Monitoring Influenza Virus Survival Outside the Host Using Real-Time Cell Analysis
Summary
Reported here is a protocol for the quantification of infectious viral particles using real-time monitoring of electrical impedance of infected cells. A practical application of this method is presented by quantifying influenza A virus decay under different physicochemical parameters mimicking environmental conditions.
Abstract
Methods for virus particle quantification represent a critical aspect of many virology studies. Although several reliable techniques exist, they are either time-consuming or unable to detect small variations. Presented here is a protocol for the precise quantification of viral titer by analyzing electrical impedance variations of infected cells in real-time. Cellular impedance is measured through gold microelectrode biosensors located under the cells in microplates, in which magnitude depends on the number of cells as well as their size and shape. This protocol allows real-time analysis of cell proliferation, viability, morphology and migration with enhanced sensitivity. Also provided is an example of a practical application by quantifying the decay of influenza A virus (IAV) submitted to various physicochemical parameters affecting viral infectivity over time (i.e., temperature, salinity, and pH). For such applications, the protocol reduces the workload needed while also generating precise quantification data of infectious virus particles. It allows the comparison of inactivation slopes among different IAV, which reflects their capacity to persist in given environment. This protocol is easy to perform, is highly reproducible, and can be applied to any virus producing cytopathic effects in cell culture.
Introduction
The transmission of a virus relies on the combination of several factors. For a virus secreted in the environment, its transmission also depends on the ability to persist in conditions outside of the host. Studying viral inactivation in general is, therefore, a crucial step in helping national health authorities and policy makers implement control and biosafety measures.
Knowledge about virus persistence in natural and laboratory settings has increased considerably over the last decade. In the case of influenza A viruses (IAV), their transmission routes submit viral particles to a wide range of environmental conditions. Specifically, they can be transmitted via 1) fecal-oral routes through water (i.e., avian viruses), or 2) direct or indirect contact by contaminated fomites, as well as aerosols and respiratory droplets (i.e., poultry and mammalian viruses)1. In any case, IAV are submitted to various physicochemical parameters (i.e., pH, salinity, temperature, and humidity), which more or less rapidly affects their infectivity2,3,4,5,6,7,8,9. It is of great importance, especially regarding zoonotic and pandemic viruses, to assess the potential of environmental factors to affect virus dynamics and the risks of exposure and cross-species transmission.
So far, traditional virology techniques (i.e., viral titer determination through plaque assays or 50% tissue culture infectious dose estimation) have been used to assess IAV infectivity over time; but these techniques are time-consuming and require many supplies10,11,12. Measuring infected cells impedance over time with microelectrodes serves as a useful tool to monitor IAV survival in different environmental conditions, as well as viral inactivation in general. This method provides objective, real-time data that replaces subjective human observation of cytopathic effects. It can be used to determine virus titration, thus replacing traditional measurements with lower confidence intervals and avoiding labor-intensive endpoints assays.
A linear correlation exists between titration results obtained by measurement of cell impedance and by classical plaque assay or TCID50 methods. Therefore, data obtained with the impedance-based titration method can be easily transformed in TCID50 or pfu values by creating a standard curve with serial dilution of the virus13,14,15,16,17. Detection, quantification, and efficacy of neutralizing antibodies present in serum samples can also be achieved using this experimental approach18,19. More recently, impedance-based cellular assays have been used to screen and evaluate antiviral compounds against Equid alphaherpesviruses20.
This technology has been used to evaluate the persistence of IAVs in saline water at different temperatures and to identify mutations in the hemagglutinin of IAV that increase or decrease IAV persistence in the environment21. Such screening would require extensive work if using traditional titration methods. However, this methodology can be used for any virus that has an impact on cell morphology, cell number, and cell surface attachment strength. It can also be used to monitor persistence in various environmental conditions (i.e., in the air, in water, or on surfaces).
The protocol described here applies IAV survival in water as an example. Human influenza viruses are exposed to different physicochemical parameters during extended periods. Saline (35 g/L NaCl) water at 35 °C was chosen as the environmental model based on previous results9. Residual infectivity of exposed viruses is quantified at different timepoints through cell infection. MDCK cells, the reference cell type for IAV amplification, are seeded on 16 well microtiter plates coated with microelectrode sensors and infected by exposed viruses 24 h later. Cell impedance is measured every 15 min and expressed as an arbitrary unit called the cell index (CI). Cytopathic effects induced by the influenza virus, whose rate of onset directly depends on the number of infectious viral particles inoculated to the cells culture, leads to CI decrease, which is subsequently quantified as the CIT50 value. This value corresponds to the time necessary to measure a 50% reduction from the initial CI (i.e., before virus addition). CIT50 values calculated for several environmental exposure times allow for deduction of the inactivation slope of a virus after linear regression of CIT50 values.
Protocol
Handle all influenza viruses according to appropriate biosafety level requirements (BSL-2 or higher depending on the subtype). Use IAV strains with a low passage history (less than 5x on MDCK cells) to insure low variation between experiments.
1. Preparation of reagents and starting materials
- Preparation of MDCK cells and sterile cell culture medium
- Cultivate Madin-Darby Canine Kidney (MDCK) cells in modified Eagle’s medium (MEM) supplemented with 10% heat inactivated fetal calf serum (FCS) and antibiotics (100 units/mL penicillin, 100 mg/mL streptomycin).
- After thawing, passage MDCK cells at least 2x before infecting them to ensure complete recovery (but less than 30 passages to avoid any drift in cell phenotypes).
- Seed 75 cm2 tissue culture flask(s) containing 30 mL of sterile 1x MEM with 7.5 x 106 MDCK cells and incubate at 37 °C in humidified 5% CO2 incubator.
- Preparation of impedance monitoring equipment
- Place the instrument in the incubator at 35 °C and allow it to warm up for at least 2 h.
- Connect it to the control unit placed outside the incubator.
- Proceed to cleaning procedures as recommended by the manufacturer before starting the remainder of the experiment.
- Production of IAV stocks on MDCK cells
- To propagate and amplify H1N1 viruses, seed 7.5 x 106 MDCK cells on two 75 cm2 tissue culture flasks and incubate for 24 h at 37 °C to reach 90%–100% confluence.
- Decant the cell culture medium from the cell monolayer in 75 cm2 flasks. Wash the cells with 5 mL of sterile 1x PBS.
- Remove PBS and add 5 mL of 1x PBS to wash the cells again.
- Label one flask as the control and remove PBS before adding 15 mL of virus propagation media (1x MEM with 0% FCS) carefully on the monolayer. Incubate this flask in an incubator maintained at 35 °C with 5% CO2. Use it for comparison after 3 days of propagation.
- Thaw one vial of IAV stock at RT. Dilute the virus to the appropriate concentration in a 1.5 mL tube containing virus propagation media (1x MEM with 0% FCS).
- Remove 1x PBS from the 75 cm2 flask and infect MDCK cells at a multiplicity of infection (MOI) of 1 x 10-3 or 1 x 10-4 plaque forming units per cell (pfu/cell) by adding 1 mL of the diluted virus to the cell monolayer.
- Adsorb virus to MDCK cells for 45 min at RT by stirring the flask regularly every 15 min.
- Gently remove the inoculum and add 15 mL of virus propagation media per flask containing 1 µg/mL TPCK-trypsin (trypsin/L-1-tosylamide-2-phenylethyl chloromethyl ketone), to cleave the viral hemagglutinin HA0 into HA1 and HA2 subunits (an event that is required for HA fusion with the endosomal membrane and release of the viral genome)22.
- Incubate the flasks at 35 °C and 5% CO2 for at least 3 days to replicate the virus.
- Observe MDCK cells under a microscope at 40x magnification and look for cytopathic effects (CPE) on the cells (by comparing to the cell control flask). If CPE is not complete (i.e., around 80% of the cells are detached from the substrate), put the flasks back in the incubator for an additional 24 h.
- When CPE is complete, decant the cell culture supernatant and centrifuge at 300 x g for 10 min to pellet the cellular debris.
- Transfer the clarified supernatant to a 15 mL tube and aliquot progeny viruses to single-use sterile cryogenic vials. Immediately place the cryotubes at -80 °C to freeze and stock viruses.
2. Determination of appropriate cell quantity for infecting cells
NOTE: During all experiments, keep the plates on non-electrostatic surfaces at all times, such as the paper wraps from the packaging. Follow section 2 below to determine the most appropriate concentration of cells to be seeded in the electronic microtiter plate (designed as E-Plate).
- Prepare MDCK cells in 75 cm2 flasks to obtain freshly split cells (approximately 80% confluence) 24 h before the experiment.
- Wash cells with 5 mL of 1x PBS and detach them by adding 3 mL of 0.25% Trypsin-EDTA solution.
- Add 7 mL of fresh cell culture medium and count cells using an automated cell counter with trypan blue staining.
- Adjust the cell concentration to 400,000 cells/mL with cell culture media. Perform two-fold serial dilutions in additional tubes to obtain cell densities of 200,000; 100,000; 50,000; 25,000; 12,500; and 6,250 cells/mL. Adjust the dilution range of the cells according to the cell type and their growth behavior.
- Leave the E-plate (Table of Materials) at RT for several minutes and add 100 µL of cell culture media to each well using a multi-channel pipette. Do not touch the electrodes of the E-Plate.
- Unlock the cradles and insert the plate front end into the cradle pocket of the impedance measuring instrument (Table of Materials). Close the door of the incubator.
- Open the software.
- In “Default experiment pattern setup”, choose the selected cradle(s) and double-click on the top page, then enter the name of the experiment. Click “Layout” and enter the necessary sample information for each selected well of the plate; then, click “Apply” when finished. Click “Schedule” | “Steps” | “Add a step”. The software automatically adds a step of 1 s to measure the background impedance (CI).
- Click on “Start/Continue” in the “Execute” tab. Click on “Plot”, add all samples by selecting the appropriated wells, and ensure CI is between -0.1 and 0.1 before proceeding to the next step.
- Remove the plate from the cradle.
- Add 100 µL of each cell suspension from step 2.4 in duplicate to the appropriate wells and 100 µL of cell media in wells used as controls. Leave the E-plate in the laminar flow hood for 30 min at RT to allow for uniform distribution of the cells at the bottoms of the wells.
- Insert the E-plate into the cradle pocket. Click “Schedule” | “Add step” in the software and enter values to monitor cells every 30 min for 200 repetitions. Then, select “Start/Continue”.
- Check and plot the CI data by clicking on the “Plot” button in the software. Select the concentration of cells that are just before the stationary phase 24 h after seeding on the plate, in order to obtain cells that are still in a growing phase during viral infection. Stationary phase is reached when CI is at its maximum.
3. Correlation between CIT50 values and multiplicity of infection
- Add 100 µL of sterile 1x MEM culture medium in each well of the E-plate. Insert E-plate into the cradle pocket of the instrument at 35 °C. Measure the background as described in step 2.7.
- Remove the E-plate from the cradle.
- Seed 3 x 104 of freshly split MDCK cells on each well of the electronic microtiter plate and grow them for 24 h at 35 °C with 5% CO2 so that they are in a replicative phase during IAV infection.
- Infect MDCK cells with different 10-fold dilutions of a known 6 log10 TCID50/mL titer of H1N1 virus, using reverse pipetting for reproducibility by following the steps below:
- Rinse MDCK cells 2x with 100 µL of MEM with no FCS (virus propagation media). Be aware of removing all media after the second wash to avoid further dilution of the inoculum.
- Add 100 µL of viral suspension in each well using single channel pipette. To avoid contamination, proceed by starting from left-to-right then top-to-bottom in the plate, while covering the remaining wells with the lid.
- Insert the plate into the cradle pocket of the instrument at 35 °C. Be gentle to avoid sudden movements potentially leading to contaminations.
- Start to monitor cell impedance every 15 min during at least 100 h as described in step 2.10.
- After the two cycles of measurements (i.e., 30 min), pause the apparatus by clicking on “Pause” in the “Execute” tab and remove the E-plate from the cradle.
- Add 1 µg/mL TPCK-trypsin to the virus propagation media to cleave the viral hemagglutinin.
- Add 100 µL of virus propagation media containing TPCK-trypsin into each well and insert the E-plate into the cradle pocket.
- Click “Start/Continue” in the “Execute” tab.
NOTE: Do not forget to create a negative control corresponding to mock-infected cells by replacing viral suspension with virus propagation media.
4. IAV survival kinetics
- Expose IAV to saline distilled water at 35 °C and test for their infectivity over time by measuring cell impedance decrease.
- Prepare saline distilled water by adding NaCl to a final concentration of 35 g/L in distilled water. Add 900 µL of saline water into 2 mL cryotubes.
- Add 100 µL of viral stock in saline water and place the cryotubes in an incubator (35 °C, 5% CO2) for 1 h, 24 h, or 48 h.
- Seed 100 µL (containing 3 x 104) of freshly split MDCK cells on a 16 well microtiter plate and grow for 24 h at 37 °C and 5% CO2.
- Infect cells with 100 µL of exposed viruses (previously diluted 10x in culture media) using reverse pipetting method for reproducibility by repeating section 3.4.
- Monitor cell impedance every 15 min for at least 100 h.
5. Evaluation of loss of infectivity
- Determination of CIT50 values to quantify CI decrease due to virus-induced cytopathic effects with the CIT50 value.
- Click “Plot” and add all the samples by clicking “Add all”. Export the results to a spreadsheet by clicking on “Export Experiment Info”. Consider initial CI as cellular impedance value measured 5 h after cell infection by exposed viruses (i.e., 24 h after seeding of MDCK cells on the microtiter E-plate).
- Calculate CIT50 value corresponding to the necessary time to measure a 50% decrease from the initial CI. To calculate the CIT50 value, note the CI value at 5 hpi for each sample. Then, find the timepoint at which the CI value is equal to one-half the CI value at 5 hpi by using the index and match functions in the spreadsheet.
- Calculation of the mean inactivation slope
- Determine CIT50 values for each exposed viral suspension, at different exposure times (in days).
- Calculate a linear regression slope from CIT50 plotted values, referred to as the inactivation slope and expressed in CIT50.day-1.
Representative Results
Raw data obtained after 120 h with different concentrations of MDCK cells, from 15,000 to 120,000 cells per well, is shown in Figure 1. After 24 h, CI measures show that cells in wells seeded with 30,000 cells were still in the exponential phase of growth, and this cell concentration was used for further experiments. Figure 2 illustrates the linear correlation between CIT50 values and the initial multiplicity of infection. MDCK cells are cultured for 24 h, then infected with A/Paris/2590/2009 H1N1 viral strain at a different multiplicity of infection. Initial CI is measured 5 h after infection.
Figure 3 illustrates the experimental procedure used in our experiments (panel A). Typical results showing CI decrease due to virus-induced cytopathic effect are shown on panel B. These are raw data obtained after being processed by the software. CIT50 values were calculated using the spreadsheet after exporting of the data. After calculation of CIT50 values at different timepoints, a linear regression analysis allowed determination of the slope (Panel C). Viral inactivation slopes were thus obtained for each virus in each condition, then were compared to identify viruses that had the greatest stability in the studied environment.
Figure 4 shows inactivation slopes of IAV recombinant viruses bearing a genetic backbone belonging to the A/WSN/1933 H1N1 virus strain and a HA and NA from A/New Caledonia/20/1999 H1N1 virus (HA-NA/NC99). Non-synonymous mutations in the HA were introduced to study the impact on virus particle persistence outside the host in saline water.
By comparing mean inactivation slopes from different experiments performed in triplicate, we were able to identify amino acids in the HA that were sufficient to affect viral persistence outside the host. The lower the inactivation slope, the more stable the virus. As an example, the HA/F453Y substitution or HA::K147 insertion in the HA of HA-NA/NC99 virus induced a significant increase in the mean inactivation slope to 9.8 CIT50/day and 9.9 CIT50/day, respectively, compared to wild-type HA (with a mean inactivation slope of 4.85 CIT50/day), thus generating very unstable mutants. In contrast, the HA/T327A substitution did not affect viral stability at 35 °C in saline water (mean inactivation slope of 6.6 CIT50/day). The methodology was thus powerful enough to identify amino-acid residues in the HA glycoprotein that were involved in IAV survival outside the host.
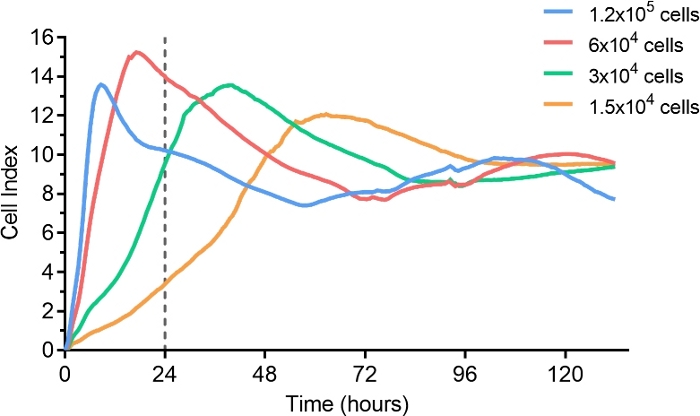
Figure 1: Cell titration in microtiter E-plate.
Serial dilutions of MDCK cells were seeded on microtiter E-plates, and cell growth was monitored for up to 100 h (200 sweeps every 30 min). Gray dots represent the standard deviations of CI measured in duplicate. The vertical line at 24 h highlights that in the described conditions, initial seeding of 3 x 104 cells/well allowed a culture of around 70% confluency at the time of the infection. Please click here to view a larger version of this figure.
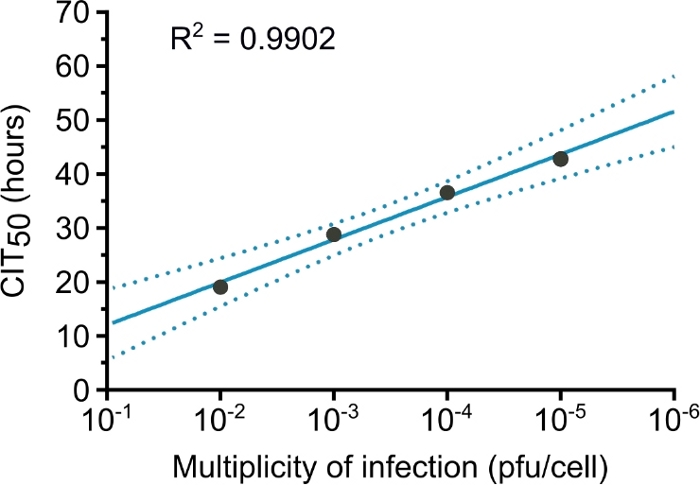
Figure 2: Linear regression between CIT50 values and initial multiplicity of infection.
Each point indicates the CIT50 value calculated for infection of cells with different multiplicities of infection. The solid line represents the linear regression slope (R2 = 0.99), and dashed lines represent the 95% confidence intervals. This figure has been modified from a previous publication21. Please click here to view a larger version of this figure.
Figure 3: Determination of inactivation slope.
(A) Viral particles were diluted in saline water at 35 °C for 0, 1, or 2 days, and CI was monitored continuously and plotted as cell index. (B) CI decrease quantified with the CIT50 values, which are represented manually on the raw data by vertical dashed lines. Each curve represents the evolution of cell impedance after infection with viruses that were exposed to saline water for increasing times (red: non-exposed virus, yellow: 24 h exposure, green: 48 h exposure, dark: mock-infected cells). Initial CI was measured 5 h after infection (C) Linear regression of CIT50 values used to calculate the inactivation slope. Please click here to view a larger version of this figure.
Figure 4: Impact of non-synonymous mutations in the HA on IAV persistence in saline water.
Inactivation slopes of reassortant viruses bearing a HA from A/New Caledonia/20/1999 H1N1 virus with (substitution or insertion) or without mutation (HAWT). Boxplots displayed the distribution of single inactivation slopes (six or eight depending on the virus, calculated from different independent experiments) obtained for each exposed virus around the mean (horizontal lines). Mean inactivation slopes were compared using an ANOVA test (ns, p > 0.05, ****p < 0.0001). Ref corresponds to the reassortant virus bearing wildtype HA, against which other viruses are compared. This figure has been modified from a previous publication21. Please click here to view a larger version of this figure.
Discussion
RTCA is an impedance-based technology that is increasingly used for real-time monitoring of cell properties, such as cell adherence, proliferation, migration and cytotoxicity. In this study, the capacity of this technology to assess IAV survival outside the host is demonstrated by measuring virus inactivation slope. Fastidious techniques such as TCID50 and plaque assays are replaced by objective real-time assessment of cell viability, thus reflecting cytopathic effects induced by the virus. Similar to the TCID50 or plaque forming unit (pfu), the CIT50 is also linearly correlated with the multiplicity of infection (Figure 2). Among the limitations of this approach, this method fails to precisely monitor cells infected with non-cytopathogenic virus.
As an example, introduction of new mutation into the viral genome can strongly attenuate a virus and decrease its cytopathogenicity. In contrast, the inactivation slope calculated here is independent of virus replication and a potential virus attenuation, as this slope is derived from CIT50 values measured after different timepoints of inactivation of the same virus. Here, it is thus assumed that viral particles that are still able to infect a cell after this inactivation protocol share the same replication phenotype as viruses before the inactivation.
Despite higher costs, the workload using this approach is considerably reduced, which is advantageous when a project requires performance of kinetics, with measure frequent timepoints and over a long period of time. As in every protocol, some steps are critical, and manipulations must be carried out with care and precision. Reverse pipetting is a crucial step to ensure precise dispensing of the media, and special attention must be paid to avoid any contamination. Likewise, the initial cell quantity must be the same between each experiment and must be assessed using an automated cell counter with trypan blue staining. During monitoring of the CI by the apparatus, the opening of the incubator should be limited as much as possible.
If care and attention are provided during the experiment with limited movement, then the contamination risk becomes low, and the results are highly reproducible. The distribution of inactivation slopes among different viruses are significantly different, so a non-parametric statistical test is used to compare virus persistence. Mean inactivation slopes can be calculated for any viruses producing cytopathic effects in cell culture, such as enteric viruses, ebolavirus, or coronaviruses, whose persistence in environmental conditions is currently being studied (and likely using traditional virology methods). In the future, this method can be also be used to compare the replication of different viruses, investigate virus tropism for several cell lines at the same time, and study specific steps of the virus cycle.
Divulgazioni
The authors have nothing to disclose.
Materials
| 0.25%Trypsin | ThermoFisher | 25200056 | |
| 75 cm2 tissue culture flask | Falcon | 430641U | |
| E-Plate 16 (6 plates) | ACEA Biosciences, Inc | 5469830001 | E-plates are avalible in different packaging |
| FCS | Life technologies (gibco) | 10270-106 | |
| MEM 1X | Life technologies (gibco) | 31095029 | |
| PBS 1X | Life technologies (gibco) | 14040091 | |
| Penicillin-Streptomycin | Life technologies (gibco) | 11548876 | |
| TPCK-Trypsin | Worthington | LS003740 | |
| xCELLigence Real-Time Cell Analysis Instrument S16 | ACEA Biosciences, Inc | 380601310 | The xCELLigence RTCA S16 instruments are available in different formats (16-well, 96-well, single or multi-plate) |
Riferimenti
- Killingley, B., Nguyen-Van-Tam, J. Routes of influenza transmission. Influenza and Other Respiratory Viruses. 7, 42-51 (2013).
- Sooryanarain, H., Elankumaran, S. Environmental Role in Influenza Virus Outbreaks. Annual Review of Animal Biosciences. 3 (1), 347-373 (2015).
- Keeler, S. P., Dalton, M. S., Cressler, A. M., Berghaus, R. D., Stallknecht, D. E. Abiotic factors affecting the persistence of avian influenza virus in surface waters of waterfowl habitats. Applied and Environmental Microbiology. 80 (9), 2910-2917 (2014).
- Stallknecht, D. E., Kearney, M. T., Shane, S. M., Zwank, P. J. Effects of pH, temperature, and salinity on persistence of avian influenza viruses in water. Avian Diseases. 34 (2), 412-418 (1990).
- Poulson, R. L., Tompkins, S. M., Berghaus, R. D., Brown, J. D., Stallknecht, D. E. Environmental Stability of Swine and Human Pandemic Influenza Viruses in Water under Variable Conditions of Temperature, Salinity, and pH. Applied and Environmental Microbiology. 82 (13), 3721-3726 (2016).
- Zhang, G., et al. Evidence of influenza A virus RNA in Siberian lake ice. Journal of Virology. 80 (24), 12229-12235 (2006).
- Nazir, J., et al. Long-Term Study on Tenacity of Avian Influenza Viruses in Water (Distilled Water, Normal Saline, and Surface Water) at Different Temperatures. Avian Diseases. 54 (1), 720-724 (2010).
- Brown, J. D., Swayne, D. E., Cooper, R. J., Burns, R. E., Stallknecht, D. E. Persistence of H5 and H7 avian influenza viruses in water. Avian Diseases. 51, 285-289 (2007).
- Dublineau, A., et al. Persistence of the 2009 pandemic influenza A (H1N1) virus in water and on non-porous surface. PLoS ONE. 6 (11), 28043 (2011).
- Titration of Influenza Viruses. Springer Nature Experiments Available from: https://experiments.springernature.com/articles/10.1007/978-1-4939-8678-1_4 (2020)
- Szretter, K. J., Balish, A. L., Katz, J. M. Influenza: Propagation, Quantification, and Storage. Current Protocols in Microbiology. 3 (1), 1-22 (2006).
- Gaush, C. R., Smith, T. F. Replication and Plaque Assay of Influenza Virus in an Established Line of Canine Kidney Cells. Applied Microbiology. 16 (4), 588-594 (1968).
- Witkowski, P. T., et al. Cellular impedance measurement as a new tool for poxvirus titration, antibody neutralization testing and evaluation of antiviral substances. Biochemical and Biophysical Research Communications. 401 (1), 37-41 (2010).
- Lebourgeois, S., et al. Development of a Real-Time Cell Analysis (RTCA) Method as a Fast and Accurate Method for Detecting Infectious Particles of the Adapted Strain of Hepatitis A Virus. Frontiers in Cellular and Infection Microbiology. 8, 335 (2018).
- Teng, Z., Kuang, X., Wang, J., Zhang, X. Real-time cell analysis–a new method for dynamic, quantitative measurement of infectious viruses and antiserum neutralizing activity. Journal of Virological Methods. 193 (2), 364-370 (2013).
- Fang, Y., Ye, P., Wang, X., Xu, X., Reisen, W. Real-time monitoring of flavivirus induced cytopathogenesis using cell electric impedance technology. Journal of Virological Methods. 173 (2), 251-258 (2011).
- Charretier, C., et al. Robust real-time cell analysis method for determining viral infectious titers during development of a viral vaccine production process. Journal of Virological Methods. 252, 57-64 (2018).
- Tian, D., et al. Real-Time Cell Analysis for Measuring Viral Cytopathogenesis and the Efficacy of Neutralizing Antibodies to the 2009 Influenza A (H1N1) Virus. PLoS ONE. 7 (2), 31965 (2012).
- Teng, Z., Kuang, X., Wang, J., Zhang, X. Real-time cell analysis–a new method for dynamic, quantitative measurement of infectious viruses and antiserum neutralizing activity. Journal of Virological Methods. 193 (2), 364-370 (2013).
- Thieulent, C. J., et al. Screening and evaluation of antiviral compounds against Equid alpha-herpesviruses using an impedance-based cellular assay. Virology. 526, 105-116 (2019).
- Labadie, T., Batéjat, C., Manuguerra, J. -. C., Leclercq, I. Influenza Virus Segment Composition Influences Viral Stability in the Environment. Frontiers in Microbiology. 9, 1496 (2018).
- Wiley, D. C., Skehel, J. J. The structure and function of the hemagglutinin membrane glycoprotein of influenza virus. Annual Review of Biochemistry. 56, 365-394 (1987).

