Retroviral Overexpression of CXCR4 on Murine B-1a Cells and Adoptive Transfer for Targeted B-1a Cell Migration to the Bone Marrow and IgM Production
Summary
Here we describe a method for retroviral overexpression and adoptive transfer of murine B-1a cells to examine in vivo B-1a cell migration and localization. This protocol can be extended for diverse downstream functional assays including quantification of donor B-1a cell localization or analysis of donor cell-derived secreted factors post-adoptive transfer.
Abstract
As cell function is influenced by niche-specific factors in the cellular microenvironment, methods to dissect cell localization and migration can provide further insight on cell function. B-1a cells are a unique B cell subset in mice that produce protective natural IgM antibodies against oxidation-specific epitopes that arise during health and disease. B-1a cell IgM production differs depending on B-1a cell location, and therefore it becomes useful from a therapeutic standpoint to target B-1a localization to niches supportive of high antibody production. Here we describe a method to target B-1a cell migration to the bone marrow by retroviral-mediated overexpression of the C-X-C motif chemokine receptor 4 (CXCR4). Gene induction in primary murine B cells can be challenging and typically yields low transfection efficiencies of 10-20% depending on technique. Here we demonstrate that retroviral transduction of primary murine B-1a cells results in 30-40% transduction efficiency. This method utilizes adoptive cell transfer of transduced B-1a cells into B cell-deficient recipient mice so that donor B-1a cell migration and localization can be visualized. This protocol can be modified for other retroviral constructs and can be used in diverse functional assays post-adoptive transfer, including analysis of donor cell or host cell phenotype and function, or analysis of soluble factors secreted post B-1a cell transfer. The use of distinct donor and recipient mice differentiated by CD45.1 and CD45.2 allotype and the presence of a GFP reporter within the retroviral plasmid could also enable detection of donor cells in other, immune-sufficient mouse models containing endogenous B cell populations.
Introduction
Recent studies have demonstrated considerable immune cell, and specifically B cell, phenotypic and functional heterogeneity depending on cell localization1,2,3,4,5. B-1a cells are one such population with heterogeneous capacity to produce protective IgM antibodies; bone marrow B-1a cells secrete IgM constitutively and contribute significantly to plasma IgM titers6, while peritoneal B-1a cells have low-level IgM secretion at homeostasis and instead can be activated through innate toll-like receptor (TLR) or cytokine-mediated signaling to rapidly proliferate, migrate, and secrete IgM7,8,9,10. B-1a cell IgM antibodies recognize oxidation-specific epitopes (OSE) that are present on pathogens, apoptotic cells, and oxidized LDL, and IgM binding to OSE can prevent inflammatory downstream signaling in diseases like atherosclerosis11. Therefore, strategies to increase IgM production via increasing peritoneal B-1a cell migration to sites like the bone marrow may be therapeutically useful. However, it is important for such strategies to be targeted and cell-type specific, as off-target effects may negatively impact immune function or health.
Here we describe a method for targeted and long-term overexpression of CXCR4 in primary murine B-1a cells and subsequent adoptive transfer to visualize cell migration and functional IgM antibody production (Figure 1). Genetic manipulation of primary B cells is limited by low transfection efficiencies compared to transfection of transformed cell lines. However, as transformed cell lines can significantly deviate from primary cells12,13, the use of primary cells is likely to provide results that more closely align to normal physiology. Several techniques have been described for gene transfer in primary murine B cells, including retroviral transduction, adenoviral transduction, lipofection, or electroporation-based transfection, which have varying levels of efficiency, transience, and impact on cell health13,14,15. The following method utilized retroviral transduction as it yielded adequate gene transfer efficiency of >30% while minimally impacting cell viability. The CXCR4-expressing retrovirus was generated using the previously described retroviral construct murine stem cell virus-internal ribosomal entry site-green fluorescent protein (MSCV-IRES-GFP; MigR1)16, into which the mouse CXCR4 gene was sub-cloned4. MigR1 (control(Ctl)-GFP) and CXCR4-GFP retroviral particles were generated using calcium phosphate transfection as described in previously published protocols4,14.
Successfully transduced B-1a cells were then intravenously transferred into lymphocyte-deficient Rag1-/- mice. Both donor and recipient mice additionally contained knockout of the apolipoprotein E (ApoE) gene, which results in increased OSE accumulation and atherosclerosis, thereby providing a model for in vivo B-1 cell activation and IgM production. Moreover, donor and recipient mice differed in CD45 allotype; donor B-1 cells came from CD45.1+ ApoE-/- mice and were transferred into Rag1-/- CD45.2+ ApoE-/- recipients. This allowed differentiation of donor CD45.1 from recipient CD45.2 B cells post-transfer without the need to additionally stain for B cell markers during flow cytometry analysis. The results provided here demonstrate that targeted CXCR4 overexpression on B-1a cells associates with increased ability of B-1a cells to migrate to the bone marrow, which associates with increased plasma anti-OSE IgM. We additionally provide a method for the enrichment of peritoneal B-1 cells through negative selection and demonstrate the requirement of B-1 cell activation for efficient transduction. This method can be adapted for other retroviral constructs to study the effect of protein overexpression on B-1a cell migration, phenotype, or function. Moreover, the use of CD45.1 versus CD45.2 allotype distinction could theoretically allow transfer into other immune-sufficient murine models containing endogenous B cells.
Protocol
All animal protocols were approved by the Animal Care and Use Committee at the University of Virginia.
1. Magnetic separation and enrichment of peritoneal B-1 cells
- Euthanize a 12−14-week-old, male, CD45.1+ApoE-/- mouse using CO2.
- Make a superficial cut in the abdomen using straight surgical scissors and peel back skin using curved scissors to expose the peritoneal wall. Flush peritoneal cavity with 10 mL of 37 °C RPMI-1640 medium using a 10 mL syringe and 25 G needle. Shake mouse to disengage cells by grasping the tail and moving the mouse side to side thoroughly for 15−20 s.
NOTE: Massaging the lavaged peritoneum can also maximize cell recovery. - Collect peritoneal fluid using a 10 mL syringe and 25 G needle by drawing up fluid at the lower right side of the peritoneum just above the level of the hip, near the intestines. Avoid disrupting epidydimal fat depots and underlying organs. Avoid drawing fluid from the mouse’s left side as the omental fat can easily be drawn into the syringe.
- Once ~6−7 mL of fluid is collected, dispense into a 50 mL conical tube placed on ice. Next, elevate the mouse vertically by holding the peritoneal wall above the diaphragm using forceps so that any remaining fluid remains at the bottom of the peritoneal cavity. Make a small cut in the peritoneal wall using surgical scissors above the liver (make sure not to cut the liver) and collect any remaining peritoneal fluid using a glass pipet and bulb.
- Pool peritoneal washout cells from all CD45.1+ApoE-/- mice (n = 15−20) into 50 mL conical tubes, and store on ice.
NOTE: For calculating the number of mice needed: B-1a cells comprise 5−10% of the total peritoneal population and yield roughly 2.5−5 x 105 B-1a cells per mouse in authors’ hands. Transduction efficiency is ~30−40%, as shown below. - Count live cells using a viability dye such as trypan blue and a hemocytometer (dilute samples 1:5 in trypan blue and load 10 µL into the hemocytometer chamber). Pool cells at up to 1 x 108 cells per tube. Centrifuge cells at 400 x g for 5 min at 4 °C, then aspirate supernatant.
- Resuspend up to 1 x 108 cells in 1 mL of anti-CD16/CD32 antibody (Table of Materials) diluted 1:50 in assay buffer (1x phosphate buffered saline [PBS], 0.5% bovine serum albumin [BSA], 2 mM EDTA) in order to block Fc receptors. Scale as necessary based on cell count. Incubate on ice for 10 min at 4 °C.
- Prepare a 2x master mix of the biotinylated antibodies (Table 1) in assay buffer for depletion. The 2x master mix accounts for the volume of the liquid the cells are already in when incubating with anti-CD16/CD32. For example, if cells are incubating in 500 µL of diluted anti-CD16/CD32, then add 500 µL of 2x master mix containing 10 µL of biotinylated Ter119, Gr-1, CD23, and NK1.1 antibodies, and 25 µL of biotinylated F4/80 antibody to achieve the final concentrations given in Table 1. Add 2x master mix to cells and stain for 20 min at 4 °C.
- Wash cells with 5 mL of assay buffer and centrifuge at 400 x g for 5 min at 4 °C and aspirate supernatant.
- Resuspend and incubate cells with anti-biotin microbeads (Table of Materials) diluted in assay buffer as per the concentration and protocol recommended by the manufacturer.
- Wash with 5 mL of assay buffer and centrifuge at 400 x g for 5 min at 4 °C. Aspirate supernatant and resuspend up to 1 x 108 cells in 500 µL of assay buffer.
- Prime magnetic selection columns (Table of Materials) with 3 mL of assay buffer. Transfer cells onto primed magnetic selection columns and collect the eluent containing enriched B-1 cells in a 15 mL conical tube on ice. Wash the magnetic selection column with additional assay buffer till the overall volume collected is 10 mL.
NOTE: An aliquot of the pre-purified and post-purified cell fractions should be separately analyzed by flow cytometry for purification efficiency by staining for CD19+ B cells, F480+ macrophages, and CD5+ T cells as shown in Figure 2. - Count the post-purified cell fraction (the eluent containing enriched B-1 cells) by counting live cells as in step 1.6, and resuspend at 1 x 106 cells/mL in B cell culture medium (RPMI-1640, 10% heat-inactivated fetal bovine serum [FBS], 10 mM HEPES, 1x non-essential amino acids, 1 mM sodium pyruvate, 50 µg/mL gentamicin, 55 µM β-mercaptoethanol).
2. Peritoneal B-1 cell stimulation
- Split pooled cells for the two transduced conditions (Ctl-GFP and CXCR4-GFP), while setting aside and plating at least 10 x 106 cells for a non-transduced control.
- Plate up to 150 µL (150,000 cells) per well into 96-well round-bottom plates.
- Add 100 nM TLR9 agonist ODN1668 to all wells to stimulate cell proliferation.
- Incubate for 16−18 h at 37 °C, 5% CO2.
3. Retroviral transduction of peritoneal B cells
- Generate Ctl-GFP (MigR1) and CXCR4-GFP retroviral particles using calcium phosphate transfection as described in previously published protocols14.
NOTE: This will take several days, so prepare and titer retroviral stocks and store aliquots at -80 °C prior to starting this protocol. - Thaw retrovirus stocks on ice. Use immediately and do not re-freeze as virus titer significantly diminishes during freeze-thaw cycles. Add Ctl-GFP or CXCR4-GFP retroviral supernatants at 20:1 multiplicity of infection (MOI) to cells, in the presence of 8 µg/mL polybrene and fresh β-mercaptoethanol at 55 µM final concentration. Do not add viral stocks to the cells set aside for the non-transduced control.
- Perform spinfection by centrifuging plates at 800 x g for 90 min at room temperature.
- Incubate plates with retrovirus at 37 °C, 5% CO2 for an additional 3 h.
- Harvest and replate cells in fresh B cell medium and incubate at 37 °C, 5% CO2 overnight.
4. Cell sorting of transduced peritoneal B-1a cells
- Harvest cultured cells and pool into three separate 50 mL conical tubes for each condition: non-transduced, Ctl-GFP transduced, and CXCR4-GFP transduced.
- Count live cells as in step 1.6, then centrifuge at 400 x g for 5 min at 4 °C and aspirate supernatant.
- Resuspend cells at 100,000 cells/µL in sort buffer (PBS + 1% BSA) containing 1:50 anti-CD16/CD32 antibody (Table of Materials) in order to block Fc receptors. Incubate on ice for 10 min at 4 °C.
- Aliquot cells for compensation controls (~30,000 cells per compensation control): for unstained and single stain controls aliquot from non-transduced sample and for GFP single stain control aliquot from transduced samples.
NOTE: Commercially available compensation beads can alternatively be used for single stain controls if non-transduced cell number is low. - Prepare a 2x antibody master mix containing the fluorophore-conjugated antibodies in sort buffer (Table 1). Add 2x master mix to non-transduced, CTL-GFP transduced, and CXCR4-GFP transduced samples. Add individual antibodies to single stain controls. Incubate for 20 min at 4 °C in the dark.
NOTE: The 2x master mix accounts for the volume of liquid the cells are already in when incubating with anti-CD16/CD32, as in step 1.8. An aliquot of cells from each condition can separately be stained for CXCR4 to confirm CXCR4 overexpression. - Wash samples with 1 mL of sort buffer and strain through 70 µm filters into polypropylene tubes.
- Centrifuge at 400 x g for 5 min at 4 °C and aspirate supernatant. Resuspend samples at 50,000 cells/µL in sort buffer.
- Prior to cell sorting prepare labeled fluorescence-activated cell sorting (FACS) collection tubes containing 1 mL of collection medium (RPMI-1640, 20% heat-inactivated FBS, 10 mM HEPES, 1x non-essential amino acids, 1 mM sodium pyruvate, 50 µg/mL gentamicin, 55 µM β-mercaptoethanol) for each population to be sorted.
- Prior to running samples on the cell sorter add 2x DAPI (prepared as 1:5000 dilution in sort buffer) for dead cell discrimination.
- Sort GFP+ B-1a cells into FACS tubes containing collection medium as DAPI– CD19+ GFP+ B220mid-lo CD23– IgM+ CD5+ cells from the CTL-GFP and CXCR4-GFP samples. Use the non-transduced sample to set the GFP+ gate and to sort DAPI– CD19+ GFP– B220mid-lo CD23– IgM+ CD5+ non-transduced B-1a cells.
NOTE: Alternatively, non-transduced cells can also be sorted from the CTL-GFP and CXCR4-GFP samples by separately gating B-1a cells within the GFP- fraction. Transduced or non-transduced B-1b cells can also be sorted using this sort strategy as DAPI– CD19+ GFP+/- B220mid-lo CD23– IgM+ CD5– cells.
5. Adoptive transfer
- After cell sorting, centrifuge cells at 400 x g for 5 min at 4 °C and aspirate supernatant carefully.
- Resuspend cells in cold sterile 1x PBS at 1,000 cells/µL.
- Anesthetize male Rag1-/- ApoE-/- mice using isoflurane and inject 100 µL (100,000 cells) per mouse via intravenous retro-orbital or tail vein injection using an ultra-fine insulin syringe. Inject a few mice with 1x PBS as a control.
6. Quantification of donor cells and plasma IgM
- At the desired time post-adoptive transfer, analyze transferred cells in recipient mice by bone marrow and spleen tissue harvest4 and flow cytometry. Quantify donor cell localization and CXCR4 overexpression by staining for CD45.1, CD45.2, and CXCR4 and analyze flow cytometry results using a software such as Flowjo.
NOTE: See Table 1 for antibodies used in this step. Ensure not to use an antibody conjugate that fluoresces in the FITC channel as GFP will be present on transduced donor cells. - Isolate plasma by adding 10 µL of 0.5 M EDTA to whole blood collected via cardiac puncture at the time of animal sacrifice. Centrifuge whole blood at 7,000 x g and aliquot plasma into separate 1.5 mL centrifuge tubes. Store at -80 °C until performing analysis of secreted factors such as IgM by ELISA4.
Representative Results
An overview of the protocol is given in Figure 1. Figure 2 displays enrichment of peritoneal B-1a cells after magnetic depletion of other peritoneal cell types. Live singlet cells in the post-depletion fraction have a greater proportion of CD19+ B cells compared to F4/80+ macrophages, lack CD5hi CD19– T cells, and contain an increased frequency of CD19+ CD5mid B-1a cells compared to the pre-depletion fraction. Figure 3 displays the requirement of B cell activation for successful retroviral B cell transduction, and a dose-dependent increase in the frequency of successfully transduced GFP+ B cell subsets with increasing virus MOI using Ctl-GFP retrovirus. Table 2 displays increased transduction efficiency using 96 well round-bottom plates compared to 24-well or 6-well plates. Figure 4 displays successful CXCR4 overexpression (>40%) on B-1 cells and increased B-1 cell migration towards CXCL12 in vitro after transduction with CXCR4-GFP retrovirus, without a significant impact on B cell viability. Figure 5 displays the gating strategy for sorting of live, singlet, CD19+ CD23- IgM+ CD5+ B-1a cells from either a non-transduced condition (GFP-), or the two transduced conditions (GFP+). Note that CD23+ B-2 cells are not present in these samples due to prior magnetic depletion. Transduced live, singlet, CD19+ CD23- IgM+ CD5- B-1b cells can also be sorted using this gating strategy. Figure 6 displays transferred CD45.1+ donor cells and sustained CXCR4 overexpression on donor cells recovered from bone marrow and spleen of CD45.2 recipient mice 17 weeks post-cell transfer. Table 3 displays a positive association between CXCR4 expression and donor cell localization to the bone marrow, but not spleen. Table 4 displays a positive association between donor cell number in the bone marrow and plasma amount of anti-MDA-LDL IgM.
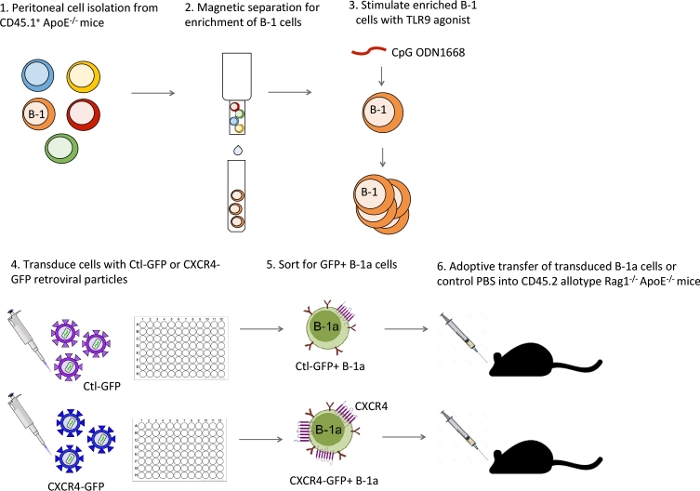
Figure 1: Schematic of experimental design for retroviral transduction and adoptive transfer. Peritoneal cells isolated from CD45.1 allotype mice are enriched for B-1 cells through magnetic depletion using biotinylated antibodies and anti-biotin microbeads. Enriched peritoneal B-1 cells are activated to stimulate cell proliferation with TLR9 agonist CpG oligodeoxynucleotide. Activated cells are transduced with Ctl-GFP or CXCR4-GFP retroviral particles. Successfully transduced GFP+ B-1a cells are sorted using FACS and adoptively transferred into CD45.2 allotype host mice. Please click here to view a larger version of this figure.
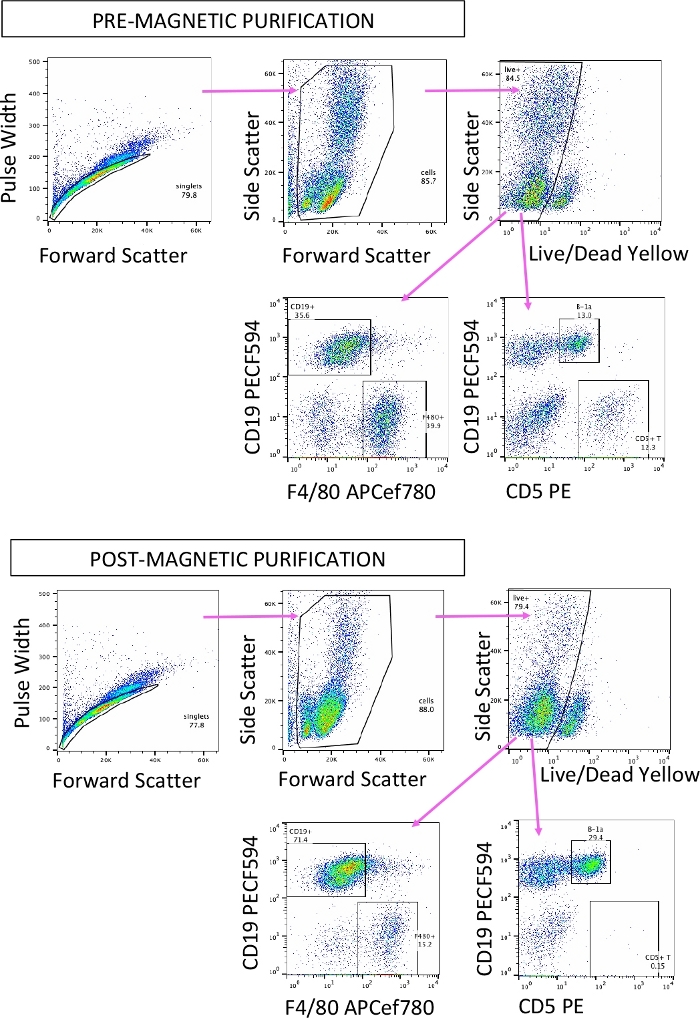
Figure 2: Enrichment for peritoneal B-1 cells. Representative flow cytometry plots of peritoneal cells pre-magnetic enrichment (top) and post-magnetic enrichment (bottom) for CD19+ B cells. CD19+ F4/80- cells are B cells, CD19- F4/80+ cells are macrophages, CD19+ CD5mid cells are B-1a cells, and CD19- CD5hi cells are T cells. Please click here to view a larger version of this figure.
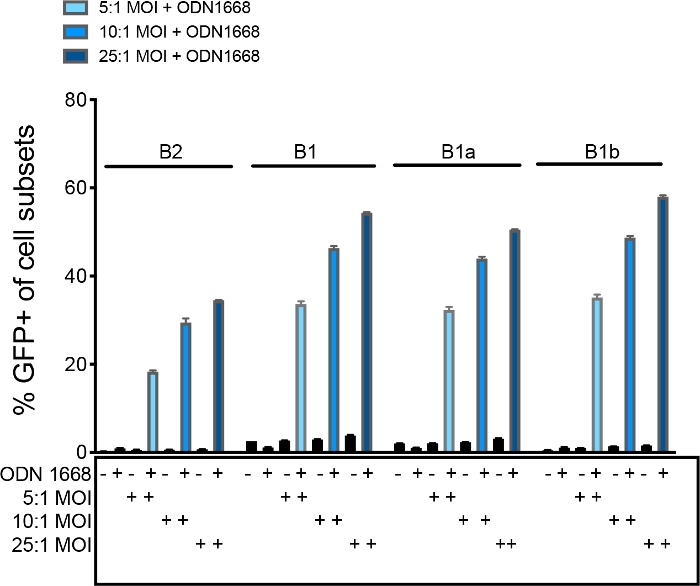
Figure 3: Retroviral transduction requires B cell activation. Peritoneal B cells were transduced with Ctl-GFP retrovirus at 5:1, 10:1, or 25:1 MOI or left non-transduced in the presence or absence of TLR9 agonist CpG ODN1668. The frequency of successfully transduced GFP+ B2, B1, B-1a, or B-1b cells was quantified by flow cytometry 18 h post-transduction. Error bars represent mean ± SEM. Please click here to view a larger version of this figure.
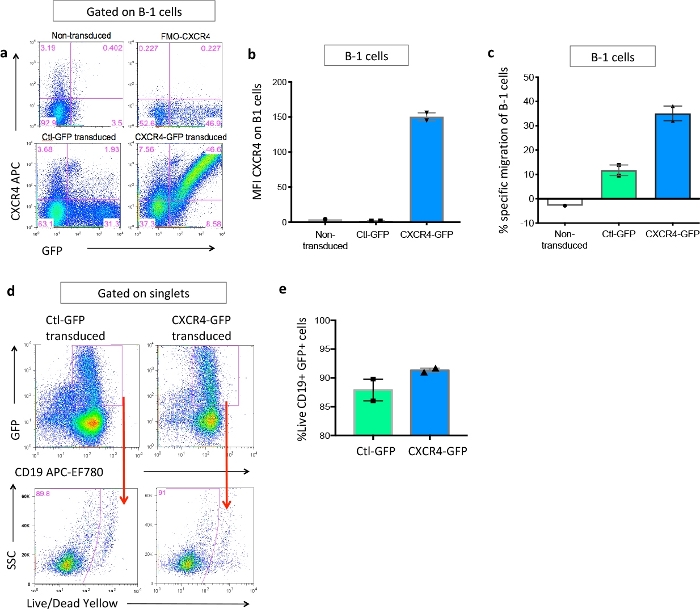
Figure 4: Confirmation of CXCR4 overexpression and increased B-1a migration in vitro. Peritoneal B cells from ApoE-/- mice with B cell-specific deficiency of CXCR4 were isolated and transduced with Ctl-GFP or CXCR4-GFP retrovirus, or cultured without transduction. (a) Representative flow plots of CXCR4 and GFP expression on B-1 cells from non-transduced (upper left), Ctl-GFP transduced (lower left), or CXCR4-GFP transduced (lower right) conditions. FMO-CXCR4 (upper right) used to set CXCR4 positive gate. (b) Quantification of the MFI of CXCR4 on GFP+ B-1 cells from non-transduced (n = 1), Ctl-GFP transduced (n = 2), or CXCR4-GFP transduced (n = 2) conditions. (c) Frequency of non-transduced (n = 1), Ctl-GFP transduced (n = 2), or CXCR4-GFP transduced (n = 2) B-1 cells that migrated towards CXCL12 as a percentage of the total number of B-1 cells loaded in transwell. (d) Representative gating strategy for quantification of viable cells within the successfully transduced B cell population (CD19+GFP+). (e) Frequency of live B cells after transduction with Ctl-GFP (n = 2) or CXCR4-GFP retrovirus (n = 2). Error bars represent mean ± SEM. This figure has been modified from our previous publication4. Please click here to view a larger version of this figure.
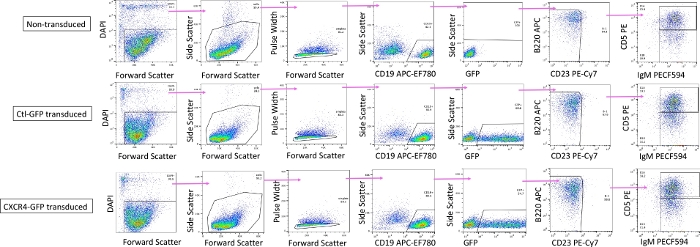
Figure 5: Gating strategy for sorting transduced GFP+ B-1a cells. Representative flow cytometry plots for sorting GFP+ or GFP- B-1a cells from a non-transduced sample (top), a Ctl-GFP transduced sample (middle), and a CXCR4-GFP transduced sample (bottom). B-1a cells defined as live, singlet, CD19+ CD23- IgM+ CD5+ cells. Please click here to view a larger version of this figure.
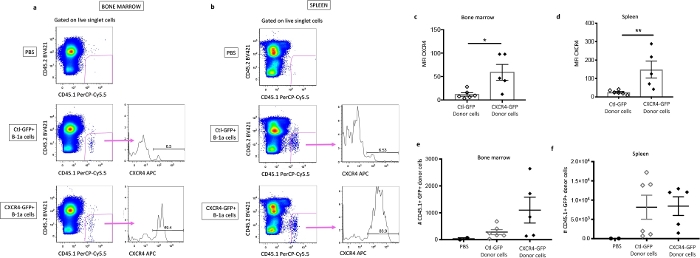
Figure 6: Quantification of transferred donor cells. Representative flow cytometry plots displaying CD45.1+ CD45.2- donor cells from one PBS control (top), one Ctl-GFP+ B-1a cell recipient (middle), and one CXCR4-GFP+ B-1a cell recipient (bottom), and subsequent analysis of CXCR4 expression on donor cells in bone marrow (a), or spleen (b). Quantification of CXCR4 expression (mean fluorescence intensity, MFI) on donor cells from bone marrow (c) or spleen (d). Quantification of the number of donor cells recovered in bone marrow (e) or spleen (f) of recipients. *P < 0.05 or **P < 0.01 by Mann-Whitney test. Error bars represent mean ± SEM. This figure has been modified from our previous publication4. Please click here to view a larger version of this figure.
| Protocol step | Antibody | Final concentration |
| Step 1.8 | Ter119 biotin | 1 µL per 100 µL final volume |
| CD3e biotin | 1 µL per 100 µL final volume | |
| Gr-1 biotin | 1 µL per 100 µL final volume | |
| CD23 biotin | 1 µL per 100 µL final volume | |
| NK1.1 biotin | 1 µL per 100 µL final volume | |
| F4/80 biotin | 2.5 µL per 100 µL final volume | |
| Antibody | Final concentration | |
| Step 4.5 | CD5 PE | 1 µL per 100 µL final volume |
| IgM PECF594 | 1 µL per 100 µL final volume | |
| CD23 PECy7 | 1 µL per 100 µL final volume | |
| B220 APC | 1 µL per 100 µL final volume | |
| CD19 APCef780 | 1 µL per 100 µL final volume | |
| Antibody | Final concentration | |
| Step 6.1 | CD45.1 PerCP Cy5.5 | 1 µL per 100 µL final volume |
| CD45.2 BV421 | 1 µL per 100 µL final volume | |
| CXCR4 APC | 2.5 µL per 100 µL final volume |
Table 1: Antibodies and their final concentrations used in the protocol.
| Condition | %GFP+ of total population | %GFP+ of CD19+ B cells |
| 96-well round-bottom plate | 30.9% | 52.7% |
| 24-well plate | 8.4% | 21.2% |
| 6-well plate | 16.2% | 27.3% |
Table 2: Plate optimization. Frequency of successfully transduced total GFP+ cells or GFP+ CD19+ B cells after transduction of 6 x 106 enriched peritoneal B-1 cells in either a 96-well round-bottom plate (40 wells at 150,000 cells per well), a 24-well plate (6 wells at 1 x 106 cells per well), or a 6-well plate (3 wells at 2 x 106 cells per well) at a 20:1 MOI with Ctl-GFP retrovirus.
| Variable | MFI of CXCR4 on donor B-1a cells | |
| r-value | p-value | |
| # of donor B-1a cells in bone marrow | 0.71 | *0.014 |
| # of donor B-1a cells in spleen | 0.43 | 0.18 |
Table 3: Association between CXCR4 expression on donor cells and donor cell localization. The mean fluorescence intensity (MFI) of CXCR4 on donor B-1a cells correlated with the number of donor B-1a cells in bone marrow or spleen of Rag1-/- ApoE-/- recipient mice 17 weeks post-adoptive transfer. Data presented as correlation coefficient (r) and statistical significance (p). This table has been modified from our previous publication4.
| Variable | # of donor cells in bone marrow | |
| r-value | p-value | |
| Plasma anti-MDA-LDL IgM | 0.67 | *0.028 |
| Plasma E06/T15 IgM | 0.56 | 0.076 |
| Plasma 1,3-dextran IgM | 0.29 | 0.39 |
Table 4: Association between donor cell localization and plasma amount of anti-OSE IgM. The number of donor B-1a cells in bone marrow of Rag1-/- ApoE-/- recipient mice 17 weeks post-adoptive transfer correlated with circulating amount of anti-MDA-LDL IgM, E06/T15 IgM, or anti-1,3-dextran IgM. Data presented as correlation coefficient (r) and statistical significance (p). This table has been modified from our previous publication4.
Discussion
The method provided here enables stable and relatively efficient primary B-1a cell gene delivery, in vivo adoptive transfer, and identification and localization of injected cells. Cells were able to be detected 17 weeks post-cell transfer and retained increased CXCR4 expression. Retrovirus-mediated delivery yielded 30-40% transduction efficiency of primary murine B-1a cells with minimal impact on cell viability in our hands (Figure 4e). This is in line with results from a previous study by Moghimi and colleagues which compared techniques for gene transfer into primary murine B cells including retroviral infection, adenoviral infection, nucleofection, or lipofectamine15. However, we found that the range of CXCR4 overexpression varied considerably within recipients receiving CXCR4-GFP transduced B-1a cells (Figure 6c,d). Therefore, we utilized associative analysis to demonstrate that increased CXCR4 expression correlated with increased B-1a migration and localization to the bone marrow, which associated with increased plasma IgM (Table 3 and Table 4).
Limitations of this method include the large number of mice required to get sufficient numbers of successfully transduced B-1a cells, and the variability in transduction efficiency from one experiment to another. Transduction efficiency is improved by higher titers of viral stocks, which should be at least 2 x 107 infectious particles/mL14. The use of older mice, aged 12−16 weeks can also improve peritoneal B-1 cell yield, as peritoneal B-1 cell numbers increase with age17.
It is also important to note that the amount of IgM secreted by transduced B-1a cells post-adoptive transfer was ~5-fold less than the amount secreted by non-transduced B-1a cells after adoptive transfer into the same Rag1-/- ApoE-/- model (data not shown). This may be due to the requirement of B-1 cell activation with TLR9 agonist prior to retroviral transduction (Figure 3), which may limit secondary activation and IgM production in response to OSE in vivo post-adoptive transfer. Therefore, for studies that require robust IgM production by transferred B-1a cells, alternative gene transfer techniques that do not require prior B-1a cell activation, such as lentiviral delivery18,19, may prove useful. Alternatively, modifications to this protocol that involve activation strategies to induce proliferation but not B-1a differentiation into IgM-secreting cells might also be sufficient for successful retroviral transduction without impacting secondary B cell activation. IL-5 is an important cytokine mediating B-1a cell proliferation and survival, and may be an effective alternative to TLR9 stimulation20,21.
Prior studies have utilized splenic B cells isolated through positive or negative selection strategies using antibodies against B220 (B cell marker) or Thy1.2 (T cell marker)13,14. However, B220+ splenic B cells are a heterogeneous population containing B-1 and B-2 cell subsets. Moreover, B-1 cell frequency within the total splenic CD19+ B cell population is low (1−2%). In contrast, this method utilizes the peritoneal cavity as a B-1 cell source for transduction, as B-1 cells comprise 60−70% of total CD19+ B cells in this compartment22, and uses CD23 as a marker for depleting peritoneal B-2 cells. Subsequent sorting of successfully transduced B-1a cells based on GFP, CD19, B220, CD23, IgM, and CD5 expression further allows transfer of a more specifically defined cell type. The magnetic depletion strategy to enrich peritoneal B-1 cells effectively depleted T cells, and reduced F4/80 peritoneal macrophage frequency by ~50% in our hands (Figure 2), though further optimization and troubleshooting of this critical step could increase transduction efficiency. For example, using a higher concentration of biotinylated F4/80 antibody for better macrophage depletion might further increase B-1a cell transduction efficiency, as there would be less “off-target” retroviral transduction of other cell types. The use of 96-well round-bottom plates for transduction, instead of flat-bottom 24-well or 6-well plates additionally considerably improved transduction efficiency (Table 2), though increases handling and pipetting time.
Overall, this method provides a useful proof-of-concept approach for determining whether targeted gene delivery to B-1a cells can alter B-1a cell localization and functional IgM production. Future applications of this technique could include ex vivo delivery of retroviral constructs targeting other proteins, and adoptive transfer to determine its effect on donor or host cell processes in vivo, including cell survival, migration, proliferation, or function. Adoptive transfer into immunocompetent hosts, rather than lymphocyte-deficient hosts, would also be possible with this technique since donor cells (CD45.1+ GFP+) could be differentiated from host cells (CD45.2+ GFP-). Targeting other chemokine receptors using this method could further support the hypothesis that targeting B-1a cell migration towards niches permissive of high IgM production can effectively boost levels of protective IgM.
Divulgazioni
The authors have nothing to disclose.
Acknowledgements
This work was supported by 1R01 HL107490, 1R01 HL136098, Project 3 of P01 HL055798, P01 HL136275-01 (C.A. McNamara), and R01GM100776 (T.P. Bender). A. Upadhye was supported by American Heart Association Pre-doctoral fellowship 16PRE30300002 and 5T32AI007496-20. We thank Joanne Lannigan, Mike Solga, and Claude Chew from the University of Virginia Flow Cytometry Core for their excellent technical assistance.
Materials
| 70 micron filter caps | Falcon | 352235 | |
| anti-biotin microbeads | Miltenyi Biotec | 130-090-485 | |
| anti-CD16/CD32, or Fc block | Life Technologies | MFCR00 | |
| B220 APC | eBioscience | 17-0452-83 | Clone: RA3-6B2 |
| Beta-mercaptoethanol | Gibco | 21985-023 | |
| CD19 APCef780 | eBioscience | 47-0193-82 | Clone: eBio1D3 |
| CD23 biotin | eBioscience | 13-0232-81 | Clone: B3B4 |
| CD23 PECy7 | eBioscience | 25-0232-82 | Clone: B3B4 |
| CD3e biotin | eBioscience | 13-0033-85 | Clone: eBio500A2 |
| CD45.1 ApoE-/- mice | N/A | N/A | Bred in house |
| CD45.1 PerCP-Cy5.5 | BD Biosciences | 560580 | Clone: A20 |
| CD45.2 BV421 | BD Biosciences | 562895 | Clone: 104 |
| CD45.2 Rag1-/- ApoE-/- mice | N/A | N/A | Bred in house |
| CD5 PE | eBioscience | 12-0051-83 | Clone: 53-7.3 |
| Ctl-GFP retrovirus | N/A | N/A | Generated in house using GFP-expressing retroviral plasmid MigR1 provided by Dr. T.P. Bender |
| CXCR4 APC | eBioscience | 17-9991-82 | Clone: 2B11 |
| CXCR4-GFP retrovirus | N/A | N/A | Generated in house by cloning mouse CXCR4 into MigR1 retroviral plasmid |
| F4/80 biotin | Life Technologies | MF48015 | Clone: BM8 |
| Flowjo Software v. 9.9.6 | Treestar Inc. | License required | |
| Gentamicin | Gibco | 15710-064 | |
| Gr-1 biotin | eBioscience | 13-5931-82 | Clone: RB6-8C5 |
| heat-inactivated fetal bovine serum | Gibco | 16000-044 | |
| HEPES | Gibco | 15630-080 | |
| IgM PECF594 | BD Biosciences | 562565 | Clone: R6-60.2 |
| Insulin syringes | BD Biosciences | 329461 | |
| Isoflurane | Henry Schein Animal Health | 029405 | |
| Live/Dead Yellow | Life Technologies | L34968 | |
| LS columns | Miltenyi Biotec | 130-042-401 | |
| NK1.1 biotin | BD Biosciences | 553163 | Clone: PK136 |
| Non-essential amino acids | Gibco | 11140-050 | |
| ODN 1668 | InvivoGen | tlrl-1668 | |
| PBS | Gibco | 14190-144 | |
| RPMI-1640 | Gibco | 11875-093 | |
| Sodium pyruvate | Gibco | 11360-070 | |
| Ter119 biotin | eBioscience | 13-5921-82 | Clone: Ter119 |
Riferimenti
- Baumgarth, N. B-1 Cell Heterogeneity and the Regulation of Natural and Antigen-Induced IgM Production. Frontiers in Immunology. 7, 324 (2016).
- Holodick, N. E., Vizconde, T., Rothstein, T. L. B-1a cell diversity: nontemplated addition in B-1a cell Ig is determined by progenitor population and developmental location. Journal of Immunology. 192 (5), 2432-2441 (2014).
- Prohaska, T. A., et al. Massively Parallel Sequencing of Peritoneal and Splenic B Cell Repertoires Highlights Unique Properties of B-1 Cell Antibodies. Journal of Immunology. 200 (5), 1702-1717 (2018).
- Upadhye, A., et al. Diversification and CXCR4-Dependent Establishment of the Bone Marrow B-1a Cell Pool Governs Atheroprotective IgM Production Linked to Human Coronary Atherosclerosis. Circulation Research. 125 (10), 55-70 (2019).
- Yang, Y., et al. Distinct mechanisms define murine B cell lineage immunoglobulin heavy chain (IgH) repertoires. Elife. 4, 09083 (2015).
- Choi, Y. S., Dieter, J. A., Rothaeusler, K., Luo, Z., Baumgarth, N. B-1 cells in the bone marrow are a significant source of natural IgM. European Journal of immunology. 42 (1), 120-129 (2012).
- Holodick, N. E., Tumang, J. R., Rothstein, T. L. Immunoglobulin secretion by B1 cells: Differential intensity and IRF4-dependence of spontaneous IgM secretion by peritoneal and splenic B1 cells. European Journal of Immunology. 40 (11), 3007-3016 (2010).
- Moon, H., Lee, J. G., Shin, S. H., Kim, T. J. LPS-induced migration of peritoneal B-1 cells is associated with upregulation of CXCR4 and increased migratory sensitivity to CXCL12. Journal of Korean Medical Science. 27 (1), 27-35 (2012).
- Wang, H., et al. Expression of plasma cell alloantigen 1 defines layered development of B-1a B-cell subsets with distinct innate-like functions. Proceedings of the National Academy of Sciences of the United States of America. 109 (49), 20077-20082 (2012).
- Yang, Y., Tung, J. W., Ghosn, E. E., Herzenberg, L. A., Herzenberg, L. A. Division and differentiation of natural antibody-producing cells in mouse spleen. Proceedings of the National Academy of Sciences of the United States of America. 104 (11), 4542-4546 (2007).
- Tsiantoulas, D., Gruber, S., Binder, C. J. B-1 cell immunoglobulin directed against oxidation-specific epitopes. Frontiers in Immunology. 3, 415 (2012).
- Kaur, G., Dufour, J. M. Cell lines: Valuable tools or useless artifacts. Spermatogenesis. 2 (1), 1-5 (2012).
- McMahon, S. B., Norvell, A., Levine, K. J., Monroe, J. G. Transient transfection of murine B lymphocyte blasts as a method for examining gene regulation in primary B cells. Journal of Immunological Methods. 179 (2), 251-259 (1995).
- Lin, K. I., Calame, K. Introduction of genes into primary murine splenic B cells using retrovirus vectors. Methods in Molecular Biology. 271, 139-148 (2004).
- Moghimi, B., Zolotukhin, I., Sack, B. K., Herzog, R. W., Cao, O. High Efficiency Ex Vivo Gene Transfer to Primary Murine B Cells Using Plasmid or Viral Vectors. Journal of Genetic Syndromes and Gene Therapy. 2 (103), 1000103 (2011).
- DeKoter, R. P., Lee, H. J., Singh, H. PU.1 regulates expression of the interleukin-7 receptor in lymphoid progenitors. Immunity. 16 (2), 297-309 (2002).
- Holodick, N. E., Vizconde, T., Hopkins, T. J., Rothstein, T. L. Age-Related Decline in Natural IgM Function: Diversification and Selection of the B-1a Cell Pool with Age. The Journal of Immunology. 196 (10), 4348-4357 (2016).
- Laurie, K. L., et al. Cell-specific and efficient expression in mouse and human B cells by a novel hybrid immunoglobulin promoter in a lentiviral vector. Gene Therapy. 14 (23), 1623-1631 (2007).
- Warncke, M., et al. Efficient in vitro transduction of naive murine B cells with lentiviral vectors. Biochemical and Biophysical Research Communications. 318 (3), 673-679 (2004).
- Moon, B. G., Takaki, S., Miyake, K., Takatsu, K. The role of IL-5 for mature B-1 cells in homeostatic proliferation, cell survival, and Ig production. Journal of Immunology. 172 (10), 6020-6029 (2004).
- Takatsu, K., Kouro, T., Nagai, Y. Interleukin 5 in the link between the innate and acquired immune response. Advances in Immunology. 101, 191-236 (2009).
- Baumgarth, N. The double life of a B-1 cell: self-reactivity selects for protective effector functions. Nature Reviews Immunology. 11 (1), 34-46 (2011).

