Incremental Temperature Changes for Maximal Breeding and Spawning in Astyanax mexicanus
Summary
This article outlines the basic laboratory conditions and protocols for an incremental temperature regime to stimulate maximal spawning in the Mexican tetra Astyanax mexicanus, which is an emerging model for developmental and evolutionary studies.
Abstract
The Mexican tetra, Astyanax mexicanus, is an emerging model system for studies in development and evolution. The existence of eyed surface (surface fish) and blind cave (cave fish) morphs in this species presents an opportunity to interrogate the mechanisms underlying morphological and behavioral evolution. Cave fish have evolved novel constructive and regressive traits. The constructive changes include increases in taste buds and jaws, lateral line sensory organs, and body fat. The regressive changes include loss or reduction of eyes. melanin pigmentation, schooling behavior, aggression, and sleep. To experimentally interrogate these changes, it is crucial to obtain large numbers of spawned embryos. Since the original A. mexicanus surface fish and cave fish were collected in Texas and Mexico in the 1990s, their descendants have been routinely stimulated to breed and spawn large numbers of embryos bimonthly in the Jeffery laboratory. Although breeding is controlled by food abundance and quality, light-dark cycles, and temperature, we have found that incremental temperature changes play a key role in stimulating maximal spawning. The gradual increase of temperature from 72 °F to 78 °F in the first three days of a breeding week provides two-three consecutive spawning days with maximal numbers of high-quality embryos, which is then followed by a gradual decrease of temperature from 78 °F to 72 °F during the last three days of the spawning week. The procedures shown in this video outline the workflow before and during a laboratory breeding week for incremental temperature stimulated spawning.
Introduction
The teleost Astyanax mexicanus has an eyed surface-dwelling (surface fish) form and many different blind cave-dwelling (cave fish) forms1,2. Cave fish have evolved in perpetual darkness and under food limitations, resulting in the appearance of novel constructive and regressive traits3. The constructive traits include increases in taste buds and jaw size, sensory organs of the lateral line, and fat reserves. The regressive traits include the loss or reduction of melanin pigmentation, eyes, and behaviors, such as sleep, schooling, and aggression. An attribute of the Astyanax system is complete fertility between the two forms, allowing the use of quantitative trait loci (QTL) mapping to determine the genomic region(s) associated with constructive and regressive evolution4,5,6,7. A. mexicanus offers an advantageous system to study development because it can be induced to spawn frequently in the laboratory. The embryos of A. mexicanus are translucent, slightly larger than those of zebrafish, produced in large quantities, and develop into sexually mature adults in approximately 8-12 months. Their period of maximal spawning capacity is about 5 years. This protocol describes the workflow needed in an A. mexicanus culture facility during a typical breeding week and includes the details of fish system maintenance and the temperature control regime for maximal spawning.
A. mexicanus is a tropical fish that lives in rivers originating in limestone plateaus (surface fish) and in pools in limestone caves (cave fish)8. Limestone dissolves to produce hard water, and A. mexicanus thrives in hard water. Fish adapted to hard water conditions can tolerate a range of salty conditions, but generally breed in specific ones9. Induction of spawning behavior is accomplished by a combination of factors. Because fish are cold-blooded and rely on their environment to maintain homeostasis, their metabolism is sensitive to environmental changes and they react more quickly to stressors10. A. mexicanus should be cultured in aquatic systems under carefully regulated conditions of water flow, pH, conductivity, osmotic pressure, lighting, and water temperatures.
In the Jeffery laboratory, fish are maintained in two running water systems: (1) a “baby system” for young adult fish prior to sexual maturity and (2) an adult (or main) system for sexually mature, breeding adults. The “baby system” consists of 8 L and 15 L tanks supplied with running water. The “baby system” is seeded by fry and young metamorphosized juveniles grown from larvae in smaller (1-10 L) tanks, in which water is exchanged weekly. Larvae, fry, and juveniles are extremely food dependent and must be fed live food (brine shrimp) once a day to ensure a high rate of survival. Young juveniles from the “baby system” are placed into the adult system after about 1-1.5 years. At first, they are fed pulverized tetra flakes, and after further growth they are transferred to the regular adult feeding regime. Sexual maturity can be assessed by abdominal volume in females, and methods for determining sex have been described11. In the adult system, water is exchanged automatically in 42 L tanks 3 times per 24-hour period. The adult system is monitored daily by visual inspection and automatic temperature, pH, and conductivity readings from probes. The optimal pH is around 7.4 and can range between 6.8-7.5, the base temperature of the system is 72/73 °F, and the ideal conductivity ranges between 600-800 mS. Automatic readings are displayed on a controller screen, and visual checks of water pressure are read at flow meters distributed throughout the system. Independent checks on water quality are made weekly by testing temperature and measuring water quality parameters for pH, ammonia, and nitrate using a colorimetric test. Ammonia and nitrate levels are kept at or close to zero by adding beneficial bacteria (e.g., Nutafin Cycle) to the system. Room lighting is controlled by a timer adjusted to 14-hour light and 10-hour dark periods. In addition to the overall water quality parameters mentioned above, the following considerations need special attention during a breeding week.
The first consideration is photoperiod, as fish (even cave fish in the laboratory) depend on light cycles to set their circadian clock. Circadian rhythms can affect everything from breeding and feeding to immune system health12,13 and must be consistent for maximum health benefits. Fish are maintained in a running-water system on a 14-hour light and 10-hour dark photoperiod. The surface fish generally begin spawning an hour after the system has been darkened, and light introduced during this period can interfere with and terminate spawning. The spawning of blind cave fish is less disturbed by light. Compared to surface fish spawning, cave fish spawning is delayed, usually beginning four to five hours after the system has been darkened.
The second consideration is nutrition. Adult fish are normally fed a diet of tetra flakes once a day. Prior to spawning, fish are fed a protein-rich diet supplemented with extra amounts of tetra flakes and other food: egg yolk flakes and occasionally living California blackworms (Lumbriculus variegatus) to compensate for protein loss due to egg production during the previous spawning cycle. During the breeding week, fish are fed twice per day, once in the morning and again in the afternoon/evening. Fish feeding only once a day but with a single very large portion of food should be avoided, as this can cause malnutrition14.
The third consideration is space. The space requirements are based on the average body mass of an adult as well as behavioral considerations, such as whether the fish have schooling behavior or aggressive behavior. Over or under-crowding tanks can lead to heightened aggression and constant stress, making fish vulnerable to injury from their tank mates and reluctant to participate in spawning15. We typically house 10-20 fish per 42 L tank.
The fourth consideration is temperature. As mentioned above, fish are cold-blooded animals and rely on the environment to maintain body temperature. Because temperature has a direct effect on metabolic processes, changes in temperature can trigger behavioral alterations in fish16. This breeding program consists of two-week cycles in temperature: the first week introduces a temperature spike to 78 °F, and the next week maintains a static temperature of 72 °F. During the first (breeding) week, plastic-edged breeding nets are placed at the bottom of the tanks each evening. The breeding nets serve as a barrier between the fish in the tanks and the spawned eggs, which would otherwise be consumed. The temperature is raised by 2 °F per day to a maximum of 78 °F by mid-week, and spawning is induced according to the light cycle in the first 2-3 evenings of this week. The temperature is then lowered by 2 °F increments to 72 °F during the remaining days of the week, and base temperature is maintained until the beginning of the next breeding week. Breeding is usually stimulated no more than twice a month to allow the fish time to recover.
Overall, this method allows for the spawning of large quantities of the highest quality embryos over a longer period of time.
Protocol
This procedure has been approved by Institutional Animal Care guidelines of the University of Maryland, College Park (Currently IACUC 469 #R-NOV-18-59; Project 1241065-1).
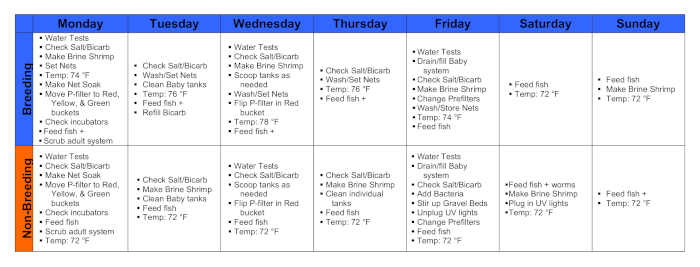
Figure 1. Calendars during a breeding week and a non-breeding week. Please click here to view a larger version of this figure.
1. Monday
- At 9-10 AM, perform water tests and steps 1.1.1-4 below.
- Record the room, tank, and reservoir temperatures using a thermometer.
- Record the ammonia, nitrate, and nitrate levels with a colorimetric test kit.
- Record the pH from the monitoring system as well as the colorimetric test kit.
- Record the conductivity from the carboy monitor and the main system monitor.
- Beginning at 10 AM, feed all fish.
- Feed all fish in the adult system with tetra flakes, crushing the flakes in the tanks with young fish. Feed only as many flakes as a tank of fish can consume completely in 3-5 minutes, about a “finger pinch”.
- Check the incubator used to house fingerbowls of developing embryos and change the water if needed.
- Open the embryo incubator and check the water levels in all reservoirs. If they are running low on water, add system water. Check incubator temperature settings. Raise embryos at 73-77 °F (23-25 °C).
- Clean live feed.
- To clean the blackworms, remove the uncovered Tupperware basins containing their cultures from the live feed refrigerator and pour off the excess water above the worm clusters into the sink. Using distilled water, suspend and rinse the worms repeatedly until the pour-off water is clear.
- Add enough clean water so that the worm clusters are about half covered. Replace remaining worms in the live feed refrigerator, uncovered.
- Feed fish.
- At least 30 min after the first feeding, feed fish in the tanks in which breeding is desired with egg yolk flakes, blackworms, or both. Add a “fingerpinch” of yolk flakes per tank. Add enough blackworm clusters to allow each fish in the tank to consume about 5-10 worms.
- At 10 AM- 1 PM, set the water temperature to 74 °F.
- Scrub the breeding tanks as needed and set the breeding nets.
- Clean the tanks and place the nets at least one hour after the last feeding. Clean all tanks that nets are placed in beforehand. Set breeding nets carefully so as not to interfere with the air supply to the tank.
2. Tuesday
- Collect the embryos and wash all breeding nets.
- At 9-10 AM, remove the breeding nets from the bottom of adult system tanks. Gently rinse the embryos into a hand-held net using the hose attached to the carboy, and invert the hand-held net into a fingerbowl of clean system water to expel the embryos.
- Collect and wash each set of embryos, and then place in a fingerbowl of clean system water containing 0.00003% methylene blue (blue water). If there is an exceptionally large number of embryos from a single tank, separate them into multiple bowls. The concentration of living embryos should be about 100 per 200 mL of blue water in each fingerbowl.
- Estimate the time of fertilization by staging the embryos under a microscope using the published A. mexicanus developmental time table17.
- Monitor the fingerbowls containing embryos frequently. Remove dead or deformed embryos and debris, such as uneaten food or feces, with a Pasteur pipette. Change the blue water in fingerbowls frequently.
- Place the fingerbowls in an incubator for 5-7 days. At this time, the yolk has been used up and feeding cultures with living brine shrimp is necessary for further development.
- Take spawning data.
- For every tank that drops embryos, record the following information.
- Record the date and the tank number.
- Record the approximate number of embryos dropped (Figure 2):
High (500+)
Medium (200-500)
Low(<200) - Record the quality of embryos dropped (Figure 2):
High (>75% alive)
Medium (25-50% alive)
Low (<25% alive) - Estimate the original spawning time by consulting the Astyanax mexicanus staging table17.
- Record the temperature that the system was set at when the fish spawned.
- For every tank that drops embryos, record the following information.
- Feed all fish.
- Set the water temperature to 76 °F.
- Prepare live feed.
- Feed fish #2.
- Scoop excess food and debris from tanks and scrub before resetting nets.
3. Wednesday
- Repeat steps 2.1-2.2. Collect embryos and wash all breeding nets.
- Take spawning data as before.
- Perform water tests as before.
- Feed all fish.
- Set the water temperature to 78 °F.
- Prepare the live feed.
- Check on embryos in the incubator.
- Clean and change the water of the embryos in fingerbowls that will eventually be used to replenish the general adult breeding stock. Use methylene blue-treated system water.
- Feed fish again.
- Clean tanks as needed and reset nets.
4. Thursday
- Repeat steps 2.1-2.2. Collect embryos and wash and store breeding nets.
- Take spawning data as before.
- Set water temperature to 76°F.
- Clean the live feed.
- Clean all individual tanks.
- Check on embryos in the incubator.
- Feed fish #2.
5. Friday
- Feed all fish.
- Set water temperature to 74 °F.
- Clean the live feed.
- Check on embryos in the incubator.
6. Saturday
- Feed fish.
7. Sunday
- Feed fish.
Representative Results
We generally breed and spawn the descendants of surface fish originally collected at Nacimiento del Rio Choy in San Luis Potosi, Mexico (Rio Choy surface fish) and San Solomon Springs in Balmorhea State Park, Texas (Texas surface fish) and cave fish derived from Cueva de El Pachón (Pachón cave fish) in Tamaulipas, Mexico, and Cueva de los Sabinos (Los Sabinos cave fish) and Sotano de la Tinaja (Tinaja cave fish) in San Luis Potosi, Mexico.
Throughout a breeding week, data is collected for various tanks. The spawned embryos in each tank are observed for quantity and quality. The quantity is recorded as high, medium, or low. A quantity of high is recorded if the number of embryos dropped is over 500, a quantity of medium is recorded if the number of embryos dropped is between 200-500, and a quantity of low is recorded if the number of embryos dropped is less than 200. The quality is similarly recorded as high, medium, or low. A quality of high is recorded if more than 75% of the embryos in the bowl are alive, a quality of medium is recorded if about 25% to 75% of the embryos in the bowl are alive, and a quality of low is recorded if less than 25% of the embryos are alive. These indications of quantity and quality are then assigned a number with high being 3, medium being 2, and low being 1. If there were no embryos spawned or no live embryos in the spawning, a number of 0 is assigned.
The breeding data from July of 2017 through March of 2020 for the Rio Choy and Texas surface fish and the Los Sabinos, Tinaja, and Pachón cave fish is shown in Figure 2. The data were analyzed by breeding week and by averaging the numbers resulting from each day of collecting embryos during a single breeding week. The data indicates breeding was continuous throughout the year in Rio Choy and Texas surface fish and in Pachón cave fish. The quantity and quality of most Rio Choy surface fish was between low and high, while the quantity and quality of most Texas surface fish and Pachón cave fish was between low and medium. The occurrence of spawning was not continuous in Tinaja or Los Sabinos cave fish: spawning was low or non-existent during the late summer (July) to autumn (October). Although the lowest levels of spawning were recorded for Los Sabinos cave fish, the quality of embryos was the best. In general, surface fish show better spawning quantity and quality than cave fish.
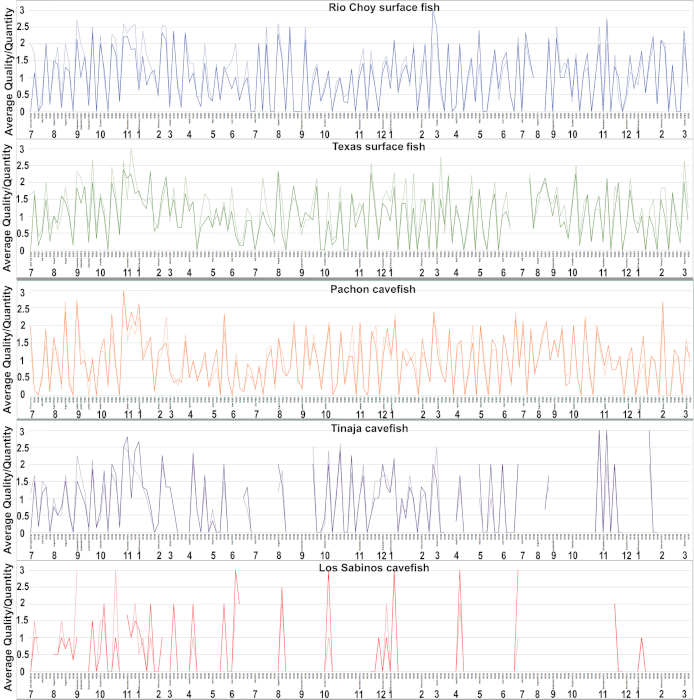
Figure 2. Breeding data for different surface fish and cave fish populations from July of 2017 through March of 2020. From top to bottom is Rio Choy surface fish, Texas surface fish, Pachón cave fish, Tinaja cave fish, and Los Sabinos cave fish. Unbroken lines: quality of spawn. Broken lines: quantity of spawn. Weeks with no lines represent periods in which spawning was not attempted. Please click here to view a larger version of this figure.
Discussion
Astyanax mexicanus is a novel biological model that spawns frequently and can be bred easily in the laboratory1,2. Because we are interested in the developmental mechanisms that underlie evolutionary changes in A. mexicanus cave fish, the production and use of embryos is vital to our research goals. The primary purpose of maintaining an adult stock of fish is the production of embryos and young fry for use in developmental experiments and for replenishment of adult breeding stocks. Occasionally, adults may also be used for physiology, behavioral, or genetic experiments. Genetic experiments require paired mating or in vitro fertilization18. For in vitro fertilization animals exhibiting spawning behavior19 may be removed from tanks and used for crosses during the temperature induced breeding regime.
To maximize the quality and quantity of embryos for research, there are operational details that require attention prior to the breeding week. When manipulating fish in the tanks, use only designated hand-held nets, immerse them in a net soaking solution between each use, and rinse them with hot tap water between uses to prevent contamination between the tanks. Carefully wash and disinfect all instruments used to care for the fish. Remove and clean the breeding nets in system water before replacing them in the tanks. At the end of the week, all breeding nets are stored dry on shelves in the fish room. During a breeding week, check each tank for embryos in the breeding nets or for fish exhibiting spawning behavior (i.e., swimming around in circles paired against each other), as the females in those tanks are likely ready to spawn. This should be done using red light during the dark hours of the photoperiod. A. mexicanus spawn in the dark by scattering eggs and sperm in clouds. A tank with a population of about 10-20 fish and a male to female ratio of about 1:1 can produce up to 500 fertilized embryos in one spawning per tank, and each tank of fish may spawn two or three times over the course of a single breeding week. In a good spawn, most embryos will survive through hatching, a critical stage. Cultures should be checked frequently and unfertilized eggs, dead or deformed embryos, or debris, including any food remnants or parasites, should be removed. Cultures with mostly living embryos should be “cleaned” every few hours during the embryonic and early larval periods by manually removing dead or abnormal embryos (which will eventually die) and debris using a Pasteur pipette. Cultures with a large proportion of dead embryos can still be used by transferring the normally developing embryos to new fingerbowls with fresh blue water. Usually, this process must be repeated several times to obtain the purest cultures of living embryos. In either case, a final concentration of about 100 embryos per 200 mL is ideal because crowding of embryos can affect development, especially for cave fish. Cultures should be further “cleaned” over time by periodically removing most of the water from fingerbowls and replacing with it with fresh blue water. Cultures “cleaned” frequently usually provide the highest quality of embryos.
In addition to natural breeding, hormonal stimulation20 or in vitro fertilization18 is also potentially useful in obtaining embryos. For these purposes, however, fish must be healthy and ready for natural spawning (exhibiting spawning behavior), and a much smaller yield of embryos than obtained under the temperature-induced spawning conditions should be expected.
A limitation of temperature-induced breeding under the conditions described above is that surface fish and cave fish spawn at different times, the former during the early evening and the latter from after midnight until early morning. This situation cannot be avoided by shifting the photoperiod because regular feeding and fish system maintenance usually need to be carried out during the light (day-time) period of the cycle. In principle, however, schedules can be adjusted so that fish spawn at similar times by maintaining the two morphs on different light-dark cycles (and in different fish rearing systems) for close-to-simultaneous spawning. Furthermore, if two temperature control systems are available, fish could be cultured in different systems, and by alternating weekly temperature rises, spawning could be done on a weekly rather than bi-monthly schedule, doubling the capacity for obtaining embryos.
Whether used for scientific research, teaching, or biotechnology, A. mexicanus is an excellent model system for exploring the fascinating questions surrounding the evolution of development. For scientific research, this system is useful for investigating molecular, genetic, and evolutional mechanisms of eye development and disease. The eye is an extraordinary organ in terms of its structure, function, and development. Vision is acquired during embryonic development as a result of the coordinated formation and growth of several different eye tissues. The precise mechanisms by which this occurs are still largely unknown. The structure of mammalian and fish eyes are similar. The natural eye phenotypes of A. mexicanus is an excellent model system to explore the molecular and cellular mechanisms and genetic pathways involved in eye development and degeneration21,22. This knowledge could be used to develop prevention strategies and treatments for inherited eye diseases. Pigmentation studies is another area in which A. mexicanus is making a valuable contribution23. In teaching, A. mexicanus embryos can be used to illustrate the general principles of embryonic development and in instructional experimentation for beginning students. In biotechnology, with the recent development of genomic DNA editing24 and especially CRISPR/Cas-9 genomic engineering technology25, A. mexicanus embryos are a valuable resource to explore gene functions. Each of these applications is aided by the spawning of large quantities of high-quality embryos, which can be achieved by the incremental temperature breeding regime described in this communication.
Divulgazioni
The authors have nothing to disclose.
Acknowledgements
We thank David Martasian, Diedre Heyser, Amy Parkhurst, Craig Foote, and Mandy Ng for valuable contributions to the Jeffery laboratory A. mexicanus culture facility. The research in the Jeffery laboratory is currently supported by NIH grant EY024941.
Materials
| Blackworms | Eastern Aquatics, Lancaster, PA | None | |
| Breeding Nets | Custom made | ||
| Brine shrimp eggs | AquaCave Lake Forest, IL. | None | |
| Colorimetric test kit | Petco | SKU:11916 | API Freshwater pH Test Kit |
| Egg yolk flakes | Pentair, Minneapolis, MN | None | |
| Fingerbowls | Carolina Biological Supply | 741004 | Culture dishes, 4.5 in, 250 mL |
| Hand held nets | Any Pet Store | ||
| Incubator for embryos | Fisher Scientific | 51-029-321HPM | 405 L |
| Instant Ocean sea salts | Spectrum Brands, Blacksburg, VA | None | |
| Methylene Blue | Sigma-Aldrich, St. Louis, MO | M9140 | |
| Pasteur Pipettes | Fisher Scientific | 13-678-20 | 5.75 in. |
| Net soaking solution | Any Pet Store | ||
| Nutrafin Cycle | Amazon | None | Bacterial boost |
| Refrigerator for live feed | Any source | ||
| Stereomicroscope | Any source | ||
| Thermometer | Any source | ||
| Tetra Tropical Crisps | Spectrum Brands, Blacksburg, VA | None |
Riferimenti
- Jeffery, W. R. Cavefish as a model system in evolutionary developmental biology. Biologia dello sviluppo. 231, 1-12 (2001).
- Jeffery, W. R. Emerging model systems in evo-devo: cavefish and mechanisms of microevolution. Evolution & Development. 10, 265-272 (2008).
- Jeffery, W. R. Evolution and development in the cavefish Astyanax. Current Topics in Developmental Biology. 86, 191-221 (2009).
- Protas, M. E., et al. Genetic analysis of cavefish reveals molecular convergence in the evolution of albinism. Nature Genetics. 38, 107-111 (2006).
- Protas, M., Conrad, M., Gross, J. B., Tabin, C. J., Borowsky, R. Regressive evolution in the Mexican cave tetra, Astyanax mexicanus. Current Biology. 17, 452-454 (2007).
- O’Quin, K. E., Yoshizawa, M., Doshi, P., Jeffery, W. R. Quantitative genetic analysis of retinal degeneration in the blind cavefish. PLoS ONE. 8 (2), 57281 (2013).
- Yoshizawa, M., et al. Distinct genetic architecture underlies the emergence of sleep loss and prey-seeking behavior in the Mexican cavefish. BMC Biology. 13, 15 (2015).
- Elliot, W. R. The Astyanax caves of Mexico. Cavefishes of Tamaulipas, San Luis Potosi, and Guerrero. Association for Mexican Cave Studies Bulletin. 26, 1 (2018).
- Luo, S., Wu, B., Xiong, X., Wang, J. Effects of total hardness and calcium:magnesium ratio of water during early stages of rare minnows (Gobiocypris rarus). Comparative Medicine. 66, 181-187 (2016).
- Balasch, J. C., Tort, L. Netting the stress responses in fish. Frontiers in Endocrinology. 10, 62 (2019).
- Borowsky, R. . Determining the sex of adult Astyanax mexicanus. , (2008).
- Paschos, G. Circadian clocks, feeding time, and metabolic homeostasis. Frontiers in Pharmacology. 6, 112 (2015).
- Scheiermann, C., Kunisaki, Y., Frenette, P. S. Circadian control of the immune system. Nature Reviews Immunology. 13, 190-198 (2013).
- Williams, M. B., Watts, S. A. Current basis and future directions of zebrafish nutrigenomics. Genes & Nutrition. 14, 34 (2009).
- Harper, C., Wolf, J. C. Morphologic effects of the stress response in fish. ILAR Journal. 50, 387-396 (2009).
- Neubauer, P., Andersen, K. H. Thermal performance in fish is explained by an interplay between physiology, behavior and ecology. Conservation Physiology. 7 (1), 025 (2019).
- Hinaux, H., et al. Developmental staging table for Astyanax mexicanus. Zebrafish. 8 (4), (2011).
- Borowsky, R. . In vitro fertilization of Astyanax mexicanus. , (2008).
- Simon, V., Hyacinthe, C., Rétaux, S. Breeding behavior in the blind Mexican cavefish and its river-dwelling conspecific. PLoS One. 14 (2), 0212591 (2019).
- Harvey, B. J., Carolsfield, J. Induced Breeding in Tropical Fish Culture. International Development Research Centre. , (1993).
- Ma, L., Parkhurst, A., Jeffery, W. R. The role of a lens survival pathway including sox2 and aA-crystallin in the evolution of cavefish eye degeneration. EvoDevo. 5, 28 (2014).
- Krishnan, J., Rohner, N. Cavefish and the basis for eye loss. Philosophical Transactions of the Royal Society B: Biological Sciences. 5 (372), 20150487 (2017).
- Bilandžija, H., Abraham, L., Ma, L., Renner, K., Jeffery, W. R. Behavioral changes controlled by catecholaminergic systems explain recurrent loss of pigmentation in cavefish. Proceedings of the Royal Society. 285, (2018).
- Ma, L., Jeffery, W. R., Essner, J. J., Kowalko, J. E. Genome editing using TALENs in blind Mexican cavefish. PLoS ONE. 1093, 0119370 (2015).
- Klaassen, H., Wang, Y., Adamski, K., Rohner, N., Kowalko, J. E. CRISPR mutagenesis confirms the role of oca2 in melanin pigmentation in Astyanax mexicanus. Biologia dello sviluppo. 441, 313-318 (2018).

