Electroporation Method for In Vivo Delivery of Plasmid DNA in the Adult Zebrafish Telencephalon
Summary
Presented here is an electroporation method for plasmid DNA delivery and ependymoglial cell labeling in the adult zebrafish telencephalon. This protocol is a quick and efficient method to visualize and trace individual ependymoglial cells and opens up new possibilities to apply electroporation to a broad range of genetic manipulations.
Abstract
Electroporation is a transfection method in which an electrical field is applied to cells to create temporary pores in a cell membrane and increase its permeability, thereby allowing different molecules to be introduced to the cell. In this paper, electroporation is used to introduce plasmids to ependymoglial cells, which line the ventricular zone of the adult zebrafish telencephalon. A fraction of these cells shows stem cell properties and generates new neurons in the zebrafish brain; therefore, studying their behavior is essential to determine their roles in neurogenesis and regeneration. The introduction of plasmids via electroporation enables long-term labeling and tracking of a single ependymoglial cell. Furthermore, plasmids such as Cre recombinase or Cas9 can be delivered to single ependymoglial cells, which enables gene recombination or gene editing and provides a unique opportunity to assess the cell’s autonomous gene function in a controlled, natural environment. Finally, this detailed, step-by-step electroporation protocol is used to obtain successful introduction of plasmids into a large number of single ependymoglial cells.
Introduction
Zebrafish are excellent animal models to examine brain regeneration after a stab wound injury. In comparison to mammals, on the evolutionary ladder, less evolved species such as zebrafish generally show higher rates of constitutive neurogenesis and broader areas of adult neural stem cell residence, leading to constant generation of new neurons throughout most brain areas in the adult life. This feature appears to correlate with significantly higher regenerative capacity of zebrafish in comparison to mammals1, as zebrafish have remarkable potential to efficiently generate new neurons in most brain injury models studied2,3,4,5,6,7,8. Here, the zebrafish telencephalon is studied, since it is a brain area with prominent neurogenesis in adulthood. These zones of adult neurogenesis are homologous to neurogenic zones in the adult mammalian brain9,10,11.
Abundant neurogenic areas in the zebrafish telencephalon are present due to the existence of radial glia like cells or ependymoglia cells. Ependymoglial cells act as resident adult neural stem cells and are responsible for generation of new neurons in both the intact and regenerating brain3,5. Lineage tracing experiments have shown that ventricular ependymoglia react to injury, then proliferate and generate new neuroblasts that migrate to the lesion site5. Due to the everted nature of zebrafish telencephalon, ependymoglial cells line the ventricular surface and build the ventral ventricular wall. The dorsal ventricular wall is formed by a dorsal ependymal cell layer (Figure 1A). Importantly, zebrafish ependymoglia embody the characteristics of both mammalian radial glia and ependymal cells. Long radial processes are a typical feature of radial glia cells, whereas cellular extensions and tight junctions (as well as their ventricular positions) are typical features of ependymal cells12,13,14. Therefore, these cells are referred to as ependymoglial cells.
To follow in vivo behavior of single ependymoglial cells during regeneration, they need to be reliably labeled. Various methods of in vivo cell labeling for fluorescent microscopy have been previously described, such as endogenous reporters or lipophilic dyes15. These methods, in contrast to electroporation, may require longer periods of time and often do not offer the possibility of single cell labeling or permanent long-term tracing. Electroporation, however (besides single cell labeling), offers the possibility of introducing new DNA into the host cell. Moreover, compared to other methods of DNA transfer into the cells, electroporation has been demonstrated to be one of the most efficient methods16,17,18,19.
Presented here is an electroporation protocol that has been refined for the purpose of labeling single ependymoglial cells in the adult zebrafish telencephalon. This protocol allows for the labelling of single ependymoglial cells in order to follow them over a long-term period20 or to manipulate specific pathways in a cell-autonomous manner21,22.
Protocol
All animals used in this protocol were kept under standard husbandry conditions, and experiments have been performed according to the handling guidelines and regulations of EU and the Government of Upper Bavaria (AZ 55.2-1-54-2532-0916).
1. Preparation of Plasmid Mixture for Electroporation
- Dilute the plasmid of interest in sterile water and add fast green stain stock solution [1 mg/mL]. Make sure that the final concentration of the plasmid is ∼1 µg/µL. Add the stain at a concentration of no more than 3%, as its only purpose is to color the solution and visualize ventricular injection.
- Once prepared, mix the plasmid solution by pipetting up and down several times or by finger tapping. Store at room temperature (RT) until usage.
NOTE: For co-electroporation of two plasmids into the same cell, ensure that the concentration of each individual plasmid used in the mixture is at least 0.8 ng/µL with a molar ratio of 1:1 to obtain 80%–90% co-electroporation efficiency.
2. Preparations for Injection and Electroporation Procedure
- Prepare the glass capillaries (outer diameter 1 mm, inner diameter 0.58 mm) necessary for the injection in the needle pulling apparatus.
- In order to inject the correct amount of plasmid (see above), pull the capillary at a temperature of 68.5 °C with two light and two heavy weights (see Table of Materials for puller specifications).
NOTE: In case a different puller is used, calibrate the capillary to deliver the appropriate volume of electroporation mix. - Manually set the injection device to an injection pressure of 200 hPa (by turning the Pi knob) and constant pressure of 0 hPa. Manually set the injection time to manual mode and control the pressure with foot pedal.
- Set the electroporation device to “LV mode” with five pulses at 54–57 V (25 ms each with 1 s intervals). Connect the electrodes to the device.
- Prepare one fish tank with clean fish water, where the fish will be awakened from anesthesia after the electroporation procedure. Aerate the water by keeping the air stone attached to the air pump for the entire recovery period until the fish is fully awakened.
- Take a regular kitchen sponge and make a longitudinal cut in the sponge to hold fish into during the injection and electroporation procedure (see previous publication3).
NOTE: The kitchen sponge should be regularly washed or exchanged in order to remove potential toxic chemicals. - Place a small amount of highly conductive multi-purpose ultrasound gel next to the injection and electroporation setup.
NOTE: This will ensure adequate electrical conductivity, and consequently, distribution of electroporated cells throughout the entire telencephalon.
3. Zebrafish Anesthesia
- Prior to anesthetization, prepare a stock solution of anesthesia with 0.2% MS222 in distilled water and adjust the pH to 7 with Tris-HCl buffer. Dilute this stock 1:10 (i.e., to 0.02% MS222) using fish water.
- Anesthetize the fish by keeping them in this working solution until the movement of the body and gills subsides (typically for a couple of minutes).
4. Plasmid Solution Injection
- Fill the prepared glass capillary with 10 µL of plasmid solution using microloader tips. Avoid the formation of air bubbles inside the capillary.
- Press Menu/Change Capillary on the injection device. Insert and secure the needle into the needle holder.
- Under a stereomicroscope with a magnification of 3.2x or 4x, cut only the tip of the capillary using fine-end forceps. Switch the injection device from Change Capillary mode into Inject mode, then apply pressure with a foot pedal to ensure that the plasmid solution is running easily out of the needle and without hindrance.
- Transfer the fish from the husbandry tank to the container (plastic box) with anesthetic solution. Wait for a few minutes until movement of the gills subsides.
- Place fish into the pre-wetted sponge with the dorsal side facing up. Perform all the following injection steps under the stereomicroscope to ensure the accuracy of procedure.
- Using a dissecting micro-knife from stainless steel with 40 mm cutting edge and 0.5 mm thickness, create a small hole in the fish skull at the posterior side of the telencephalon (Figure 1B), just next to the border with optic tectum.
NOTE: This step should be performed carefully since the hole should be very small and superficial, penetrating solely the skull, to avoid brain damage. - Tilt the fish as necessary and orient the tip of the glass capillary towards the skull in the correct angle to facilitate penetration of the capillary tip through the hole.
- Insert the tip of the capillary through the hole in the skull carefully until it reaches the telencephalic ventricle (see Figure 1B). This will require penetration through the dorsal ependymal cell layer. Be especially careful not to insert the capillary too deeply to avoid contact with the brain tissue. Keep the capillary precisely in between the hemispheres, remaining inside the ventricle just after piercing the dorsal ependymal layer.
NOTE: This is a very delicate step. Accuracy of this procedure is improved using pigmentation mutant lines such as brassy24, allowing better visualization of glass capillary position during injection. - With the capillary tip inside of the ventricle, inject the plasmid solution by applying pressure with the foot pedal for about 10 s, which corresponds to approximately 1 µL of plasmid solution.
NOTE: If changing the needle puller, capillaries or injector, the system should be calibrated in order to always deliver 1 µL of plasmid solution. Calibration can be performed by measuring the diameter of the plasmid droplet expelled into a mineral oil (e.g., paraffin oil) and subsequently calculating the volume of the droplet. After 10 s of injection, there should be ~1 µL of plasmid liquid expelled into the mineral oil. - Confirm success of the previous step by observing the spread of liquid throughout the ventricle.
5. Electroporation
- Remove the fish from the injection set-up while still holding it in the sponge.
- Immerse the inner side of the tip of the electrodes in the ultrasound gel.
- Cover the fish telencephalon with a small amount of ultrasound gel.
- Position the fish head between the electrodes, placing the positive electrode at the ventral side of the fish’s head and the negative electrode on the dorsal side (Figure 1C), while still holding the fish’s body in the sponge. This sets the direction of the flow of the current necessary to electroporate ependymoglia positioned at subventricular zone.
- Press the electrodes gently and precisely against the telencephalon (Figure 1C). Administer the current with the foot pedal. Hold the electrodes in place until all five pulses are finished.
6. Fish Recovery
- Let the fish recover in the previously prepared, continuously aerated tank until it wakes up. Lidocaine gel could be applied on the skull in order to relieve any possibly developed pain.
Representative Results
The described electroporation method allows delivery of plasmid DNA into ependymoglial cells, which are located superficially in the zebrafish telencephalon and just under the dorsal ependymal cell layer (Figure 1A).
If the result of electroporation is positive, labeled single ependymoglial cells (red cells in Figure 2A,B) can be observed among other ependymoglial cells (white in Figure 2A,B). Depending on the efficiency of the electroporation process, a higher (Figure 2A) or lower (Figure 2B) number of ependymoglial cells may be labeled. Nevertheless, this protocol yields higher number of labeled cells than previously published20, which is apparent in Figure 3A and Video 1. It is worth mentioning that the highest density of labeled cells tends to emerge mostly at the inner, ventricular side of both hemispheres (Figure 3A), due to the way in which the injected plasmid liquid distributes between the hemispheres. In Video 1, one hemisphere of the zebrafish telencephalon is presented in 3D, and the ependymoglial cells with radial processes can be seen from the side. The cells are co-electroporated and labeled with two plasmids, tdTomato-mem (red fluorescent protein anchored to the cell membrane) and H2B-YFP plasmid (labelling nuclei). Cell division of two nuclei surrounded by yellow circles should be noted.
Unsuccessful electroporation results in very low number or no labeled ependymoglial cells. This outcome can be generally explained by inaccurate injection, in which the tip of the capillary does not penetrate the dorsal ependymal cell layer. In this case, plasmid solution spreads above the dorsal ependymal cell layer instead of filling telencephalic ventricle. This leads to ependymal cells solely being labeled (Figure 3B). Dorsal ependymal cells (blue arrows in Figure 3B) differ morphologically from ependymoglial cells (yellow arrow in Figure 3A). Their soma is larger and cuboid, and they do not possess radial, elongated processes. This is evident from comparing a side view of the ependymoglial cell layer (Figure 4A,B). TdTomato-mem labeled cells are most likely ependymal cells, which are located above the layer of ependymoglia (Figure 4B). In contrast, in Figure 4A, a tdTomato-mem expressing plasmid is introduced to individual ependymoglial cells. Thus, they express tdTomato-mem in addition to their initial labeling (in this case, transgenic gfap:GFP fish line, seen here in white).
This protocol enables the labelling and subsequent following of the behaviour of ependymoglial cells in zebrafish telencephalon after injury over a short-21 or long-term3 period of time. This is accomplished through live, in vivo imaging and helps address the question of their roles in regeneration and neurogenesis.
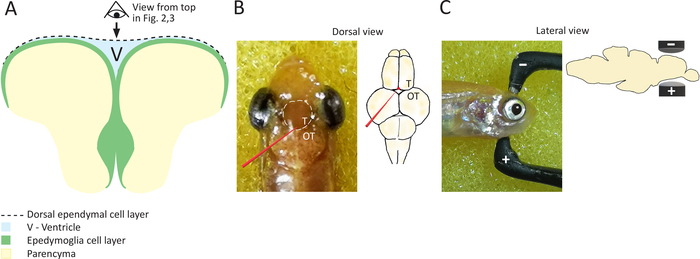
Figure 1: Schematic representation of coronal section of the everted zebrafish telencephalon. (A) Scheme of a coronal section of zebrafish telencephalon, highlighting the position of ependymoglial cells, which are lining ventricular surface and building the ventral ventricular wall. Dorsal ependymal layer is bridging the two hemispheres and covering the ventricle (V), located in between two cell layers: ependymoglial and ependymal. Black arrow and the representation of the eye indicate view are shown in Figures 2 and Figure 3. (B) On the left, a photograph of the zebrafish head taken from above, highlighting the position of telencephalon with a white dashed line. A glass capillary is depicted in red, along with the target site for capillary insertion. Pictured on the right is a schematic of zebrafish brain showing the position of plasmid injection in red. It should be noted that the glass capillary does not touch the telencephalon and that the plasmid is injected just above the telencephalon into the ventricle. T = telencephalon, OT = optic tectum. (C) Depicted on the left is a photograph of the zebrafish head (side view), and on the right is a depiction of the head (side view) showing the position of electrodes in order to target the telencephalon. Please click here to view a larger version of this figure.
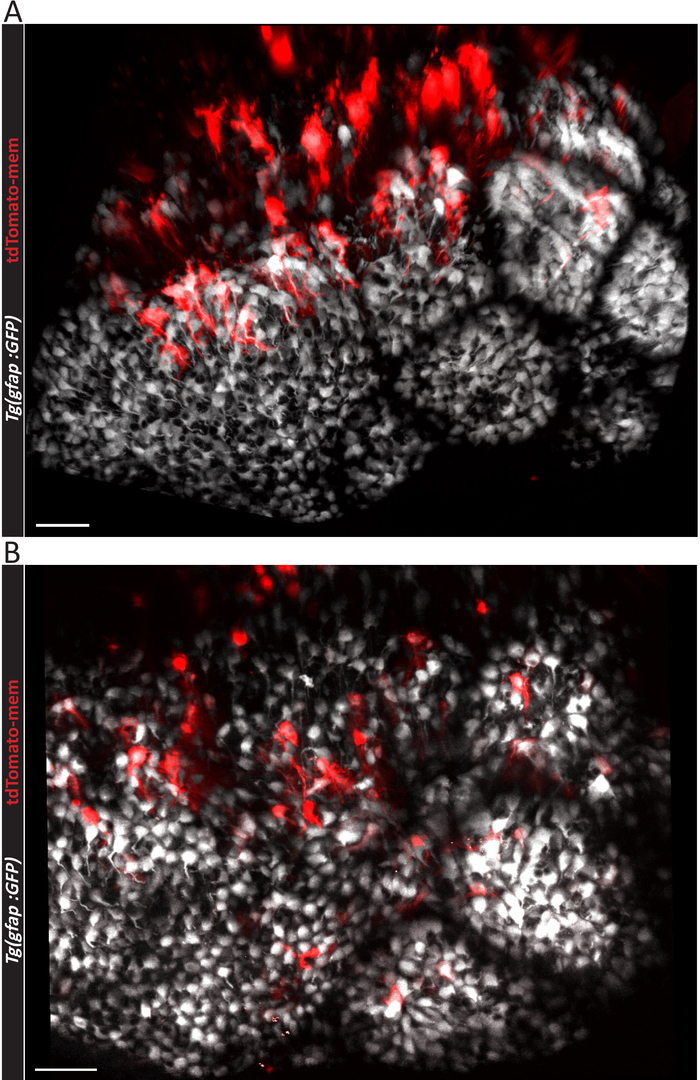
Figure 2: Micrographs showing effective electroporation outcomes. Three-dimensional representation of a (A) larger or (B) smaller number of electroporated ependymoglial cells in the adult zebrafish telencephalon, as viewed from above. The electroporation were done in Tg (gfap:GFP) fish line expressing GFP fluorescent protein (depicted in white) in all ependymoglial cells. Individual electroporated cells are labelled with pCS2-tdTomato-mem plasmid3. Scale bars = 50 µm. Please click here to view a larger version of this figure.
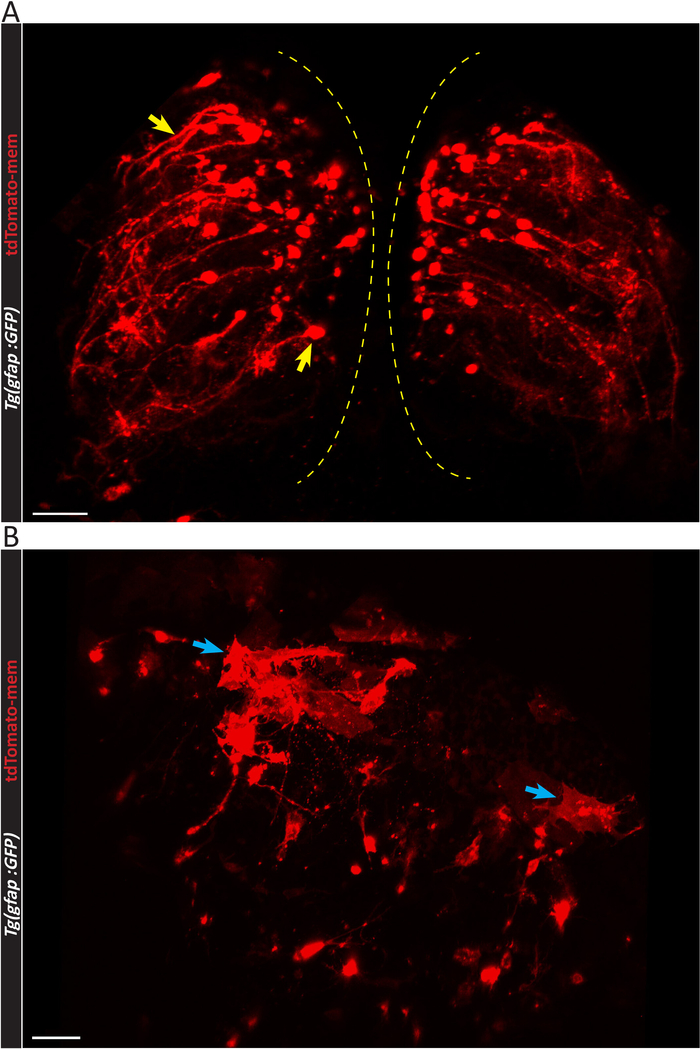
Figure 3: Confocal micrographs depicting the difference between successful and unsuccessful electroporation. (A) Three-dimensional confocal image of BABB-cleared zebrafish telencephalon (REF) with a large number of pCS-tdTomato-mem electroporated ependymoglial cells. The morphology of the ependymoglial cells with long, elongated processes (yellow arrows) should be noted. Both telencephalic hemispheres, highlighted with yellow dashed lines, can be observed. (B) Confocal image of unsuccessful electroporation of pCS2-tdTomato-mem plasmid. Mostly dorsal ependymal cells are labelled, and few ependymoglial cells express tdTomato-mem plasmid. The clear difference in the morphology of ependymal cells (blue arrows) should be noted. Scale bars = 50 µm. Please click here to view a larger version of this figure.
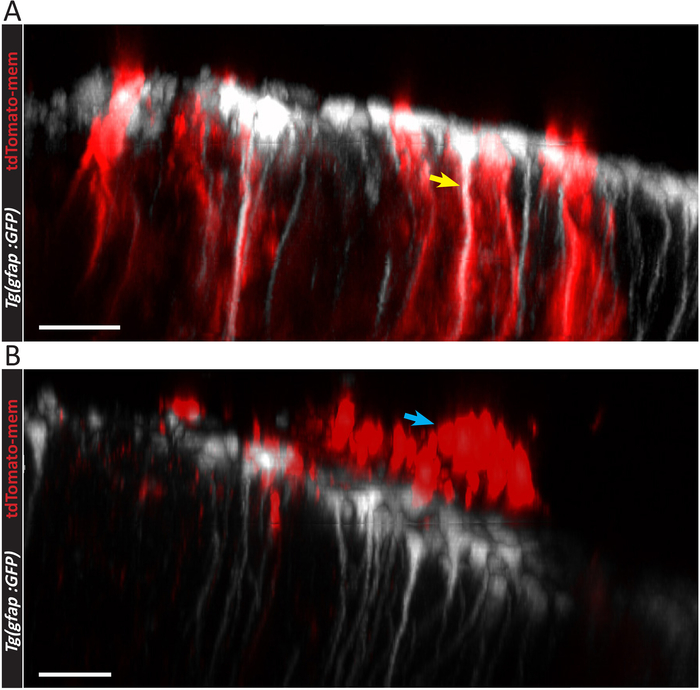
Figure 4: 3D lateral views of electroporated and non-electroporated ependymoglial cells. (A) Three-dimensional lateral representation electroporated ependymoglial cells, positive for both Tg(gfap:GFP) and pCS2-tdTomato-mem (yellow arrow). (B) 3D lateral representation of unsuccessful electroporation. The location of pCS2-tdTomato-mem positive cells above the Tg(gfap:GFP) ependymoglia layer should be noted. Most likely dorsal ependymal layer cells were electroporated (blue arrow). Scale bars = 30 µm. Please click here to view a larger version of this figure.
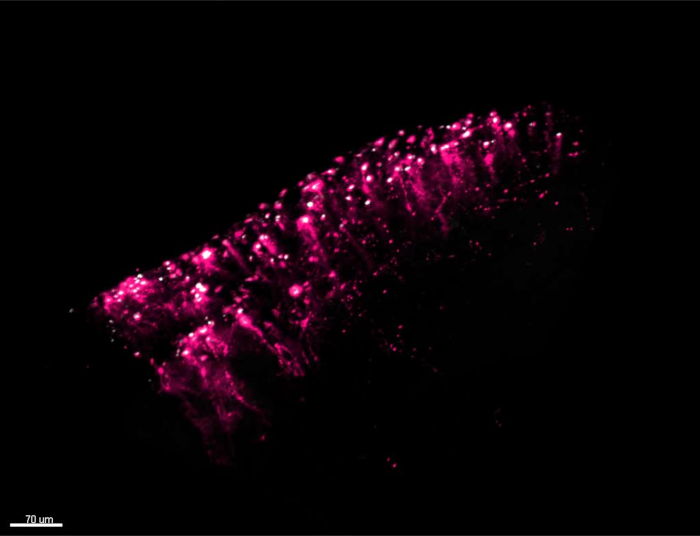
Video 1: 3D movie of zebrafish telencephalon and electroporated ependymoglial cells. Video shows one hemisphere of the zebrafish telencephalon in 3D. Ependymoglial cells are co-electroporated with two plasmids: tdTomato-mem (red fluorescent protein anchored to the cell membrane) and H2B-YFP plasmid (labelling nuclei). Individually labelled ependymoglia with radial processes that can be observed from aside. Yellow circles highlight ependymoglial cell with mitotic figure visualized by H2B-YFP labelled chromatin. Please click here to view this video. (Right-click to download.)
Discussion
This electroporation protocol is a reliable in vivo method of labelling individual ependymoglial cells. The protocol may need a further adaptation to label other cell types such as neurons or oligodendrocytes. To achieve successful labelling, plasmids containing different promoters can be used. Chicken-beta actin promoter, eF1alpha, CMV and ubiquitin promoter have been previously used to drive the expression of different transgenes in ependymoglia and their progeny23. However, different kinetics of transgene expression was observed which should be taken into the account. For example, CMV promoter driven transgene expression is very fast (expression could be seen after 24 h), while eF1alpha takes longer. Apart from labeling, the electroporation protocol can be used as a fast and straightforward platform of gene editing with the use of Cre recombinase or CRISPR Cas9 techniques22. Moreover, the use of flip-cassette25-containing plasmids and their electroporation in cell type-specific Cre lines may serve as a clear extension of a method allowing labelling or gene-editing in the ependymoglial progeny generated after electroporation.
This protocol has several critical steps. First, during the injection step, the experimental needs to be careful that the injected plasmid amount is equal for each individual fish, such that the number of labelled cells remains comparably similar. This can be achieved by controlling the size of the glass capillary opening, meaning that the cut of each tip should be constant among different capillaries or calibration should be performed for each individual capillary. Additionally, the duration of injection, regulated by a foot pedal, should be the same for each individual injection. Second, the proper position of a hole in the skull made with the micro-knife is crucial for the proper dispersion of the plasmid liquid throughout the telencephalon. It is equally important to penetrate the dorsal ependymal cell layer with the capillary tip, as stated in the protocol. Moreover, it is also essential that the hole created is not too large to prevent the plasmid mixture and cerebrospinal fluid from leaking out of the telencephalon. Another critical step is the strength of the applied electrical current. It is important to make sure that the electroporation device is operating as precisely as possible, such that the strength of the applied current does not deviate much from the voltage appearing on the screen, which is not always accurate. If these values are not consistent, it is necessary to adjust the strength of the current on the electroporation device, since an administered current higher than the recommended 54-57 V may compromise fish survival.
Compared to the other methods for a plasmid delivery and cell labelling commonly used in the field, electroporation has obvious advantages. Despite the critical steps mentioned above, it happens very rarely that no electroporated cells can be observed or that the dorsal ependymal cells are mistakenly electroporated. Generally, the success rate of this electroporation protocol is 90%-95% and we observe almost no TUNEL+ cells after electroporation. In contrast to lipofection for example, during electroporation, cationic liposomes (e.g., lipofectamine) are not used and thus toxicity connected with its usage is completely avoided26. It was previously reported that lipofection and electroporation have equal efficiency rates (20-50 cells per telencephalon)27. However, this optimized protocol generally yields 100-200 cells per telencephalon. In comparison to viral vectors, biosafety is not an issue with electroporation.
In addition, commonly used AAVs or lentiviruses fail to produce detectable expression of transgenes in zebrafish brain17,28. Finally, although the Cre-lox system is nowadays commonly used in zebrafish, plasmid electroporation is faster since it does not require long waiting times necessary for fish breeding and growing and allows individual cell labelling and tracing. However, such a technique requires highly skilled scientists to achieve successful electroporation and high survival rates of experimental animals (we typically experience survival rates of ~70%-80%). This rate also tends to fluctuate depending on the experimenter. Learning the procedure requires practice and typically takes three trials. However, this is dependent on the manual dexterity of the individual and may take longer in some cases.
The presented electroporation protocol is a fast, highly efficient method for electroporating a large number of ependymoglial cells with the necessary precautions to obtain optimal results. Electroporation of the adult zebrafish telencephalon is crucial for visualizing individual ependymoglial cells and studying their roles in neurogenesis and regeneration processes. Recently, success has been achieved in the simultaneous disruption of multiple genes of the adult zebrafish telencephalon through gene editing via the electroporation and StagR-Cas9 techniques22. This opens many possibilities and future applications of electroporation for a broad range of genetic manipulations.
Divulgazioni
The authors have nothing to disclose.
Acknowledgements
Special thanks to James Copti for editing of the manuscript. We also gratefully acknowledge funding to JN from the German Research foundation (DFG) by the SFB 870 and SPP “Integrative Analysis of Olfaction” and SPP 1738 “Emerging roles of non-coding RNAs in nervous system development, plasticity & disease”, SPP1757 “Glial heterogeneity”, and Excellence Strategy within the framework of the Munich Cluster for Systems Neurology (EXC 2145 SyNergy – ID 390857198).
Materials
| Reagent/Material | |||
| Fast Green | Sigma-Aldrich | F7258-25G | For coloring plasmid solution |
| MS222 | Sigma-Aldrich | A5040-25G | MS222 should be stored at RT (up to two weeks) and protected from light |
| Ultrasound gel | SignaGel, Parker laboratories INC. | 15-60 | Electrode Gel |
| Equipment | |||
| Air pump | TetraTec APS 50, 10l-60l | Can be bought in the pet shops | |
| BTX Tweezertrodes Electrodes | Platinum Tweezertrode, BTX Harvard Apparatus | 45-0486 | 1mm diameter |
| Electroporation device | BTX ECM830 Square Wave Electroporation System, BTX Harvard Apparatus | 45-0662 | |
| Injection device | FemtoJet 4i, Eppendorf | 5252000013 | |
| Standard Wall Borosillicate Glass Capillary | Warner Instruments | 64-0766 | Model No: G100-4 |
| Microloader tips | Eppendorf | 5242956003 | |
| Micro-knife | Fine Science Tools | 10056-12 | |
| Joystick micromanipulator | Narishige Japan | MN – 151 | |
| Needle holder | FemtoJet 4i, Eppendorf | 5252000013 | Needle holder comes together with the injection device |
| Needle pulling device | Narishige Japan | Model No: PC-10 | The PC-10 was discontinued by Narishige in 2017 and replaced by the PC-100 |
| Petri dishes | Greiner Bio-One International | 633161 |
Riferimenti
- Kaslin, J., Ganz, J., Brand, M. Proliferation, neurogenesis and regeneration in the non-mammalian vertebrate brain. Philosophical Transactions of the Royal Society of London Series B Biological Sciences. 363 (1489), 101-122 (2008).
- Baumgart, E. V., Barbosa, J. S., Bally-Cuif, L., Gotz, M., Ninkovic, J. Stab wound injury of the zebrafish telencephalon: a model for comparative analysis of reactive gliosis. Glia. 60 (3), 343-357 (2012).
- Barbosa, J., et al. Live imaging of adult neural stem cell behavior in the intact and injured zebrafish brain. Science. 346 (6236), 789-793 (2015).
- Kishimoto, N., Shimizu, K., Sawamoto, K. Neuronal regeneration in a zebrafish model of adult brain injury. Disease Model and Mechanisms. 5 (2), 200-209 (2012).
- Kroehne, V., Freudenreich, D., Hans, S., Kaslin, J., Brand, M. Regeneration of the adult zebrafish brain from neurogenic radial glia-type progenitors. Development. 138 (22), 4831-4841 (2011).
- Marz, M., Schmidt, R., Rastegar, S., Strahle, U. Regenerative response following stab injury in the adult zebrafish telencephalon. Developmental Dynamics. 240 (9), 2221-2231 (2011).
- Ayari, B., Elhachimi, K. H., Yanicostas, C., Landoulsi, A., Soussi-Yanicostas, N. Prokineticin 2 expression is associated with neural repair of injured adult zebrafish telencephalon. Journal of Neurotrauma. , (2010).
- Skaggs, K., Goldman, D., Parent, J. M. Excitotoxic brain injury in adult zebrafish stimulates neurogenesis and long-distance neuronal integration. Glia. 62 (12), 2061-2079 (2014).
- Schmidt, R., Strahle, U., Scholpp, S. Neurogenesis in zebrafish – from embryo to adult. Neural Development. 8 (3), (2013).
- Alunni, A., Bally-Cuif, L. A comparative view of regenerative neurogenesis in vertebrates. Development. 143 (5), 741-753 (2016).
- Kizil, C., Kaslin, J., Kroehne, V., Brand, M. Adult neurogenesis and brain regeneration in zebrafish. Developmetal Neurobiology. 72 (3), 429-461 (2012).
- Than-Trong, E., Bally-Cuif, L. Radial glia and neural progenitors in the adult zebrafish central nervous system. Glia. 63 (8), 1406-1428 (2015).
- Lyons, D. A., Talbot, W. S. Glial cell development and function in zebrafish. Cold Spring Harbor Perspectives in Biology. 7 (2), a020586 (2014).
- Obermann, J., et al. The Surface Proteome of Adult Neural Stem Cells in Zebrafish Unveils Long-Range Cell-Cell Connections and Age-Related Changes in Responsiveness to IGF. Stem Cell Reports. 12 (2), 258-273 (2019).
- Progatzky, F., Dallman, M. J., Lo Celso, C. From seeing to believing: labelling strategies for in vivo cell-tracking experiments. Interface Focus. 3 (3), 20130001 (2013).
- Kusumanto, Y. H., et al. Improvement of in vivo transfer of plasmid DNA in muscle: comparison of electroporation versus ultrasound. Drug Delivery. 14 (5), 273-277 (2007).
- Zou, M., De Koninck, P., Neve, R. L., Friedrich, R. W. Fast gene transfer into the adult zebrafish brain by herpes simplex virus 1 (HSV-1) and electroporation: methods and optogenetic applications. Frontiers in Neural Circuits. 8 (41), (2014).
- Van Tendeloo, V. F., et al. Highly efficient gene delivery by mRNA electroporation in human hematopoietic cells: superiority to lipofection and passive pulsing of mRNA and to electroporation of plasmid cDNA for tumor antigen loading of dendritic cells. Blood. 98 (1), 49-56 (2001).
- Mars, T., et al. Electrotransfection and lipofection show comparable efficiency for in vitro gene delivery of primary human myoblasts. The Journal of Membrane Biology. 248 (2), 273-283 (2015).
- Barbosa, J. S., Di Giaimo, R., Gotz, M., Ninkovic, J. Single-cell in vivo imaging of adult neural stem cells in the zebrafish telencephalon. Nature Protocols. 11 (8), 1360-1370 (2016).
- Di Giaimo, R., et al. The Aryl Hydrocarbon Receptor Pathway Defines the Time Frame for Restorative Neurogenesis. Cell Reports. 25 (12), 3241-3251 (2018).
- Breunig, C. T., et al. One step generation of customizable gRNA vectors for multiplex CRISPR approaches through string assembly gRNA cloning (STAgR). PLoS One. 13 (4), e0196015 (2018).
- Barbosa, J. S. . In vivo single cell analysis reveals distinct behavior of neural stem and progenitor cells in homeostasis and regeneration in the adult brain. , (2014).
- Kelsh, R. N., et al. Zebrafish pigmentation mutations and the processes of neural crest development. Development. 123, 369-389 (1996).
- Torper, O., et al. In Vivo Reprogramming of Striatal NG2 Glia into Functional Neurons that Integrate into Local Host Circuitry. Cell Reports. 12 (3), 474-481 (2015).
- Nguyen, L. T., Atobe, K., Barichello, J. M., Ishida, T., Kiwada, H. Complex formation with plasmid DNA increases the cytotoxicity of cationic liposomes. Biological and Pharmaceutical Bulletin. 30 (4), 751-757 (2007).
- Chapouton, P., Godinho, L., Detrich, H. W., Westerfield, M., Zon, L. . Neurogenesis. Chapter IV in The Zebrafish: Cellular and Developmental Biology, Part A Developmental Biology. 100, (2010).
- Zhu, P., et al. Optogenetic Dissection of Neuronal Circuits in Zebrafish using Viral Gene Transfer and the Tet System. Frontiers in Neural Circuits. 3 (21), (2009).

