Simulator Training for Endovascular Neurosurgery
Summary
Simulation of complex, high-risk procedures is critical to the education of medical trainees. A protocol for simulator-based endovascular neurosurgery training in a controlled academic environment is described. The protocol includes stepwise guidelines for trainees of varying levels, with a discussion of the advantages and limitations of this model.
Abstract
Simulation-based training has become common practice across medical specialties, especially for learning complex skills performed in high-risk environments. In the field of endovascular neurosurgery, the demand for consequence- and risk-free learning environments led to the development of simulation devices valuable for medical trainees. The goal of this protocol is to provide instructive guidelines for the use of an endovascular neurosurgery simulator in an academic setting. The simulator provides trainees with the opportunity to receive realistic feedback on their knowledge of anatomy, as well as haptic feedback indicative of their success in handling the catheter-based systems without negative consequences. The utility of this specific protocol in relation to other neuroendovascular training modalities is also discussed.
Introduction
Simulation-based training is an established educational tool for medical trainees and is particularly beneficial in high-risk fields such as endovascular neurosurgery. Multiple virtual reality training devices utilizing catheter-based systems exist, such as the ANGIO Mentor simulator (Simbionix Ltd., Airport City, Israel) and VIST-C and VIST G5 simulators (Mentice AB, Gothenburg, Sweden), with a significant body of data demonstrating the utility of training on procedural aptitude1. In spite of the usefulness of the simulators, step-by-step procedural instructions for their use are lacking.
Presented is a detailed protocol for use of the ANGIO Mentor simulator, a system that supports competency improvements in common endovascular neurosurgery procedures including diagnostic cerebral angiograms, mechanical thrombectomies, and aneurysm coil embolizations2. Prior work shows that after trainees of all levels performed five simulated angiograms, five thrombectomies, and ten aneurysm coil embolizations on the ANGIO Mentor simulator, they displayed significant improvements in procedural time, fluoroscopy and contrast doses, and adverse technical events2.
The following step-by-step instructions are divided into case scenarios and can easily be integrated into an academic training curriculum for medical students, residents, or fellows2. It should nonetheless be noted that a basic understanding of cerebral arterial anatomy, angiography, and stroke and aneurysm treatments is needed to optimize the educational potential of the simulation device.
All procedures described below (i.e., diagnostic cerebral angiogram, coiling of carotid terminus aneurysm, mechanical thrombectomy) can be performed by a single operator using the ANGIO Mentor simulator (Simbionix Ltd.) (Figure 1). This training device allows neurosurgical trainees of all skill levels to gain exposure to endovascular techniques in a preclinical setting, with the three patient scenarios utilized based on a previously published curriculum for simulator-based angiography training2. To reproduce endovascular techniques with high fidelity, the simulator utilizes actual catheters and wires introduced through a port similar to the diaphragm of a femoral artery sheath. The wires and catheters engage internal rollers that record both rotational and translational motions, which are displayed on the monitors. Device selections and patient vital signs are also visible to the simulator operator.
Protocol
1. Simulator setup
- Prior to all procedures, assemble the simulator as shown in Figure 1 and turn on. Refer to Table 1 for the full list of simulator equipment needed to complete each simulation.
- Select the patient scenario using the software interface on the attached laptop (Figure 1C).
- Select the appropriate arterial sheath or guide catheter from the drop-down menu. This does not need to be physically inserted as part of the simulation, but will act as the femoral access site and allow subsequent entry of wires and catheters into the system (Figure 1D). Specific sheath/guide sizes for each scenario are discussed below.
- Select the appropriate catheter(s), guidewire, and/or microsystem based on the specific scenario as discussed below (Figure 1D).
- Turn on A (PA) and B plane (lateral) fluoroscopy on the software interface. Activate the fluoroscopy with the foot pedals (Figure 1H) and adjust both the patient and image intensifier positions with the joysticks (Figure 1I) until the correct PA and lateral views are obtained.
2. First patient scenario: Four-vessel angiography
NOTE: This scenario depicts a 52-year-old male with an unruptured left carotid terminus aneurysm found incidentally on a non-contrast computed tomography (CT) scan of the head.
- Select a 5-French femoral sheath, a 0.035 in guidewire, and a 4-French diagnostic catheter from the drop-down menu as tools to be used in this simulation.
- Insert the guidewire into the simulator machine (Figure 1D) until it registers on the simulation screen, signaling that access has been gained. Advance the guidewire until it is visualized in the descending thoracic aorta and continues into the aortic arch.
- When the guidewire is safely in the aortic arch, hold the guidewire in place and insert a diagnostic catheter over the guidewire through the simulated femoral sheath to the aortic arch.
- Remove the guidewire and utilize the fluoroscopy puff technique by gently pressing the contrast syringe (Figure 1E) to simulate contrast injection and briefly opacify the vessels as the catheter is advanced into the desired artery.
- Next, create a roadmap guide injecting contrast with the contrast syringe (Figure 1E) while the fluoroscopy foot pedal is depressed (Figure 1H). Next, reinsert the wire to selectively catheterize the desired vessel, advancing the catheter over the wire. Remove the wire for subsequent angiography runs. The right and left internal and external carotid arteries and the right and left vertebral arteries are all catheterized using this technique.
- Using the diagnostic catheter and the simulator contrast syringe (Figure 1E), perform angiograms of each of the above circulations by depressing the fluoroscopy pedal (Figure 1H) while injecting contrast with the syringe. Obtain high-magnification views of the aneurysm, if necessary. Review angiograms for adequacy prior to removing the catheter.
- Once the necessary images are obtained, remove the diagnostic catheter/guidewire from the simulation sheath. Simulated closure of the femoral arteriotomy site is not performed.
3. Second patient scenario: Carotid terminus aneurysm coiling
NOTE: This scenario depicts a 52-year-old male with a known ruptured left carotid terminus aneurysm, severe headache, nonfocal exam, and a Glasgow Coma Scale score of 15.
- Select a 6-French guide catheter, 0.035 in guidewire, and a 4-French diagnostic catheter, from the drop-down menu.
- Insert a diagnostic catheter over a guidewire into the aortic arch as in steps 2.2–2.3.
- Insert a guide catheter over the diagnostic catheter through the femoral access site (Figure 1D) to the aortic arch.
- Remove the guidewire and create a roadmap guide of the left common carotid artery by roadmap flourscopy foot pedal injecting contrast with the contrast syringe (Figure 1E) while the fluoroscopy foot pedal (Figure 1H) is depressed.
- Reinsert the guidewire and selectively catheterize the left common carotid artery and internal carotid artery using fluoroscopy and the roadmap overlay visualized on the image projection monitor (Figure 1B) by leading with the guidewire and advancing the diagnostic catheter and guide catheter once safe access is gained.
- When the guide catheter is within the internal carotid artery, remove the diagnostic catheter and wire and perform angiographic runs of the left internal carotid cerebral circulation by depressing the fluoroscopy pedal (Figure 1H) while injecting contrast with the syringe (Figure 1E).
- Measure the aneurysm using the calculation option on the software interface (Figure 1C). Keeping in mind that the coil diameter for the first coil should be 1 mm wider than the mean aneurysm diameter, select an appropriate coil.
- Select a microcatheter and microwire from the drop-down menu.
- Insert the microcatheter and microwire through the femoral access site (Figure 1D), and under roadmap guidance obtained as in step 3.6, selectively catheterize the aneurysm with the microsystem.
- Remove the microwire, insert the previously selected coil through the femoral access site (Figure 1D), and advance it slowly into the aneurysm.
- Once the coil is fully inserted, perform a diagnostic cerebral angiogram by depressing the fluoroscopy pedal (Figure 1H) while injecting contrast with the syringe and assess the patency of the parent artery and aneurysm filling. The goal is to maintain patency of the parent artery and either completely embolize the aneurysm or provide sufficient coverage of the dome or presumed rupture point to appropriately reduce rupture risk.
- Detach the coil on the software interface (Figure 1C) and remove the coil wire. If necessary, repeat steps 3.11 and 3.12 with additional coils until ~30% aneurysm occlusion is obtained.
- Remove the microcatheter and guide catheter from the simulation sheath site (Figure 1D). Simulated closure of the femoral arteriotomy site is not performed.
4. Third patient scenario: Left middle cerebral artery thrombectomy
NOTE: This scenario depicts a 64-year-old female with a National Institutes of Health Stroke Scale (NIHSS) score of 12 for aphasia and right-sided weakness who was last known to be normal 4 h earlier. Head CT revealed a hyperdense left middle cerebral artery (MCA) sign and an Alberta Stroke Program Early CT score (ASPECTS) of 10, but no hemorrhage. A CT angiogram demonstrated a left M1 segment complete occlusion.
- Select a 6-French guide catheter, 0.035 in guidewire, and a 4-French diagnostic catheter, from the drop-down menu.
- Insert the guide catheter into the left internal carotid artery and perform angiographic runs of the left internal carotid cerebral circulation as described in steps 3.2–3.6.
- Select a microcatheter/microwire and a stent retriever device from the drop down menu.
- Insert the microcatheter and microwire into the simulated femoral access site (Figure 1D) and into the left internal carotid artery.
- Under roadmap guidance obtained as in step 3.5, advance the microwire and microcatheter into the left MCA and carefully past the area of occlusion. Potential complications during this maneuver include vascular perforations and/or embolizing a clot downstream.
- Remove the microwire and insert a stent retriever device into the simulated femoral access site (Figure 1D) and advance into the MCA distal to the occlusion. Then, remove the microcatheter, leaving the stent retriever in place at the level of the occlusion.
- Turn on simulated aspiration on the software interface (Figure 1C), and retract the stent retriever device into the guide catheter by pulling back on the microwire.
- Remove both the stent retriever from the simulated femoral access site (Figure 1D).
- Perform an angiogram through the guide catheter by depressing the fluoroscopy pedal (Figure 1H) while injecting contrast with the syringe to ensure removal of the occlusion.
- Remove the guide catheter from the simulation sheath site (Figure 1D). Simulated closure of the femoral arteriotomy site is not performed.
Representative Results
The ANGIO Mentor simulator was previously shown to improve the skills of surgical trainees with varying neuroendovascular experience when performing simulated diagnostic angiograms, thrombectomies, and ruptured aneurysm coil embolizations in an academic setting2. In this study, performance metrics for the aforementioned procedures were established over the course of 30 days in one medical student, one neurosurgery resident, two diagnostic neuroradiology fellows, and one endovascular neurosurgery fellow. After 120 minutes of didactic instruction and a single viewing of each procedure, the trainees performed 10 sessions of each procedure (i.e., 30 total). Procedural evaluations were performed by an experienced neurointerventional attending based on total procedural time, fluoroscopy time, contrast dose, frequency of technically unsafe events (e.g., movements with insufficient leading wire, rapid forward/non-visualized device movements, accidental vessel catheterizations, coil deployments outside of the aneurysm, and number of intraprocedural ruptures), packing densities, number of coils used, and number of stent retriever passes attempted.
Based on analysis of variance (ANOVA) and Tukey’s Honest Significant Difference (HSD) testing, statistically significant improvements were seen among all participants in specific performance metrics for all three procedures, including contrast utilization, fluoroscopy time, and total procedural time (Figure 2), in addition to significantly increased Likert Scale scores, an evaluation gauge in which a score of 1 corresponds to failure and 5 corresponds to excellence based on procedural technique (Figure 3). Notably, training on diagnostic angiograms resulted in an 86% reduction in total procedure time, a 75% reduction in fluoroscopy time, a 68% reduction in contrast utilization, and a 64% improvement in the overall Likert Scale performance scale (p < 0.05 for all variables based on performance improvements in the first five angiograms). After mechanical thrombectomy simulation, trainees demonstrated a 35% reduction in total procedure time, a 41% reduction in fluoroscopy time, a 49% reduction in contrast utilization, and a 67% improvement in overall Likert Scale performance (p < 0.05 for all variables based on performance improvements in the first five procedures). Participants also showed statistically significant improvements in performance after simulated aneurysm coilings, with a 42% reduction in total procedure time, a 57% reduction in fluoroscopy time, a 21% reduction in contrast utilization, and a 58% improvement in Likert Scale score (p < 0.05 for all variables based on performance improvements in the first five procedures). A reduction in the occurrence of unsafe events was also seen across all scenarios. Based on these data, at our institution all neuroendovascular trainees perform five simulated angiograms, five simulated thrombectomies, and ten simulated aneurysm permanent coil embolizations (the higher number or embolizations based on the technical nuances of this procedure), prior to participating in a surgery with real neuroendovascular cases.
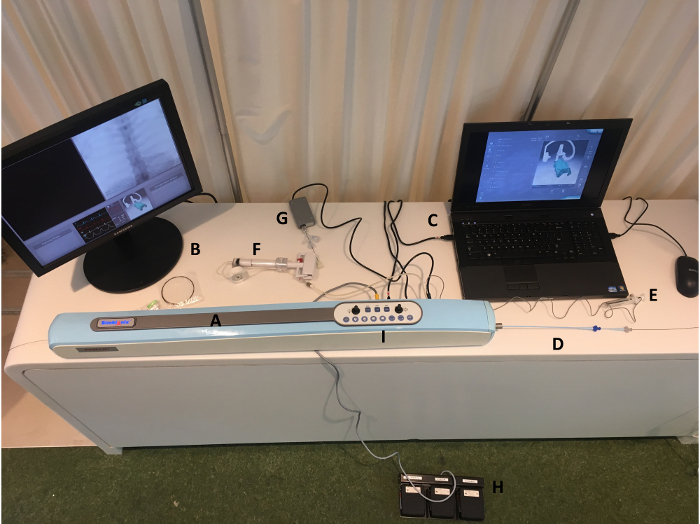
Figure 1: ANGIO Mentor Simulator complete assembly. The setup for the ANGIO Mentor simulator includes the simulator housing (A); an external monitor for image projection (X-ray, angiography) (B); a laptop for interfacing with the Simbionix Software (C); the simulated femoral artery sheath with an outer guide catheter, inner diagnostic microcatheter, and guidewire shown (D); a contrast syringe (E); an insufflator for balloon inflation not used in these patient scenarios (F); a stent delivery device not used in these patient scenarios (G); foot pedals for fluoroscopy, roadmap guidance, and angiographic runs (H); and the operator control panel on the simulator housing where the operator is able to control patient and image intensifier positioning (I). The image was obtained by the authors after setting up the simulator. Please click here to view a larger version of this figure.
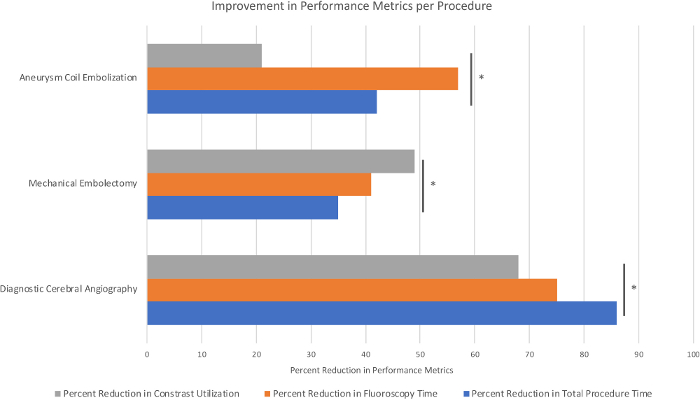
Figure 2: Performance evaluation represented as percent reduction in associated measured procedure metrics with simulator training. Sample size, n = 5 trainees, performing 10 simulations per procedure (Pannell, et al.)2. *p < 0.05 based on analysis of variance (ANOVA) and Tukey’s Honest Significant Difference (HSD) testing. Please click here to view a larger version of this figure.
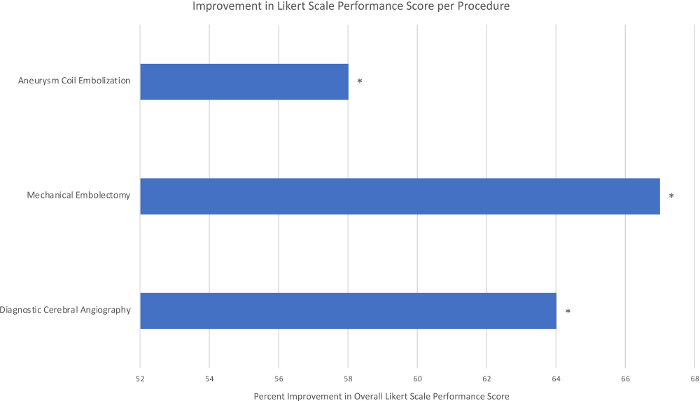
Figure 3: Performance evaluation represented as percent improvement in overall Likert Scale Score with simulator training. Sample size, n = 5 trainees, performing 10 simulations per procedure (Pannell, et al.).2 *p < 0.05 based on analysis of variance (ANOVA) and Tukey’s Honest Significant Difference (HSD) testing. Please click here to view a larger version of this figure.
| Patient Scenario #1 |
| 1) 5-French femoral sheath |
| 2) 0.035 inch guidewire |
| 3) 4-French diagnostic catheter |
| Patient Scenario #2 |
| 1) 0.035 inch guidewire |
| 2) 4-French diagnostic catheter |
| 3) 6-French guide catheter |
| 4) Microcatheter/microwire |
| 5) Coils |
| Patient Scenario #3 |
| 2) 0.035 inch guidewire |
| 3) 4-French diagnostic catheter |
| 4) 6-French guide catheter |
| 6) Microcatheter/microwire |
| 7) Stent retriever device |
Table 1: Materials used for each scenario.
Discussion
Endovascular surgery is an expanding field that offers a minimally invasive treatment approach to a variety of pathologies. The significant risks associated with vascular injuries nonetheless provides unique educational challenges. With advances in simulation-based training, the education of trainees now allows practice in a risk-free environment that mimics real-life cases. Accordingly, endovascular simulation-based training has been shown to consistently improve performance metrics such as procedure time, fluoroscopy time, and contrast volume in a wide range of participants (e.g., patients, medical students, residents, and surgeons)1,3. Commonly utilized simulation training systems include the ANGIO Mentor simulator (Simbionix Ltd., Airport City, Israel) and the VIST-C and VIST G5 simulators (Mentice AB, Gothenburg, Sweden).
Repetitive simulator training with the ANGIO mentor simulator allows for improvements in basic angiography/catheter skills as well as in performance metrics such as total procedure time, fluoroscopy time, contrast utilization, image quality, reduction in unsafe techniques, and overall Likert scale performance scores2,4,5,6. Improvements in such metrics previously reported were attained by following critical steps in the above protocol. Utilizing a stepwise approach, wherein diagnostic procedures are practiced first, allows for acquisition of the basic angiographic skills that are prerequisites for the performance of more complex procedures such as aneurysm coilings, thrombectomies, and embolizations of arteriovenous malformations (AVMs). Selection of the correct toolset is an additional important component of endovascular neurosurgery, and simulator-based learning of tool selection allows trainees to gain practice in material selection in parallel to technical learning.
Advantages of the ANGIO mentor simulator include its accuracy when performing procedural sequences, starting from the initial selection of tools to the use of simulated aspiration catheters and stent retrievers to provide both a visual and tactile educational experience. Additionally, although outside of this protocol, when poor angiographic technique is used, simulated complications that may require additional procedural steps, such as arterial dissections or aneurysm ruptures, can occur. Patient-specific data can also be uploaded to the ANGIO mentor via the PROcedure Rehearsal Studio, allowing the user to rehearse a procedure prior to its real-world performance. Other training systems nonetheless have similar educational value despite minor variations in their specific technical capacities6,7,8. For example, the VIST®-C and VIST® G5 simulators from Mentice also offer training on a variety of cerebrovascular pathologies; the ability to cause and manage complications such as arterial dissections, vasospasm, and aneurysm ruptures; and uploading of patient-specific data. The utility of this system as compared to traditional in vivo clinical training for teaching carotid angiography to experienced non-neurointerventionalists was demonstrated in a prospective, randomized, and blinded trial8.
An important technical component of endovascular neurosurgery is a refined tactile sense to avoid vessel wall dissections and perforations. In parallel to ongoing research into the development of early warning systems for dangerous levels of force buildup at the catheter tip9, haptic feedback is an important but challenging aspect of endovascular neurosurgery simulation. While the ANGIO mentor simulator includes a haptic feedback system that is linked to complications with poor technique or use of excessive force, the tactile fidelity of this system does not completely replicate the real-world experience. Other potential future improvements of the ANGIO mentor simulator include the addition of performance metrics for procedures of higher complexity, such as stent-assisted embolectomies of tandem occlusions and the addition of liquid embolization techniques.
Given its relatively high cost, difficulties in obtaining the ANGIO Mentor simulator or other simulator platforms outside of large academic centers or in developed nations potentially limits the widespread applicability of this protocol. This protocol is nonetheless likely to be very useful for senior medical students, residents, or endovascular neurosurgery trainees with a baseline knowledge of cerebrovascular anatomy and common interventional devices or procedures who generally have an academic affiliation. Of additional note, despite the continued evolution of aspiration catheters that has recently limited the need for mechanical thrombectomy for large vessel occlusions, practicing this skillset in a controlled setting remains critical in preparation for cases refractory to aspiration alone.
Future areas of study with this technology include correlating simulator performance metrics with the real-life technical performance of diagnostic cerebral angiograms, mechanical embolectomies, and aneurysm coil embolizations, as well as patient outcomes. The use of simulation platforms for procedural competence assessments for interventionalist credentialing has also been suggested, although variability in technical discrimination between users of differing experience levels suggests further study is needed prior to utilization of simulators in this setting10.
Divulgazioni
The authors have nothing to disclose.
Acknowledgements
The authors thank all the clinical teams contributing daily to the care of neurovascular patients at UCSD.
Materials
| ANGIO Mentor simulator | Simbionix Ltd., Airport City, Israel | N/a | The setup for the ANGIO Mentor simulator includes the simulator housing as pictured in Figure 1: (A), an external monitor for image projection (x-ray, angiography; B), a laptop for interfacing with the Simbionix Software (C), the simulated femoral artery sheath (with an outer guide-catheter, inner diagnostic microcatheter and guidewire shown; D), a contrast syringe (E), an insufflator for balloon inflation (F), a stent delivery device (G; not used in these patient scenarios), foot pedals for fluoroscopy, roadmap guidance, and angiographic runs (H), and the operator control panel on the simulator housing where the operator is able to control patient and image intensifier positioning (I). |
Riferimenti
- See, K. W., Chui, K. H., Chan, W. H., Wong, K. C., Chan, Y. C. Evidence for Endovascular Simulation Training: A Systematic Review. European Journal of Vascular and Endovascular Surgery. 51 (3), 441-451 (2016).
- Pannell, J. S., et al. Simulator-Based Angiography and Endovascular Neurosurgery Curriculum: A Longitudinal Evaluation of Performance Following Simulator-Based Angiography Training. Cureus. 8 (8), 756 (2016).
- Liebig, T., et al. Metric-Based Virtual Reality Simulation: A Paradigm Shift in Training for Mechanical Thrombectomy in Acute Stroke. Stroke. 49 (7), 239-242 (2018).
- Spiotta, A. M., et al. Diagnostic angiography skill acquisition with a secondary curve catheter: phase 2 of a curriculum-based endovascular simulation program. Journal of Neurointerventional Surgery. 7 (10), 777-780 (2015).
- Spiotta, A. M., Rasmussen, P. A., Masaryk, T. J., Benzel, E. C., Schlenk, R. Simulated diagnostic cerebral angiography in neurosurgical training: a pilot program. Journal of Neurointerventional Surgery. 5 (4), 376-381 (2013).
- Fargen, K. M., et al. Experience with a simulator-based angiography course for neurosurgical residents: beyond a pilot program. Neurosurgery. 73, 46-50 (2013).
- Fargen, K. M., et al. Simulator based angiography education in neurosurgery: results of a pilot educational program. Journal of Neurointerventional Surgery. 4 (6), 438-441 (2012).
- Cates, C., Lönn, L., Gallagher, A. G. Prospective, randomised and blinded comparison of proficiency-based progression full-physics virtual reality simulator training versus invasive vascular experience for learning carotid artery angiography by very experienced operators. BMJ Simulation and Technology Enhanced Learning. 2, 1-5 (2016).
- Guo, J., Jin, X., Guo, S. Study of the Operational Safety of a Vascular Interventional Surgical Robotic System. Micromachines. 9 (3), 119 (2018).
- Tedesco, M. M., et al. Simulation-based endovascular skills assessment: the future of credentialing. Journal of Vascular Surgery. 47 (5), 1008 (2008).

