Reagent | |
| Pentano Isso | Merk | 1060561000 | Reagent; Caution – highly flammable |
| Power SYBR® Green | Applied Biosystems | 4309155 | Reagent |
| Purified rat anti-mouse CD31 | Pharmingen | 550274 | Reagent |
| RNeasy Micro kit | Qiagen | 74004 | Reagent |
| Surgic-Pro 6.0 | Medtronic (Coviden) | VP733X | Suture |
| VECTASTAIN ABC HRP Kit (Peroxidase, Rat IgG) | Vectastain ABC kit; Vector Laboratories | PK-4004 | Reagent |
| Vicryl5.0/ Vicryl 6.0 | Medtronic (Covidien) | UL202/ UL101 | Suture |
| Zeiss PALM MicroBeam Laser Microdissection System | Carl Zeiss Microscopy, Germany | 1023290916 | Instrument |
| Stereotaxic microscope | Carl Zeiss Microscopy, Germany | Instrument | |
| Digital camera | Linux | Instrument |
Riferimenti
- Becker, F., et al. Chapter I: Definitions, epidemiology, clinical presentation and prognosis. European Journal of Vascular and Endovascular Surgery. 42 Suppl 2, S4-S12 (2011).
- Fowkes, F. G., et al. Peripheral artery disease: epidemiology and global perspectives. Nature Reviews Cardiology. 14 (3), 156-170 (2017).
- Abu Dabrh, A. M., et al. The natural history of untreated severe or critical limb ischemia. Journal of Vascular Surgery. 62 (6), 1642-1651 (2015).
- Lejay, A., et al. A new murine model of sustainable and durable chronic critical limb ischemia fairly mimicking human pathology. European Journal of Vascular and Endovascular Surgery. 49 (2), 205-212 (2015).
- Sprengers, R. W., Lips, D. J., Moll, F. L., Verhaar, M. C. Progenitor cell therapy in patients with critical limb ischemia without surgical options. Annals of Surgery. 247 (3), 411-420 (2008).
- Lotfi, S., et al. Towards a more relevant hind limb model of muscle ischaemia. Atherosclerosis. 227 (1), 1-8 (2013).
- Masaki, I., et al. Angiogenic gene therapy for experimental critical limb ischemia: acceleration of limb loss by overexpression of vascular endothelial growth factor 165 but not of fibroblast growth factor-2. Circulation Research. 90 (9), 966-973 (2002).
- Limbourg, A., et al. Evaluation of postnatal arteriogenesis and angiogenesis in a mouse model of hind-limb ischemia. Nature Protocols. 4 (12), 1737-1746 (2009).
- Hellingman, A. A., et al. Variations in surgical procedures for hind limb ischaemia mouse models result in differences in collateral formation. European Journal of Vascular and Endovascular Surgery. 40 (6), 796-803 (2010).
- Brevetti, L. S., et al. Exercise-induced hyperemia unmasks regional blood flow deficit in experimental hindlimb ischemia. Journal of Surgical Research. 98 (1), 21-26 (2001).
- Ministro, A., et al. Low-dose ionizing radiation induces therapeutic neovascularization in a pre-clinical model of hindlimb ischemia. Cardiovascular Research. 113 (7), 783-794 (2017).
- Pereira, A. R., et al. Therapeutic angiogenesis induced by human umbilical cord tissue-derived mesenchymal stromal cells in a murine model of hindlimb ischemia. Stem Cell Research Therapy. 7 (1), 145 (2016).
- Azaripour, A., et al. A survey of clearing techniques for 3D imaging of tissues with special reference to connective tissue. Progress in Histochemistry and Cytochemistry. 51 (2), 9-23 (2016).

