Paper-Based Preconcentration and Isolation of Microvesicles and Exosomes
Summary
Presented is a protocol to fabricate a paper-based device for the effective enrichment and isolation of microvesicles and exosomes.
Abstract
Microvesicles and exosomes are small membranous vesicles released to the extracellular environment and circulated throughout the body. Because they contain various parental cell-derived biomolecules such as DNA, mRNA, miRNA, proteins, and lipids, their enrichment and isolation are critical steps for their exploitation as potential biomarkers for clinical applications. However, conventional isolation methods (e.g., ultracentrifugation) cause significant loss and damage to microvesicles and exosomes. These methods also require multiple repetitive steps of ultracentrifugation, loading, and wasting of reagents. This article describes a detailed method to fabricate an origami-paper-based device (Exo-PAD) designed for the effective enrichment and isolation of microvesicles and exosomes in a simple manner. The unique design of the Exo-PAD, consisting of accordion-like multifolded layers with convergent sample areas, is integrated with the ion concentration polarization technique, thereby enabling fivefold enrichment of the microvesicles and exosomes on specific layers. In addition, the enriched microvesicles and exosomes are isolated by simply unfolding the Exo-PAD.
Introduction
Microvesicles and exosomes are small membrane vesicles measuring 0.2−1 μm and 30−200 nm, respectively. They are secreted into the extracellular environment by several different cell types1,2,3,4,5. They contain parental cell information in the form of subsets of DNA, mRNA, miRNA, proteins, and lipids, and circulate throughout the body via various body fluids such as serum, plasma, urine, cerebrospinal fluid, amniotic fluid, and saliva6,7,8,9. Thus, techniques for efficient isolation of microvesicles and exosomes from biological fluids can provide extensive opportunities in the fields of the diagnosis, prognosis, and real-time monitoring of disease, as well as in the development of new therapeutics.
However, the conventional isolation method for microvesicles and exosomes based on ultracentrifugation is extremely time-consuming and causes significant loss and contamination of the sample. This is because it involves several cumbersome pipetting and loading steps and discarding of various reagents with repeated ultracentrifugation5,6,10,11,12. Moreover, the high shear stress induced by ultracentrifugation (~100,000 x g) can cause the physical lysis of microvesicles and exosomes, yielding poor recovery rates (5−23%)6,13,14. Therefore, a highly efficient, unobtrusive isolation technique for microvesicles and exosomes must be developed to reduce damage and loss, thereby achieving higher recovery rates.
An origami-paper-based device (Exo-PAD) was developed for simpler, gentler, and highly efficient isolation of microvesicles and exosomes6. The design of the Exo-PAD is a multifolded paper with serially connected sample areas that gradually decrease in diameter. The ion concentration polarization (ICP) technique, which is a nano-electrokinetic phenomenon that preconcentrates charged biomolecules, was integrated with this unique design. Using the Exo-PAD resulted in fivefold enrichment of the microvesicles and exosomes in specific layers and their isolation by simply unfolding the device. This article describes the Exo-PAD in detail, from the overall device fabrication and operation to analysis of its use, to illustrate the method and show representative results6.
Protocol
1. Device fabrication
- Define the region to be printed on paper using printer software (Table of Materials).
NOTE: The design has 12 wax-patterned layers in which the diameters of the circular sample areas gradually narrow from 5 mm to 2 mm (Figure 1A). - Print hydrophobic wax on the designated regions on both sides of the cellulose paper (Table of Materials) using a commercial wax printer (Table of Materials) (Figure 1B).
- Place the wax-printed paper in a laboratory oven for 80 s at 120 °C.
NOTE: This step allows the wax to reach the inside of the paper by achieving a thermal reflow of the printed wax. With this incubation protocol, the resolution of the pattern is ~2 mm. Thus, be careful not to print patterns of less than 2 mm. Otherwise the patterns will be blocked by the wax. - Cut the wax-printed paper with a cutter to make individual devices (Figure 1C).
- Drop 5 μL and 2 μL of permselective membrane (e.g., Nafion; Table of Materials) onto the sample areas in the leftmost and rightmost layers, respectively (Figure 1D).
- Place the layers coated with the permselective membrane on a hot plate at 70 °C for 30 min to evaporate the permselective membrane solvent.
- Seal the outermost surface of the coated layer facing the buffer solution with pressure-sensitive tape, leaving a small hole.
- Fold the printed individual device (i.e., Exo-PAD) back and forth along the white lines.
NOTE: By folding the device, all sample areas become convergently connected (Figure 1E). This convergent design focuses the electric field lines when the voltage is applied for ICP, achieving more intensive preconcentration of the microvesicles and exosomes.
2. Enrichment and spatial focusing of microvesicles and exosomes by ion concentration polarization
- Load 15 μL of the microvesicle and exosome sample (~3 x 1011 particles/mL in 0.1x phosphate buffered saline [PBS] with 0.05% Tween 20) in the convergent sample areas by pipetting and wait a few seconds to ensure complete wetting of all sample areas (Figure 1F).
- Place two acrylic chambers at both ends of the Exo-PAD and clamp the Exo-PAD securely with small binder clips to prevent unfolding (Figure 1G).
- Fill the chambers with 110 μL of 0.1x PBS and insert two Ag/AgCl electrodes (Figure 1H).
- Apply 30 V to the electrodes for 20 min using a current-voltage source measurement system (Table of Materials).
NOTE: The applied voltage generates the ICP phenomenon and hence preconcentrates the microvesicles and exosomes on layers 8 and 9 of the Exo-PAD.
3. Isolation of the enriched microvesicles and exosomes
- Separate the folded Exo-PAD from the acrylic chambers and unfold the device to isolate the enriched microvesicles and exosomes from the other layers (Figure 1I).
- Punch out the sample areas in layers 8 and 9, where the microvesicles and exosomes are enriched, for downstream analysis (Figure 1J).
4. Scanning electron microscopy analysis
- Fix the enriched microvesicles and exosomes by immersing the punched areas in 2.5% glutaraldehyde in 0.1 M sodium cacodylate buffer for 1 h and in 1% osmium tetroxide in 0.1 M sodium cacodylate for 1 h.
CAUTION: Osmium tetroxide is highly poisonous and hazardous chemical. Because osmium tetroxide can penetrate plastics, it must be stored in glass. Any handling of osmium tetroxide must be performed in a chemical fume hood with double nitrile gloves. - Dehydrate the fixed microvesicles and exosomes with ascending grades of 200 proof ethanol (i.e., 50%, 70%, 90%, and 100%) for 30 min each.
- Chemically dry the sample by immersing it in hexamethyldisilazane in a desiccator for 30 min.
CAUTION: Hexamethyldisilazane is a flammable and moisture-sensitive chemical. It must be stored in a dry and well-ventilated area away from ignition sources. - Coat the completely dried microvesicles and exosomes with gold/palladium (~20 nm) via sputtering and capture scanning electron microscopy (SEM) images.
5. Nanoparticle tracking analysis
- Punch out the sample areas in layers 6, 8, and 10 using a biopsy punch after 20 min of device operation.
- Immerse each punched-out area in buffer solution (0.1x PBS with 0.01% Tween 20).
- Vortex for 10 min and centrifuge for 30 s at 6,000 rpm to resuspend the enriched microvesicles and exosomes.
- Remove the punched-out areas from the solution and measure the concentration of microvesicles and exosomes by a nanoparticle tracking analysis (NTA) instrument (Table of Materials).
Representative Results
The operation time must be optimized to achieve the maximum recovery yield of the enriched microvesicles and exosomes. Insufficient time does not allow sufficient migration of the microvesicles and exosomes, which decreases the enrichment, whereas excessive time deteriorates the spatial focusing and hence disperses the microvesicles and exosomes. Thus, through the time optimization step, the maximum preconcentration factor of microvesicles and exosomes and the final location where microvesicles and exosomes are most enriched can be identified. To find the final location of the microvesicles and exosomes, time-lapse migration of fluorescently labeled microvesicles and exosomes was observed with a microscope (Figure 2A) and the fluorescence intensities in all sample areas were quantified using ImageJ software (Figure 2B). Before the enrichment process (Figure 2A, 0 min), microvesicles and exosomes were dispersed in all sample areas with a gradual decrease in the intensity because of the slight filtering action of the paper matrix (Figure 2B, 0 min). After 10 min of processing (Figure 2A, 10 min), microvesicles and exosomes migrated electrokinetically and an instant preconcentration plug appeared on layer 7. Further processing achieved greater preconcentration of microvesicles and exosomes. When the process time reached 20 min, microvesicles and exosomes were strongly focused on layers 8 and 9 (Figure 2A, 20 min) and enriched fivefold, based on the fluorescence intensities (Figure 2B, 20 min).
After completing the preconcentration process, the enriched microvesicles and exosomes were isolated by unfolding the device and the sample area in layer 8 was punched out for further analysis. SEM images were obtained to investigate the integrity of the enriched microvesicles and exosomes. As shown in the SEM images (Figure 3A), significantly more nondamaged microvesicles and exosomes were observed after the enrichment process. Using the obtained SEM images, the size distribution of the enriched microvesicles and exosomes was analyzed (Figure 3B), where the exosomes and microvesicles were distinguished based on a threshold diameter of 200 nm. In other words, the size of exosomes ranged from 95–195 nm with a mean diameter of 134 nm and that of the microvesicles from 214–377 nm with a mean diameter of 315 nm.
Subsequently, the actual concentration of the enriched microvesicles and exosomes on the sample areas in layers 6, 8, and 10 was analyzed by NTA. Before enrichment, the concentration was ~2.24 x 1011 particles/mL in layer 8 (Figure 3C, layer 8). After enrichment, the concentration was ~1.25 x 1012 particles/mL in layer 8 (Figure 3C, layer 8), indicating that the microvesicles and exosomes were preconcentrated by a factor of ~5.58. Noticeable preconcentration was not observed on the other layers (Figure 3C, layers 6 and 10). Taken together, these results demonstrate that the proposed paper-based device can enrich microvesicles and exosomes by approximately fivefold.
The isolation efficiency of the device was evaluated as follows: The sample volume that can be accommodated by layer 8 and 9 is ~2.2 μL out of a total 15 μL of sample volume in 10 layers because of the narrowing design of the Exo-PAD. If we assume an ideal case in which all of the microvesicles and exosomes are preconcentrated on layer 8 and 9 and none are lost, then the ideal preconcentration factor should be ~6.8 (15 μL/2.2 μL = ~6.8). However, the experimental preconcentration factor obtained by NTA (Figure 3c) was ~5.58, which means a portion of 1.22 microvesicles and exosomes were lost by lysis or nonspecific binding to the paper matrix.
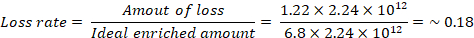
where 2.24 x 1012 particles/mL is the initial concentration of microvesicles and exosomes. From this calculation, the expected loss rate of the Exo-PAD is about 18%; hence, the isolation efficiency is about 82%. This is a significant improvement compared to the poor yields of ultracentrifugation (5%–23%).
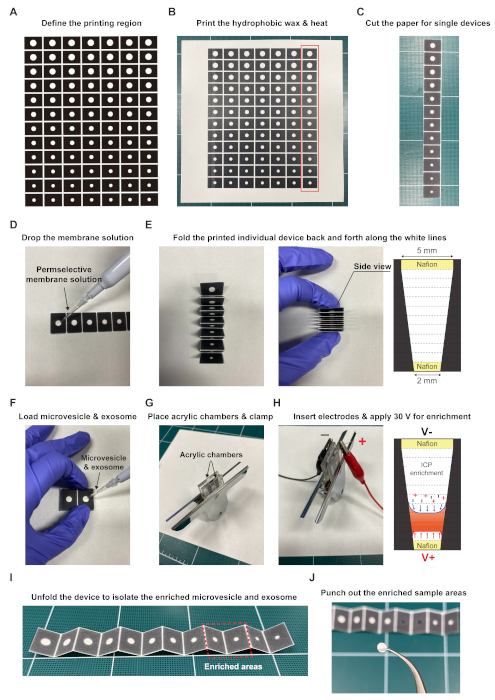
Figure 1: Overall Exo-PAD procedures, from assembly to operation. (A) Software design to guide the printing of hydrophobic wax on cellulose paper. (B) The wax-printed paper after incubation in an oven for 80 s at 120 °C. (C) The individual devices are cut out using a cutter. (D) Permselective membrane solution is dropped on the sample areas in the leftmost and rightmost layers and the solvent is evaporated on a hotplate at 70 °C for 30 min. (E) The printed Exo-PAD is folded back and forth along the white lines; all sample areas are thus convergently connected. (F) A microvesicle and exosome sample is loaded in the sample area. (G) Two acrylic chambers are placed at the ends of the device and secured with small binder clips. (H) The acrylic chambers are filled with buffer solution and two Ag/AgCl electrodes are inserted to apply a voltage of 30 V. (I) The device is unfolded to isolate the preconcentrated microvesicles and exosomes. (J) The sample areas in layers 8 and 9 are punched out for analysis. Please click here to view a larger version of this figure.
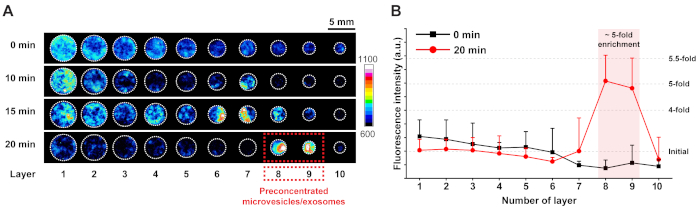
Figure 2: Observation of fluorescently labeled microvesicles and exosomes during device operation and corresponding fluorescence intensities. (A) Time-lapse observation of microvesicles and exosomes. Scale bar = 5 mm. With 20 min of processing, the microvesicles and exosomes were strongly focused on layers 8 and 9, where (B) they were enriched by approximately fivefold. Error bars represent standard deviation (n = 3). Please click here to view a larger version of this figure.
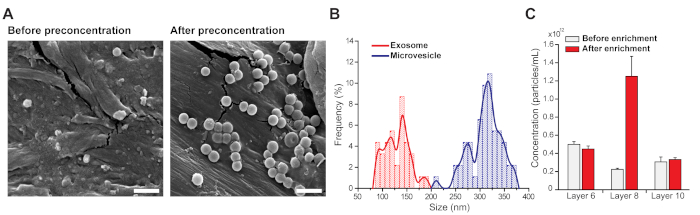
Figure 3: Analysis of the enriched microvesicles and exosomes. (A) SEM images showing the integrity of the enriched microvesicles and exosomes. After preconcentration, many more microvesicles and exosomes were observed6. Scale bar = 1 μm. (B) Size distribution of the enriched microvesicles and exosomes. The exosome sizes were 95−195 nm (mean diameter = 134 nm) and microvesicle sizes were 214−377 nm (mean diameter = 315 nm). (C) The actual concentration of the enriched microvesicles and exosomes evaluated using NTA. After enrichment, the concentration of microvesicles and exosomes increased fivefold, which is consistent with fluorescence results6. Error bars represent standard deviation (n = 3). Panels A and C are reproduced by permission from Kim et al.6. Please click here to view a larger version of this figure.
Supplementary Figure 1: Microvesicles and exosomes preconcentrated and focused on layer 8 were separated from bovine serum albumin (BSA), which stayed in layers 1–3. Please click here to download this file.
Discussion
Although the Exo-PAD was used successfully for the enrichment and isolation of microvesicles and exosomes, several critical points should be carefully considered: 1) the oven incubation time and temperature during the device preparation, 2) processing time, 3) application of voltage with varying layer numbers and sample area diameters, and 4) applicability to clinical samples.
The incubation time and temperature given in the protocol are optimized conditions to fabricate a reliable device. Longer incubation times or a higher temperature may cause distortion of the convergent design due to the excessive reflow of the printed wax. This will prevent the formation of focused electric field lines and thus increase the migration resistance during the electrokinetic enrichment process.
Processing time is also a factor to consider for maximum enrichment and isolation. A processing time of <20 min results in less preconcentration because there is not sufficient time to allow microvesicle and exosome migration. On the other hand, a processing time of >20 min deteriorates the focusing efficiency and allows dispersion of the enriched microvesicles and exosomes. According to this test, 20 min is the best processing time to achieve maximum enrichment and isolation of microvesicles and exosomes.
The applied voltage is another important factor to consider. In this protocol, considering the number of layers and the sample area diameters, 30 V is used to achieve stable preconcentration and isolation. When an ICP phenomenon is generated in the device, the operation regime can be divided into three distinct regions according to the applied voltage: (1) ohmic, (2) limiting, and (3) overlimiting regions6,15,16. For stable preconcentration and isolation, the device should be operated in the limiting region, where an ion depletion zone is generated and microvesicles and exosomes are stably preconcentrated. In this experimental setting, the operating voltage range (5−40 V) corresponds to the limiting region for the Exo-PAD. If the applied voltage is <5 V, the device operates in the ohmic region, where the electric current increases linearly, indicating that no ion depletion region is generated for preconcentration. However, if the voltage is >40 V, the ion depletion region is destroyed by strong voltage-driven electroconvection, which destabilizes preconcentration. Considering these factors, it was determined that 30 V was the optimal voltage to use for a device with 12 layers and a sample area diameter of 2−5 mm. Layer numbers or sample area diameters can be increased to enlarge the sample volume, but these factors, as well as the operating voltage, should be optimized to achieve stable enrichment of microvesicles and exosomes.
The proposed Exo-PAD can be used to isolate microvesicles and exosomes from various clinical samples, including saliva, urine, serum, and plasma. For samples with less protein, such as saliva or urine, microvesicles and exosomes can be easily isolated without major changes, because a small amount of protein in these samples will be filtered out through nonspecific binding to the paper. When operating the device within the limiting region, only the applied voltage needs to be considered, because the ionic strength of samples can be different. For protein-rich samples, such as serum or plasma, both the preconcentration of microvesicles and exosomes and their separation from abundant proteins should be carefully considered. Our recent experiment shows that separation of microvesicles and exosomes from proteins is significantly improved when carbonate buffer is used, because carbonate buffer results in a higher electrical charge difference between microvesicles and exosomes and protein due to the isoelectric point, resulting in better separation efficiency. For this experiment, a substance with abundant proteins coexisting with microvesicles and exosomes was mimicked by mixing purified microvesicles and exosomes labeled with FITC (Ex/Em: 490/520 nm) with BSA labeled with a different fluorophore (Ex/Em: 590/617 nm) in 50 mM carbonate buffer (Table of Materials). As shown in Supplementary Figure 1, most microvesicles and exosomes were preconcentrated on layer 8, as in Figure 2A and they were separated from BSA, which stayed in layers 1–3. This result suggests that enrichment from a serum or plasma sample requires an additional step adding carbonate buffer to the sample, and clearly demonstrates that the Exo-PAD can purify microvesicles and exosomes from abundant proteins.
Divulgazioni
The authors have nothing to disclose.
Acknowledgements
This study was supported by the National Research Foundation of Korea, Grant NRF-2018R1D1A1A09084044. J. H. Lee was supported by a research grant from Kwangwoon University in 2019. Hyerin Kim was supported by the “Competency Development Program for Industry Specialists” of the Korean Ministry of Trade, Industry and Energy (MOTIE), operated by the Korea Institute for Advancement of Technology (KIAT) (No. P0002397, HRD program for Industrial Convergence of Wearable Smart Devices).
Materials
| Ag/AgCl electrodes | A-M Systems, Inc. | 531500 | 0.15" diameter |
| Albumin from Bovine Serum (BSA), Alexa Fluor 594 conjugate | Thermo Fisher Scientific | A13101 | BSA conjugated with Alexa Fluor 594 (Ex/Em: 590/617 nm) |
| Carbonate-Bicarbonate Buffer | Sigma-Aldrich | C3041-50CAP | Carbonate buffer |
| CorelDraw software (Coral Co., Canada) | Corel Corporation | Printer software to define wax printing region | |
| ColorQube 8870 | Xerox Corporation | Wax printer | |
| Chromatography paper grade 1 | Whatman | 3001-861 | Cellulose paper, dimension: 20 * 20 cm |
| Fluorescent-labeled exosome standards | HansaBioMed Life Sciences, Ltd. | HBM-F-PEP-100 | Exosome labeled with FITC (Ex/Em: 490/520 nm) |
| Keithley 2410 current/voltage source-meter | Keithley Instruments, Inc. | Current–voltage source measurement system | |
| Nafion perfluorinated resin solution | Sigma-Aldrich | 31175-20-9 | Permselective membrane, 20 wt.% in the mixture of lower aliphatic alcohols and water; contains 34% water |
| NanoSight LM10 | NanoSight Technology | Nanoparticle tracking analysis (NTA) machine | |
| Phosphate-buffered saline (PBS, pH7.4) | Thermo Fisher Scientific | 10010001 |
Riferimenti
- Edgar, J. R. Q & A: What are exosomes, exactly. BMC Biology. 14 (1), 1-7 (2016).
- Contreras-Naranjo, J. C., Wu, H. J., Ugaz, V. M. Microfluidics for exosome isolation and analysis: Enabling liquid biopsy for personalized medicine. Lab on a Chip. 17 (21), 3558-3577 (2017).
- Simons, M., Raposo, G. Exosomes – vesicular carriers for intercellular communication. Current Opinion in Cell Biology. 21 (4), 575-581 (2009).
- Ståhl, A. L., Johansson, K., Mossberg, M., Kahn, R., Karpman, D. Exosomes and microvesicles in normal physiology, pathophysiology, and renal diseases. Pediatric Nephrology. 34 (1), 11-30 (2019).
- Chen, C., Lin, B. R., Hsu, M. Y., Cheng, C. M. Paper-based devices for isolation and characterization of extracellular vesicles. Journal of Visualized Experiments. (98), e52722 (2015).
- Kim, H., et al. Origami-paper-based device for microvesicle/exosome preconcentration and isolation. Lab on a Chip. 19 (23), 3917-3921 (2019).
- Raposo, G., Stoorvogel, W. Extracellular vesicles: Exosomes, microvesicles, and friends. Journal of Cell Biology. 200 (4), 373-383 (2013).
- Lee, Y., El Andaloussi, S., Wood, M. J. A. Exosomes and microvesicles: Extracellular vesicles for genetic information transfer and gene therapy. Human Molecular Genetics. 21, 125-134 (2012).
- Liu, C., et al. Field-Free Isolation of Exosomes from Extracellular Vesicles by Microfluidic Viscoelastic Flows. ACS Nano. 11 (7), 6968-6976 (2017).
- Marczak, S., et al. Simultaneous isolation and preconcentration of exosomes by ion concentration polarization. Electrophoresis. 39 (15), 2029-2038 (2018).
- Livshts, M. A., et al. Isolation of exosomes by differential centrifugation: Theoretical analysis of a commonly used protocol. Scientific Reports. 5, 1-14 (2015).
- Chiriacò, M. S., et al. Lab-on-chip for exosomes and microvesicles detection and characterization. Sensors. 18 (10), 3175 (2018).
- Lobb, R. J., et al. Optimized exosome isolation protocol for cell culture supernatant and human plasma. Journal of Extracellular Vesicles. 4 (1), 1-11 (2015).
- Taylor, D. D., Shah, S. Methods of isolating extracellular vesicles impact down-stream analyses of their cargoes. Methods. 87, 3-10 (2015).
- Han, S., et al. Electrokinetic size-based spatial separation of micro/nanospheres using paper-based 3d origami preconcentrator. Analytical Chemistry. 91 (16), 10744-10749 (2019).
- Yeh, S. H., Chou, K. H., Yang, R. J. Sample pre-concentration with high enrichment factors at a fixed location in paper-based microfluidic devices. Lab on a Chip. 16 (5), 925-931 (2016).

