Establishing Pollination Requirements in Japanese Plum by Phenological Monitoring, Hand Pollinations, Fluorescence Microscopy and Molecular Genotyping
Summary
A methodology for the determination of pollination requirements in Japanese plum-type hybrids is described, which combines field- and laboratory-pollinations and observations of pollen tubes under the fluorescence microscopy with the identification of S-genotypes by PCR and the monitoring of flowering for the selection of pollinizers.
Abstract
The Japanese plum cultivars commonly grown are interspecific hybrids derived from crosses between the original Prunus salicina with other Prunus species. Most hybrids exhibit gametophytic self-incompatibility, which is controlled by a single and highly polymorphic S-locus that contains multiple alleles. Most cultivated hybrids are self-incompatible and need pollen from a compatible donor to fertilize their flowers. Establishing pollination requirements in Japanese plum is becoming increasingly important due to the high number of new cultivars with unknown pollination requirements. In this work, a methodology for the determination of pollination requirements in Japanese plum-type hybrids is described. Self-(in)compatibility is determined by hand-pollinations in both the field and in the laboratory, followed by monitoring pollen tube elongation with fluorescence microscopy, and also monitoring fruit maturation in the field. Selection of pollinizer cultivars is assessed by combining the identification of S-genotypes by PCR analysis with the monitoring of flowering time in the field. Knowing the pollination requirements of cultivars facilitates the selection of cultivars for the design of new orchards and allows the early detection of productivity problems related with pollination deficiency in established orchards.
Introduction
Japanese plum (Prunus salicina Lindl.) is native to China1. In the 19th century, this crop was introduced from Japan to the United States, where it was intercrossed with other North American diploid plums2. In the 20th century, some of these hybrids were spread to temperate regions around the world. Nowadays, the term “Japanese plum” refers to a wide range of interspecific hybrids derived from crosses between the original P. salicina with up to 15 other diploid Prunus spp.3,4,5.
Japanese plum, like other species of the Rosaceae family, exhibits Gametophytic Self-Incompatibility (GSI), which is controlled by a single and highly polymorphic S-locus containing multiple alleles6. The S-locus contains two genes that encode a ribonuclease (S-RNase) expressed in the pistil, and an F-box protein (SFB) expressed in the pollen grain7. In the self-Incompatibility reaction, when the S-allele expressed in the pollen grain (haploid) is the same as one of the two expressed in the pistil (diploid), the growth of the pollen tube across the style is arrested due to the degradation of the pollen tube RNA by the action of the S-RNase8. Since this process prevents fertilization of the female gametophyte in the ovule, GSI promotes the outcrossing between cultivars.
Although some Japanese plum cultivars are self-compatible, most cultivars currently grown are self-incompatible, and need pollen from inter-compatible donors to fertilize their flowers3. In stone fruit species of genus Prunus such as almond9, apricot10,11,12 and sweet cherry13, pollination requirements of cultivars can be established by different approaches. Self-(in)compatibility can be determined by self-pollination of flowers in the field and subsequent monitoring of fruit set, or by semi-in vivo self-pollinations at controlled conditions in a laboratory and the observation of pollen tubes under the microscope14,15,16,17,18. Incompatibility relationships among cultivars can be determined by cross pollinations in the field or the laboratory using pollen of the potential pollinizer cultivar, and by the identification of S-alleles of each cultivar by PCR analysis14,15,16,19,20,21,22. In species such as sweet cherry or almond, self-(in)compatibility can be also assessed by the identification of particular S alleles associated to self-compatibility, as S4’ in sweet cherry13 or Sf in almond23.
Several plum breeding programs from the main producing countries are releasing a number of new cultivars2,14, many of them with unknown pollination requirements. In this work, a methodology for the determination of pollination requirements in Japanese plum-type hybrids is described. Self-(in)compatibility is determined by self-pollinations in both the field and the laboratory, followed by observations of pollen tubes under the fluorescence microscopy. Selection of pollinizer cultivars combines the identification of S-genotypes by PCR analysis with the monitoring of flowering time in the field.
Protocol
1. Hand-pollination in the field
- Pollen extraction
- To obtain pollen, collect flower buds at stage D24, according to stage 57 on the BBCH scale25,26.
NOTE: More flower buds are necessary in Japanese plum than in other Prunus species because their anthers produce less pollen. - Remove the anthers using a plastic mesh (2 mm x 2 mm pore size) and place them on paper at room temperature for 24 h until anther dehiscence.
- Sieve the pollen grains through a fine mesh (0.26 mm x 0.26 mm pore size), and conserve them in a 10 mL glass tube with a cap at 4 °C until use.
- To obtain pollen, collect flower buds at stage D24, according to stage 57 on the BBCH scale25,26.
- Pollination of emasculated flowers
- When between 10%–20% of flowers are open, select and label several branches. Remove open flowers and young buds, leaving only flower buds at stage D24, according to stage 57 on the BBCH scale25,26.
- Remove the petals, sepals, and stamens of between 800 and 1,000 flower buds per treatment with either fingernails or tweezers.
- Hand pollinate the pistils with the help of a fine paintbrush 24 h after emasculation. Some branches containing half of the pistils with pollen of the same cultivar, and the other half with compatible pollen from other cultivar as a control. Be careful not to contaminate the fingertip or paintbrush with pollen grains from other cultivars.
- Record weekly counts of flowers and developing fruits to characterize fruit drop pattern and quantify the final fruit set in each pollination treatment.
- Supplementary pollination in the field
- A few days before the first flowers open, enclose selected trees in a 0.8 mm mesh cage to avoid the arrival of pollinating insects.
- When 10%–20% flowers are open, select and label several branches per pollination treatment, leaving 1,000–1,500 flowers per treatment.
- On the next day, when flowers are open, pollinate each flower with the help of a paintbrush with the corresponding pollen (pollen from the same cultivar for self-pollination, and from other compatible cultivars as cross-pollination control).
- Pollinate every other day until all flowers open.
- Record weekly counts of flowers and developing fruits from anthesis to harvest to characterize fruit drop pattern and quantify final fruit set in each pollination treatment.
2. Hand-pollinations in the laboratory
- Collect 50–100 flowers at stage D24, according to stage 57 on the BBCH scale25,26.
- In the laboratory, emasculate 30 flowers per treatment (self- and cross-pollination).
NOTE: Emasculation should be proceeded carefully to avoid any damage on the pistils. - Make a fresh cut on the base of each flower pedicel underwater before placing it on a piece of wet florist foam (one piece of foam for each pollination treatment).
- Hand pollinate each pistil 24 h later using a fine paintbrush with pollen collected previously (see section 1.1). Pollinate one set of pistils with pollen from the same cultivar, and the other set with pollen from a compatible cultivar as control.
- Leave the pollinated pistils 72 h after pollination at room temperature. The floral foam should be continuously wet with water.
- Fix the pistils in a fixative solution of ethanol/acetic acid (3:1) for at least 24 h at 4 °C. Replace the fixative with 75% ethanol. Samples can be conserved in this solution at 4 °C until use27.
NOTE: Ensure that the samples are completely submerged in the solution.
3. Microscopic observations
- Evaluation of in vitro pollen germination
- To elaborate pollen germination medium, dissolve 25 g of sucrose on 250 mL of distilled water, then add 0.075 g of calcium nitrate [Ca(NO3)2] and 0.075 g of boric acid (H3BO3).
- Add 2 g of agar to the solution and mix until completely dissolved28.
- To sterilize the medium, autoclave it at 120 °C for 20 min. Cool the medium and, before it solidifies, distribute 3 mL per sterile Petri dish (55 mm x 12 mm) in a sterile laminar flow hood. After medium solidification, conserve the Petri dishes wrapped in aluminum foil at 4 °C until use.
- Spread the pollen of each cultivar previously used as pollen donor in the controlled pollinations in two Petri dishes and incubate them at 25 °C for 24 h.
NOTE: The inoculated culture media can be observed with microscopy immediately after or stored at -20 °C until use. For this purpose, the Petri dishes should be changed from the freezer to the fridge 24 h before microscopy observations. - To observe the pollen grains, prepare 1% (v/v) aniline blue solution that stains callose. First, prepare a 0.1 N potassium phosphate tribasic (K3PO4) solution by dissolving 7.97 g of K3PO4 in 1,000 mL of distilled water. To elaborate the 1% (v/v) aniline blue solution, dissolve 1 mL of aniline blue in 100 mL of 0.1 N K3PO4.
- Add 2–3 drops of aniline blue solution to each Petri dish plate and observe after 5 min under a UV epifluorescence microscope using exciter filter BP340-390 and barrier filter LP425. Count viable and non-viable pollen grains in three fields per plate, each field containing 100-200 pollen grains, in two Petri dishes for each cultivar.
- Pollen tube growth
- Rinse the fixed pistils with distilled water three times (1 h each, 3 h in total) and transfer to 5% (w/v) sodium sulphite (Na2SO3) at 4 °C for 24 h. To prepare this solution, dissolve 5 g of sodium sulphite in 100 mL of distilled water.
- Autoclave the pistils at 120 °C for 8 min in 5% (w/v) sodium sulphite to soften the tissues.
- Squash softened pistils in a drop of 1% (v/v) aniline blue solution under a cover glass on a slide to stain callose.
- Observe pollen tube growth along the style under a microscope with UV epifluorescence using exciter filter BP340-390 and barrier filter LP425.
4. Determining incompatibility relationships
- DNA extraction from leaves
- To extract DNA, collect 3–4 young leaves of each cultivar in the field, preferably in spring.
NOTE: DNA can also be extracted from mature leaves, but DNA from young leaves has less phenolic compounds. - Isolate DNA using a commercial kit and follow the provided protocol kit (see Table of Materials).
- Quantify the DNA concentration and evaluate the quality of the DNA of each sample at 260 nm in an UV-Vis microvolume spectrophotometer. Adjust the DNA concentration to 10 ng/μL.
- To extract DNA, collect 3–4 young leaves of each cultivar in the field, preferably in spring.
- PCR conditions for fragment amplification
- Label 0.2 mL PCR tubes and caps.
- Prepare the PCR reagents according to Table 1 and let them thaw on ice.
- Set up a volume of master mix of each pair of primers in a 1.5 mL microtube according to the number of reactions plus 10% of excess, considering a volume of 16 μL per reaction. Add the reagents following the order in Table 1 and mix thoroughly.
- Aliquot 16 μL of master mix into each 0.2 mL PCR tube containing 4 μL of DNA template or 4 μL of sterilized distilled water as negative control (C-). Use DNA of cultivars with known genotype as positive controls. Mix gently, close the reaction tubes with the caps, and centrifugate at 2,000 x g for 30 s to collect the entire volume at the bottom of the reaction tube.
- Place the reaction tubes in the thermocycler and set up the PCR program using the following temperature profile: an initial step of 3 min at 94 °C, 35 cycles of 1 min at 94 °C, 1 min at 56 °C and 3 min at 72 °C, and a final step of 7 min at 72 °C20.
- Electrophoresis and estimation of fragment size
- To prepare a 1.7% (w/v) agarose mini gel, dissolve 0.68 g of agarose and 40 mL of 1x TBE buffer into a 100 mL Erlenmeyer flask. Melt the solution by heating in a microwave at 600 W at 30 s intervals to avoid boiling.
- Let the solution stay on the bench to cool down, and then add 3.5 μL of nucleic acid staining solution.
- Place the gel tray into the casting stand and put the selected comb into the gel mold. Be sure to have enough wells for each PCR product and DNA ladder.
- Pour the agarose solution into the gel mold and let it cool down until polymerization. Remove the comb of the polymerized gel. Place the gel in the horizontal electrophoresis system containing enough 1x TBE buffer to cover the surface of the gel.
- Load the first and last wells with 2 μL of the DNA molecular weight ladder (1 kb DNA Ladder). Load 3 μL of each product of the PCR in the other wells. Close the chamber, turn on the power, and run the gel at 100 V for 30 min.
- Observe the gel under UV light using a gel documentation system. Use the DNA molecular weight ladder to determine the size of the amplified fragment and compare it with the positive controls in order to identify the corresponding alleles.
5. Monitoring flower dates
- Monitor the phenology of different trees of each cultivar at flowering over different years. Establish the length of the flowering period from the first (about 5%) to the last open flowers (about 95%). Full bloom is considered when at least 50% of flowers are at stage F24, according to stage 65 on the BBCH scale25,26.
- To compare the flowering dates of inter-compatible cultivars and determine those that coincide at flowering time every year, elaborate a calendar of flowering times with data from several years.
Representative Results
Each Japanese plum flower bud contains an inflorescence with 1–3 flowers. As in other stone fruit species, each flower is made up of four whorls: carpel, stamens, petals, and sepals, which are fused forming a cup at the base of the flower. Flower structures are smaller than other stone fruits, with a short and fragile pistil surrounded by the stamens that contain a small amount of pollen grains. At full bloom, the flowers of each inflorescence appear separated on short stalks, showing the white petals forming a balloon surrounded by the green sepals (stage D, 57 BBCH) (Figure 1A) in the days before anthesis. The flower is fully open at anthesis, showing the anthers and the pistil (stage F, 65 BBCH) (Figure 2A). Like other temperate Prunus spp., the flower buds open first, and the leaf buds sprout several days later. This makes blooming trees look spectacular showing a great number of flowers but no leaves.
Hand-pollinations in Japanese plum required the collection of flowers at the balloon stage previous to anthesis (Figure 1A), in which the pistil and stamens are nearly mature, but the anthers are still undehisced. This stage prevents the arrival of insects carrying external pollen because the petals are still closed. Although the number of flowers is much higher than in other Prunus species, most of them (85%–95%) are not able to set fruit. As a result, using a high number of flowers for pollination experiments is mandatory. The undehisced anthers could be easily separated from the flower in the laboratory and extended on a piece of paper (Figure 1B), where the anthers are dehisced after 24 h at room temperature, showing the pollen grains (Figure 1C). Then, the pollen grains were easily sieved through a fine mesh (Figure 1D), and could be used immediately or stored until use, both for field (Figure 2A–D) and/or laboratory pollinations (Figure 2E,F).
The monitoring of fruit drop of hand-pollinated flowers after emasculation (Figure 2A,B) or supplementary pollination of non-emasculated flowers (Figure 2C,D) showed clear differences between treatments. Most flowers dropped 2–3 weeks after pollination in both treatments. All self-pollinated flowers dropped. However, 4% of self-pollinated flowers from the supplementary pollination treatment remained in the tree until harvest, indicating that this cultivar (Rubirosa) behaves as self-compatible (Figure 3). The behavior of cross-pollinated flowers used as control also varied between treatments. Cross-pollinated flowers with supplementary pollination dropped until 3 weeks after pollination, resulting in 7% of fruit set. However, all emasculated cross-pollinated flowers dropped coinciding with the drop of all self-pollinated flowers.
For hand pollinations in the laboratory, the pistils were placed in wet florist foam after a fresh cut on the base of each flower pedicel underwater (Figure 2E) and pollinated 24 h later with the help of a fine brush (Figure 2F). In self-pollinated flowers, self-compatible cultivars showed at least one pollen tube reaching the base of the style in most of the pistils examined, while in self-incompatible cultivars, pollen tube growth was arrested in the upper style (Table 2).
In vitro pollen germination differed significantly between cultivars (Table 3). Good pollen viability was considered when more than 20% of pollen grains showed a pollen tube longer than its length after 24 h in the culture medium (Figure 4A). However, when most of pollen grains did not germinate (Figure 4B), the cultivar was considered male sterile and not suitable as a pollinator.
Like in other Prunus, the pistil is made up of three structures: stigma, style, and ovary. The ovary has two ovules, and at least one of them should be fertilized for fruiting. During pollination, pollen grains are transferred to the stigma, where germination occurred within 24 h (Figure 4C). Each germinating pollen grain produced a pollen tube, which grew through the pistil structures. In self-incompatible cultivars in which the pollen grains have an S allele that coincides with one of the two S alleles of the pistil, the pollen tube stopped growing in the upper third of the style (Figure 4D), preventing the arrival to the ovary and the subsequent fertilization. However, when S allele of the pollen grain is different with that of the pistil, the pollen tube could grow through the style (Figure 4E), reach the ovary (Figure 4F) and fertilize an ovule.
PCR analysis (Table 1) was carried out using primers from conserved regions of S-RNase of sweet cherry and Japanese plum (Figure 5). The primer set used, PruC2-PCER and PruT2-PCER (See Table of Materials), allowed to determine the size of both S-alleles in each cultivar. The amplified fragments were run in agarose gel by electrophoresis, and seven different S-alleles (Sa, Sb, Sc, Se, Sf, Sh, Sk) were identified in the cultivars analyzed. The fragment sizes ranged between 393 and 1,580 bp using PruC2-PCER (Figure 5) and between 820 and 1,993 bp using PruT2-PCER. Six S-genotypes were identified (SaSb, SbSc, SbSf, ScSh, SeSh, SfSk).
The phenology of each cultivar allowed to calculate the length of the flowering period for a total period of four years, considering full bloom when most flowers were at stage F, stage 65 BBCH. Flowering in orchard conditions (Figure 6) allowed the comparison of flowering times between cultivars and years, and the determination of which cultivars are coincident at flowering time in each year (Figure 7).
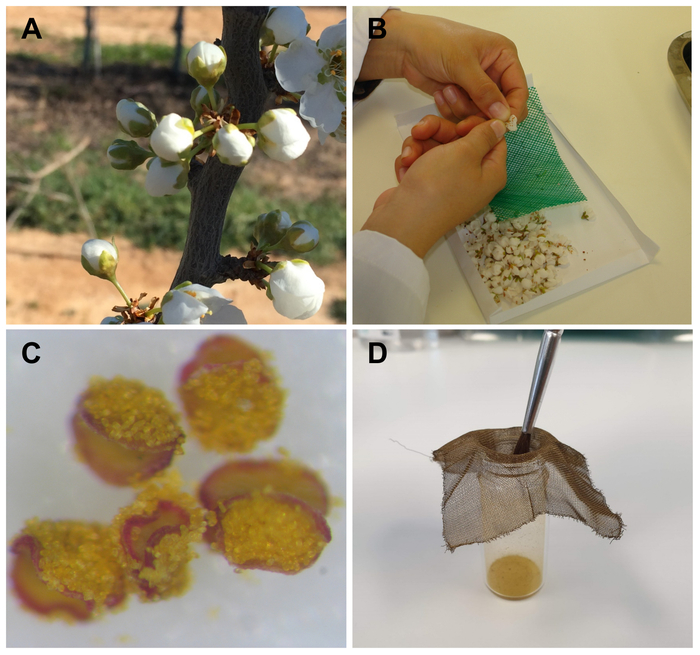
Figure 1: Pollen extraction in Japanese plum. (A) Flower buds at balloon stage D, according to Baggiolini24, stage 57 of the BBCH scale. (B) Removal of undehisced anthers from the flower. (C) Dehisced anthers showing the pollen grains. (D) Sieve of pollen grains from anthers using a fine mesh. Please click here to view a larger version of this figure.
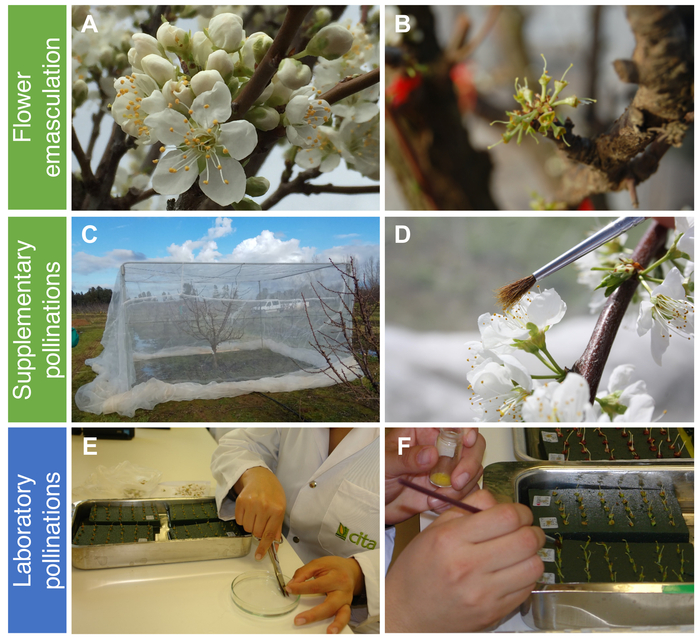
Figure 2: Hand-pollination experiments to determine self-(in)compatibility in Japanese plum. Field pollinations: (A) Flowering in Japanese plum, with flowers at anthesis and at balloon stage. (B) Flowers at balloon stage D, according to Baggiolini24, stage 57 of the BBCH scale after emasculation. (C) Caged tree to avoid the arrival of insects. (D) Supplementary hand-pollination of non-emasculated flowers. Laboratory pollinations: (E) Cut on the base of pedicel underwater and emasculated flowers placed on soaked foam. (F) Hand-pollination of the pistils with a fine paintbrush. Please click here to view a larger version of this figure.
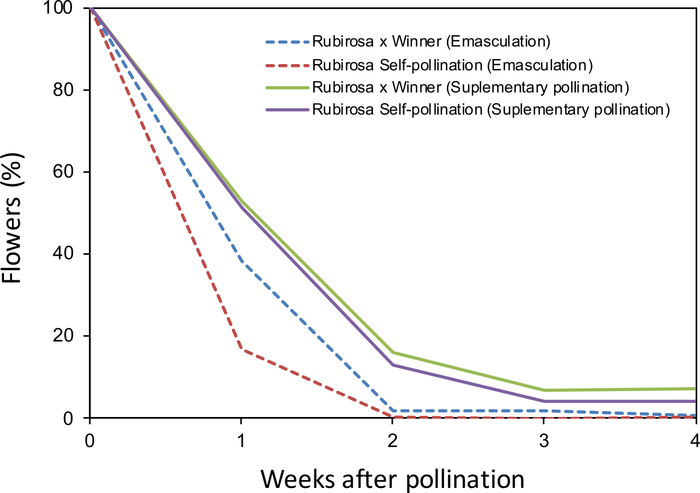
Figure 3: Fruit drop in Japanese plum as affected by different pollination treatments. Self- and cross-pollination in emasculated and non-emasculated flowers. Percentage of flowers and developing fruits from the original number of remaining flowers in the tree during the 4 weeks after pollination in 'Rubirosa'. Please click here to view a larger version of this figure.
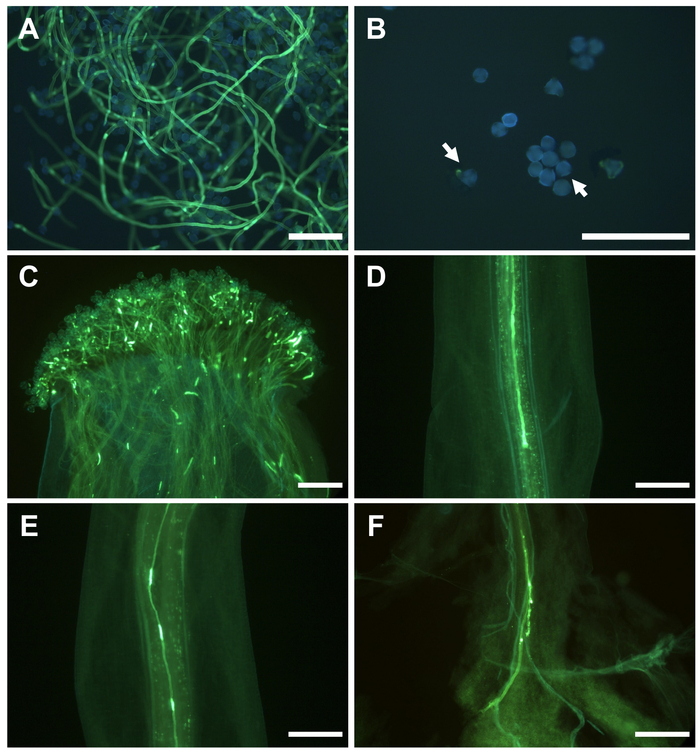
Figure 4: Pollen germination and pollen tube growth in self-pollinated flowers in Japanese plum. (A) In vitro pollen germination. (B) Non-germinated pollen grains (arrows) in vitro. (C) Pollen grain germination on the stigma surface. (D) Pollen tube arrested in the upper third of the style. (E) Pollen tube growing along the style. (F) Pollen tubes at the base of the style. Scale bars, 200 μm. Please click here to view a larger version of this figure.
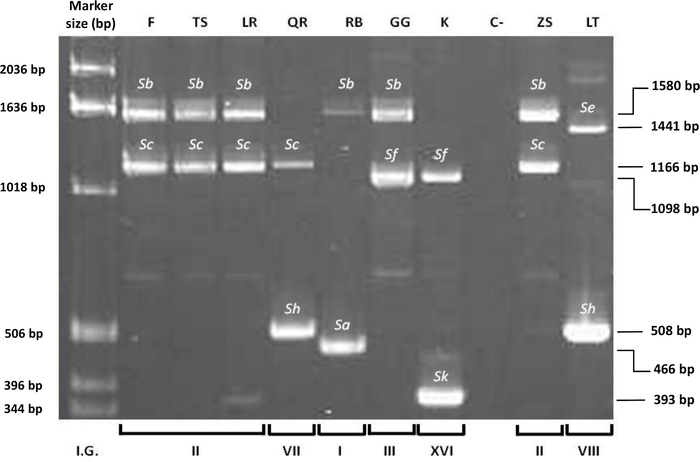
Figure 5: PCR amplification using primer set PruC2-PCER of nine Japanese plum cultivars. Identification of seven S-alleles (Sa, Sb, Sc, Se, Sf, Sh, Sk) and six S-genotypes (SaSb, SbSc, SbSf, ScSh, SeSh, SfSk). Incompatibility Group (I.G.3), ‘Fortune’ (F), ‘TC Sun’ (TS), ‘Laroda’ (LR), ‘Queen Rosa (QR), ‘Red Beaut’ (RB), ‘Golden Globe’ (GG), ‘Kelsey’ (K), Negative control (distilled water) (C-), ‘Zanzi Sun’ (ZS), ‘Laetitia’ (LT). 1 kb: Size standard. Please click here to view a larger version of this figure.

Figure 6: Monitoring of phenology of Japanese plum. Two Japanese plum cultivars with flowers in different phenological stage. Stage C24, stage 55 of BBCH scale (left) and Stage F24, stage 65 of BBCH scale (right). Please click here to view a larger version of this figure.
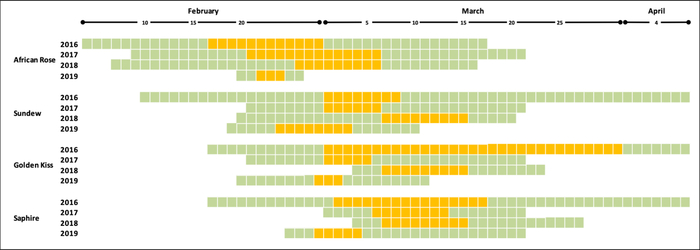
Figure 7: Flowering time in four Japanese plum cultivars over 4 years. Period from the first to the last open flowers. Yellow cells indicate the days of full bloom in which most flowers were open. Please click here to view a larger version of this figure.
| Reagents | Volume per one reaction (μL) | |
| H2O | 11.50 | |
| 10X Buffer with 20 mM MgCl2 | 2.80 | |
| dNTP mix , 10 mM each | 0.80 | |
| Primer forward | 0.40 | |
| PruC2 (5'-CTATGGCCAAGTAATTATTCAAACC-3')40 or | ||
| PruT2 (5'- TSTTSTTGSTTTTGCTTTCTT-3')40 | ||
| Primer reverse | 0.40 | |
| PCER (5'-TGTTTGTTCCATTCGCCTTCCC-3')41 | ||
| DNA template | 4.00 | |
| Taq DNA polymerase, 500 U | 0.09 | |
| Final volume | 20.0 | |
Table 1: Reaction conditions used in this protocol.
| Cultivars (S– genotype) | Number of pistils examined | Germinated pollen grains on stigma (%) | Pistils with pollen tubes at the base of style (%) | Pollen tubes at the base of style (mean number) | Self/cross -compatibility reaction |
| TC Sun (SbSc) × Larry Ann (SbSh) | 22 | 92.3 | 27 | 1.2 | + |
| TC Sun (SbSc) × Blackamber (SbSc) | 10 | 74.8 | 0 | 0.0 | – |
| TC Sun (SbSc) self-pollination | 44 | 78.3 | 7 | 1.0 | + |
| Golden Plum (SbSc) × Black Star (SeSf) | 11 | 64.7 | 36 | 1.5 | + |
| Golden Plum (SbSc) × TC sun (SbSc) | 11 | 98.4 | 0 | 0.0 | – |
| Golden Plum (ShSk) self-pollination | 38 | 85.2 | 0 | 0.0 | – |
Table 2: Pollen germination and pollen tube growth through the style for two Japanese plum cultivars after self- and cross-pollinations. Number of hand-pollinated pistils examined, percentage of germinated pollen grains on stigma, percentage of pistils with pollen tubes at the base of the style, mean number of pollen tubes at the base of the style, Self- or cross-incompatible (-) and self- or cross-compatible (+).
| Cultivar | Germination (%) | SD* |
| Earlemoon | 50 | 3.3 |
| Earliqueen | 30 | 4.2 |
| Eldorado | 10 | 1.3 |
| Friar | 52 | 2.0 |
| Golden Japan | 17 | 3.8 |
| Golden Plumza | 18 | 3.3 |
| Laroda | 46 | 3.6 |
| Larry Ann | 20 | 5.8 |
| Methley | 2 | 0.8 |
| Owen T | 16 | 0.3 |
| Primetime | 20 | 3.3 |
| Queen Rosa | 42 | 3.9 |
| Royal Diamond | 19 | 2.2 |
| Santa Rosa | 36 | 2.0 |
| TC Sun | 49 | 3.1 |
| *SD = Standard deviation | ||
Table 3: Percentage of in vitro pollen germination of 15 Japanese plum cultivars. Mean ± SD of six replicates.
Discussion
The methodology described herein for pollination requirements of Japanese plum cultivars requires determining the self-(in)compatibility of each cultivar by controlled pollinations in the field or the laboratory, and the subsequent observation of pollen tube growth with fluorescence microscopy. The incompatibility relationships are established by the characterization of the S-alleles by molecular genotyping. Finally, the selection of pollinizers is performed by the monitoring phenology to detect those cultivars that coincide at flowering every year.
Establishing pollination requirements in new Japanese plum-type cultivars is becoming increasingly important due to the high number of new cultivars with unknown pollination requirements and that most of them are self-incompatible3. The approach described herein, combining phenological monitoring, controlled pollinations, fluorescence microscopy, and molecular genotyping, has proven to be useful in determining the pollination requirements of cultivars14.
Japanese plum hybrids show a number of peculiarities that hinder the use of a single approach for determining pollination requirements as used in other Prunus species. First, the flowers are smaller and more fragile3. Pollen extraction and handling is similar to that of other Prunus spp.12, although it is necessary to collect a higher number of flowers because the anthers have less amount of pollen grains3. In some cultivars such as Rubirosa, the emasculation of the flowers cannot be used because this technique causes ovule degeneration22 and the subsequent drop of the flower21, which can result in false diagnosis of self-incompatibility. For breeding purposes, flower emasculation in sensitive cultivars used as female parental can lead to a lack of offsprings3.
The number of flowers is much higher than in other species of fruit trees, but the percentage of fruit set is very low3. This makes it necessary to use a higher number of flowers for both field and laboratory pollinations21,22. Laboratory pollinations and subsequent microscopic observations of pollen tube growth allow the assessment of self-(in)compatibility more accurately than by monitoring hand-pollinated flowers in the field until harvest, since this technique avoids environmental influence and allows the analysis of a higher number of cultivars than in field experiments. However, flower handling is more toilsome than in other Prunus species such as apricot12, since it is necessary to cut the pedicel underwater before placing the pistils in the foam. Furthermore, a higher number of pistils must be analyzed under the microscope than in other species, because in the compatible pollen-pistil relationships between Japanese plum hybrids, the pollen tubes reach the ovary in a reduced percentage of flowers3. In addition, pollen tubes are more difficult to detect under the microscope, and the number of tubes reaching the base of the style is lower15,18,20,28,29,30,31,32,33,34. In those cultivars whose flowers are especially fragile and degenerate under laboratory conditions before the pollen tubes reach the base of the style, the self-(in)compatibility should be evaluated by field pollinations.
The evaluation of pollen viability allows to know whether the pollen used in the hand-pollinations is adequate and thus to discard possible false diagnoses of self-incompatibility in those cases of low or null germination percentage. The use of this technique has reported considerable differences in pollen germination between Japanese plum cultivars21,22,35. Furthermore, this approach is also useful in detecting male sterility22,36,37, which is of great importance for discarding male-sterile cultivars as pollen donors in commercial orchards and in crosses for breeding purposes.
Although the incompatibility relationships among cultivars can be determined using the same approach used for self-(in)compatibility assessment by laboratory pollinations, that technique has some disadvantages for this purpose. Pollinations can only be performed during the flowering season, and collections or orchards with adult trees placed near the laboratory are needed for the collection of flowers, whose lifespan is very short3,38. Furthermore, the number of relationships analyzed each year is low, because each pair of cultivars require a particular cross-pollination. As an alternative, the identification of the S-alleles by PCR does not require flowers, since DNA can be extracted from any plant tissue; therefore, the period during which the samples can be collected is longer. Furthermore, unlike flowers that need to be used immediately, the leaves or other plant tissues can be stored, so the analysis is not limited to a few days in spring, but can be done throughout the year39. The identification of the two S-alleles for each cultivar by the amplification of the second S-RNase intron using the primer set PruC2-PCER and PruT2-PCER40,41 and the subsequent analysis of the size of the amplified fragments agarose gel electrophoresis20,21 allow assigning the cultivars to their corresponding incompatibility group (I.G.3). Each I.G. includes those self-incompatible cultivars with the same two S-alleles, which are therefore inter-incompatible. Cultivars from different groups, carrying at least one different S-allele, are inter-compatible.
This technique has the limitation that it does not allow the determination of the self-(in)compatibility for Japanese plum cultivars as it occurs in other Prunus species, in which self-compatibility has been associated with a particular S-allele, such as Sf in almond42 and S4’ in sweet cherry43. Some S-alleles were initially associated with self-compatibility in Japanese plum, such as Sb20,44, Se19,20,44, Sg45, and St46. However, subsequent works have reported self-incompatible cultivars that carry these S-alleles14,20,21,22,47. Therefore, further work of S-allele sequencing is required in Japanese plum to clarify whether different alleles of the same size or mutations have been erroneously identified as alleles Sb, Se, Sg, or St3. Meanwhile, the assessment of self-(in)compatibility in Japanese plum should be analyzed by field- or laboratory-pollinations and the subsequent monitoring of fruit drop in the field or the behavior of pollen tubes under the microscope.
The identification of S-alleles by PCR analysis has been shown to be adequate to establish incompatibility relationships between cultivars3. However, to choose adequate pollinizers, it is necessary to combine this information with the data on the flowering times of each cultivar in each area for several years, since the mismatch in the flowering period, even if it only occurs in some years, may cause lack of fruit set with significant reduction in harvest14.
Many of the differences observed in floral biology and agronomic behavior between cultivars may be related to their origin, since all the cultivars currently grown are hybrids derived from crosses between the original species P. salicina with other species of the same genus but with different characteristics5,48. This may be the main reason why it is necessary to combine different techniques to determine pollination requirements, unlike other fruit species. Knowing the pollination requirements of each cultivar facilitates the adequate selection of cultivars for the design of new orchards and allows the detection and resolution of production problems related to the lack of pollination in established orchards.
Divulgazioni
The authors have nothing to disclose.
Acknowledgements
This research was funded by Instituto Nacional de Investigación y Tecnología Agraria y Alimentaria (RFP2015-00015-00 and RTA2017-00003-00); Gobierno de Aragón—European Social Fund, European Union (Grupo Consolidado A12-17R), and Junta de Extremadura —Fondo Europeo de Desarrollo Regional (FEDER), Plan Regional de Investigación (IB16181), Grupo de Investigación (AGA001, GR18196). B.I. Guerrero was supported by a fellowship of Consejo Nacional de Ciencia y Tecnología of México (CONACYT, 471839).
Materials
| Acetic Acid Glacial | Panreac | 131008.1611 | |
| Agar | iNtRON Biotechnology | 25999 | |
| Aniline blue | Difco | 8504-88 | |
| Boric Acid (H3BO4) | Panreac | 131015.1210 | |
| Calcium Nitrate 4-hydrate (Ca(NO3)2·4H2O) | Panreac | 131231.1211 | |
| Coverglass | Deltalab | D102460 | 24 mm x 60 mm |
| Digital Camera | Imaging Developmet Systems | UI-1490SE | |
| Digital Camera Software Suite | Imaging Developmet Systems | 4.93.0. | |
| DNA Oligos | ThermoFisher Scientific | ||
| dNTP Mix, 10 mM each | ThermoSischer Scientific | R0193 | |
| DreamTaq Green DNA polymerase | ThermoFisher Scientific | EP0713 | |
| Ethanol 96° | VWR-Chemicals | 83804.360 | |
| 1Kb DNA Ladder (U.S. Patent No. 4.403.036) (500pb-12Kb) | Invitrogen | 15615-016 | Size: 250µg; Conc: 1.0 µg/µl |
| Gel Documentation System | Bio-Rad | 1708195 | |
| Hand Counter | Tamaco | TM-4 | |
| Image Lab Software | Bio-Rad | Image Analyse System for Gel Documentation System | |
| MetaPhor Agarose | Lonza | 50180 | |
| Microcentrifuge 5415 R | Eppendorf | Z605212 | |
| Microscope with UV epiflurescence | Leica | DM2500 | Exciter filter BP340-390, Barrier filter LP425 |
| Microslides | Deltalab | D100004 | 26 mm x 76 mm |
| Mini Electrophoresis System | Fisherbrand | 14955170 | |
| Minicentrifuge | ThermoFisher Scientific | 15334204 | |
| NanoDrop 1000 Spectrophotometer | ThermoFisher Scientific | ND1000 | |
| Petri Dishes | Deltalab | 200201 | 55 mm x 14 mm |
| Potassium Phosphate Tribasic (K3PO4·1.5H2O) | Panreac | 141513 | |
| Primer forward 'Pru C2' | ThermoFisher Scientific | ||
| Primer forward Pru T2' | ThermoFisher Scientific | ||
| Primer reverse 'PCER' | ThermoFisher Scientific | ||
| RedSafe Nucleic Acid Staining Solution | iNtRON Biotechnology | 21141 | |
| Saccharose | Panreac | 131621.1211 | |
| Sodium sulphite anhydrous (Na2SO3) | Panreac | 131717.1211 | |
| Speedtools plant DNA extraction Kit | Biotools | 21272 | |
| TBE Buffer (10X) | Panreac | A0972,5000PE | |
| Thermal Cycler T100 | Bio-Rad | 1861096 | |
| Thermomixer comfort | Eppendorf | T1317 | |
| Vertical Autoclave Presoclave II | JP Selecta | 4001725 | |
| Vortex | Fisherbrand | 11746744 |
Riferimenti
- Hendrick, U. P. . The Plums of New York. , (1911).
- Okie, W. R., Hancock, J. F., Hancock, J. F. Plums. Temperate Fruit Crop Breeding. , 337-357 (2008).
- Guerra, M. E., Rodrigo, J. Japanese plum pollination: a review. Scientia Horticulturae. 197, 674-686 (2015).
- Okie, W. R. Introgression of Prunus species in plum. New York Fruit Quarterly. 14 (1), 29-37 (2006).
- Okie, W. R., Weinberger, J. H., Janick, J., Moore, J. . Fruit Breeding, vol. 1. Tree and tropical fruits. , 559-608 (1996).
- Mccubbin, A. G., Kao, T. Molecular recognition and response in pollen and pistil interactions. Annual Review of Cell and Developmental Biology. 16, 333-364 (2000).
- Hegedűs, A., Halász, J. Recent findings of the tree fruit self-incompatibility studies. International Journal of Horticultural Science. 13 (2), 7-15 (2007).
- de Nettancourt, D. . Incompatibility and Incongruity in Wild and Cultivated Plants. , (2001).
- Tao, R., et al. Identification of stylar RNases associated with gametophytic self-incompatibility in almond (Prunus dulcis). Plant and Cell Physiology. 38 (3), 304-311 (1997).
- Halász, J., Pedryc, A., Ercisli, S., Yilmaz, K., Hegedűs, A. S-genotyping supports the genetic relationships between Turkish and Hungarian apricot germplasm. Journal of the American Society for Horticultural Science. 135 (5), 410-417 (2010).
- Lora, J., Hormaza, J. I., Herrero, M., Rodrigo, J. Self-incompatibility and S-allele identification in new apricot cultivars. Acta Horticulturae. (1231), 171-176 (2019).
- Herrera, S., Lora, J., Hormaza, J. I., Rodrigo, J. Determination of self- and inter-(in)compatibility relationships in apricot combining hand-pollination, microscopy and genetic analyses. Journal of Visualized Experiments: JoVE. (160), (2020).
- Cachi, A. M., Wunsch, A. Characterization of self-compatibility in sweet cherry varieties by crossing experiments and molecular genetic analysis. Tree Genetics & Genomes. 10, 1205-1212 (2014).
- Guerra, M. E., Guerrero, B. I., Casadomet, C., Rodrigo, J. Self-compatibility, S-RNase allele identification, and selection of pollinizers in new Japanese plum-type cultivars. Scientia Horticulturae. 261, 109022 (2020).
- Herrera, S., Lora, J., Hormaza, J. I., Herrero, M., Rodrigo, J. Optimizing production in the new generation of apricot cultivars: self-incompatibility, S-RNase allele identification, and incompatibility group assignment. Frontiers in Plant Science. 9, 1-12 (2018).
- Herrera, S., Rodrigo, J., Hormaza, J. I., Lora, J. Identification of self-incompatibility alleles by specific PCR analysis and S-RNase sequencing in apricot. International Journal of Molecular Sciences. 19 (11), 3612 (2018).
- Sociasi Company, R., Kodad, O., Fernández i Martí, J. M., Alonso, J. M. Mutations conferring self-compatibility in Prunus species: from deletions and insertions to epigenetic alterations. Scientia Horticulturae. 192, 125-131 (2015).
- Rodrigo, J., Herrero, M. Evaluation of pollination as the cause of erratic fruit set in apricot ‘Moniqui. Journal of Horticultural Science and Biotechnology. 71 (5), 801-805 (1996).
- Beppu, K., et al. Se-haplotype confers self-compatibility in Japanese plum (Prunus salicina Lindl). Journal of Horticultural Science and Biotechnology. 80 (6), 760-764 (2005).
- Guerra, M. E., Rodrigo, J., López-Corrales, M., Wünsch, A. S-RNase genotyping and incompatibility group assignment by PCR and pollination experiments in Japanese plum. Plant Breeding. 128 (3), 304-311 (2009).
- Guerra, M. E., Wunsch, A., López-Corrales, M., Rodrigo, J. Flower emasculation as the cause for lack of fruit set in Japanese plum crosses. Journal of the American Society for Horticultural Science. 135 (6), 556-562 (2010).
- Guerra, M. E., Wünsch, A., López-Corrales, M., Rodrigo, J. Lack of fruit set caused by ovule degeneration in Japanese plum. Journal of the American Society for Horticultural Science. 136 (6), 375-381 (2011).
- Fernández i Martí, A., Gradziel, T. M., Socias i Company, R. Methylation of the Sf locus in almond is associated with S-RNase loss of function. Plant Molecular Biology. 86, 681-689 (2014).
- Baggiolini, M. Les stades repérés des arbres fruitiers à noyau. Revue romande d’Agriculture et d’Arboriculture. 8, 3-4 (1952).
- Meier, U. Growth stages of mono-and dicotyledonous plants: BBCH Monograph. Federal Biological Research Centre for Agriculture and Forestry. , (2001).
- Fadón, E., Herrero, M., Rodrigo, J. Flower development in sweet cherry framed in the BBCH scale. Scientia Horticulturae. 192, 141-147 (2015).
- Fadon, E., Rodrigo, J. Combining histochemical staining and image analysis to quantify starch in the ovary primordia of sweet cherry during winter dormancy. Journal of Visualized Experiments: JoVE. (145), (2019).
- Hormaza, J. I., Pinney, K., Polito, V. S. Correlation in the tolerance to ozone between sporophytes and male gametophytes of several fruit and nut tree species (Rosaceae). Sexual Plant Reproduction. 9, 44-48 (1996).
- Burgos, L., et al. The self-compatibility trait of the main apricot cultivars and new selections from breeding programmes. Journal of Horticultural Science and Biotechnology. 72 (1), 147-154 (1997).
- Dicenta, F., Ortega, E., Cánovas, J. A., Egea, J. Self-pollination vs. cross-pollination in almond: pollen tube growth, fruit set and fruit characteristics. Plant Breeding. 121, 163-167 (2002).
- Alonso, J. M., Socias i Company, R. Differential pollen tube growth in inbred self-compatible almond genotypes. Euphytica. 144, 207-213 (2005).
- Hedhly, A., Hormaza, J. I., Herrero, M. The effect of temperature on pollen germination, pollen tube growth, and stigmatic receptivity in peach. Plant Biology. 7, 476-483 (2005).
- Hedhly, A., Hormaza, J. I., Herrero, M. Warm temperatures at bloom reduce fruit set in sweet cherry. Journal of Applied Botany. 81, 158-164 (2007).
- Milatović, D., Nikolić, D. Analysis of self-(in)compatibility in apricot cultivars using fluorescence microscopy. Journal of Horticultural Science and Biotechnology. 82, 170-174 (2007).
- Jia, H. J., He, F. J., Xiong, C. Z., Zhu, F. R., Okamoto, G. Influences of cross pollination on pollen tube growth and fruit set in Zuili plums (Prunus salicina). Journal of Integrative Plant Biology. 50, 203-209 (2008).
- Herrero, M., Salvador, J. La polinización del ciruelo Red Beaut. Información Técnica Econónomica Agraria. 41, 3-7 (1980).
- Ramming, D. W. Plum. Register of new fruit and nut varieties: Brooks and Olmo, List 37. HortScience. 30 (6), 1142-1144 (1995).
- Hartmann, W., Neümuller, M. Plum breeding. Breeding plantation tree crops: Temperate species. , 161-231 (2009).
- Guerra, M. E., López-Corrales, M., Wünsch, A. Improved S-genotyping and new incompatibility groups in Japanese plum. Euphytica. 186 (2), 445-452 (2012).
- Tao, R., et al. Molecular typing of S-alleles through identification, characterization and cDNA cloning for S-RNases in sweet cherry. Journal of the American Society for Horticultural Science. 124 (3), 224-233 (1999).
- Yamane, H., Tao, R., Sugiura, A., Hauck, N. R., Lezzoni, A. F. Identification and characterization of S-RNases in tetraploid sour cherry (Prunus cerasus). Journal of the American Society for Horticultural Science. 126, 661-667 (2001).
- López, M., Jose, F., Vargas, F. J., Battle, I. Self-(in)compatibility almond genotypes: a review. Euphytica. 150, 1-16 (2006).
- Bošković, R., Tobutt, K. R. Correlation of stylar ribonuclease zymograms with incompatibility alleles in sweet cherry. Euphytica. 90, 245-250 (1996).
- Beppu, K., Syogase, K., Yamane, H., Tao, R., Kataoka, I. Inheritance of self-compatibility conferred by the Se-haplotype of Japanese plum and development of Se-RNase gene-specific PCR primers. Journal of Horticultural Science and Biotechnology. 85, 215-218 (2010).
- Beppu, K., Kumai, M., Yamane, H., Tao, R., Kataoka, I. Molecular and genetic analyses of the S-haplotype of the self-compatible Japanese plum (Prunus salicina Lindl.) “Methley”. Journal of Horticultural Science and Biotechnology. 87 (5), 493-498 (2012).
- Beppu, K., Konishi, K., Kataoka, I. S-haplotypes and self-compatibility of the Japanese plum cultivar ‘Karari’. Acta Horticulturae. 929, 261-266 (2012).
- Sapir, G., Stern, R. A., Shafir, S., Goldway, M. S-RNase based S-genotyping of Japanese plum (Prunus salicina Lindl.) and its implication on the assortment of cultivar-couples in the orchard. Scientia Horticulturae. 118, 8-13 (2008).
- Karp, D. Luther Burbank’s plums. HortScience. 50 (2), 189-194 (2015).

