Exploring m6A and m5C Epitranscriptomes upon Viral Infection: an Example with HIV
Summary
The role of RNA modifications in viral infections is just starting to be explored and could highlight new viral-host interaction mechanisms. In this work, we provide a pipeline to investigate m6A and m5C RNA modifications in the context of viral infections.
Abstract
The role of RNA modifications in biological processes has been the focus of an increasing number of studies in the last few years and is known nowadays as epitranscriptomics. Among others, N6-methyladenosine (m6A) and 5-methylcytosine (m5C) RNA modifications have been described on mRNA molecules and may have a role in modulating cellular processes. Epitranscriptomics is thus a new layer of regulation that must be considered in addition to transcriptomic analyses, as it can also be altered or modulated by exposure to any chemical or biological agent, including viral infections.
Here, we present a workflow that allows analysis of the joint cellular and viral epitranscriptomic landscape of the m6A and m5C marks simultaneously, in cells infected or not with the human immunodeficiency virus (HIV). Upon mRNA isolation and fragmentation from HIV- infected and non-infected cells, we used two different procedures: MeRIP-Seq, an RNA immunoprecipitation-based technique, to enrich for RNA fragments containing the m6A mark and BS-Seq, a bisulfite conversion-based technique, to identify the m5C mark at a single nucleotide resolution. Upon methylation-specific capture, RNA libraries are prepared for high-throughput sequencing. We also developed a dedicated bioinformatics pipeline to identify differentially methylated (DM) transcripts independently from their basal expression profile.
Overall, the methodology allows exploration of multiple epitranscriptomic marks simultaneously and provides an atlas of DM transcripts upon viral infection or any other cell perturbation. This approach offers new opportunities to identify novel players and novel mechanisms of cell response, such as cellular factors promoting or restricting viral replication.
Introduction
It is long known that RNA molecules can be modified, and more than 150 post-transcriptional modifications have been described to date1. They consist in the addition of chemical groups, mainly methyl groups, to virtually any position of the pyrimidine and purine rings of RNA molecules2. Such post-transcriptional modifications have already been shown to be highly enriched in transfer RNA (tRNA) and ribosomal RNA (rRNA) and have recently been described on mRNA molecules as well.
The rise of new technologies, such as Next Generation Sequencing (NGS), and the production of specific antibodies recognizing definite chemical modifications allowed, for the first time, the investigation of the location and the frequency of specific chemical modifications at a transcriptome-wide level. These advancements have led to a better understanding of RNA modifications and to the mapping of several modifications on mRNA molecules3,4.
While epigenetics investigates the role of DNA and histone modifications in transcriptome regulation, epitranscriptomics in a similar fashion focuses on RNA modifications and their role. The investigation of epitranscriptomic modifications provides new opportunities to highlight novel mechanisms of regulation that may tune a variety of cellular processes (i.e., RNA splicing, export, stability and translation)5. It was thus no great surprise that recent studies uncovered many epitranscriptomic modifications upon viral infection in both cellular and viral RNAs6. Viruses investigated so far include both DNA and RNA viruses; among them, HIV can be considered as a pioneering example. Altogether, the discovery of RNA methylation in the context of viral infections may allow the investigation of yet undescribed mechanisms of viral expression or replication, thus providing new tools and targets to control them7.
In the field of HIV epitranscriptomics, modifications of viral transcripts have been widely investigated and have shown that the presence of this modification was beneficial for viral replication8,9,10,11,12,13. To date various techniques can be used to detect epitranscriptomic marks at the transcriptome-wide level. The most used techniques for m6A identification rely on immune precipitation techniques such as MeRIP-Seq and miCLIP. While MeRIP-Seq relies on RNA fragmentation to capture fragments containing methylated residues, miCLIP is based on the generation of α-m6A antibody specific signature mutations upon RNA-antibody UV crosslinking, thus allowing a more precise mapping.
Detection of m5C modification can be achieved either by antibody-based technologies similar to m6A detection (m5C RIP), or by bisulfite conversion or by AZA-IP or by miCLIP. Both Aza-IP and m5C miCLIP use a specific methyltransferase as bait to target RNA while going through RNA methylation. In Aza-IP, target cells are exposed to 5-azacytidine, resulting in the random introduction of cytidine analog 5-azacytidine sites into nascent RNA. In miCLIP, the NSun2 methyltransferase is genetically modified to harbor the C271A mutation14,15.
In this work, we focus on the dual characterization of m6A and m5C modifications in infected cells, using HIV as a model. Upon methodological optimization, we have developed a workflow that combines methylated RNA immunoprecipitation (MeRIP) and RNA bisulfite conversion (BS), allowing the simultaneous exploration of m6A and m5C epitranscriptomic marks at a transcriptome-wide level, in both cellular and viral contexts. This workflow can be implemented on cellular RNA extracts as well as on RNA isolated from viral particles.
The Methylated RNA ImmunoPrecipitation (MeRIP)16 approach allowing investigation of m6A at the transcriptome-wide level is well established and an array of m6A-specific antibodies are commercially available to date17. This method consists in the selective capture of m6A-containing RNA pieces using an m6A-specific antibody. The two major drawbacks of this technique are (i) the limited resolution, which is highly dependent on the size of RNA fragments and thus provides an approximated location and region containing the methylated residue, and (ii) the large amount of material needed to perform the analysis. In the following optimized protocol, we standardized the fragment size to about 150 nt and reduced the amount of starting material from 10 µg of poly-A-selected RNA, which is currently the advised amount of starting material, to only 1 µg of poly-A-selected RNA. We also maximized the recovery efficiency of m6A RNA fragments bound to specific antibodies using an elution by a competition approach with an m6A peptide instead of more conventional and less specific elution methods using phenol-based techniques or proteinase K. The main limitation of this RIP-based assay, however, remains the suboptimal resolution that does not allow the identification of the precise modified A nucleotide.
Analysis of the m5C mark can be currently performed using two different approaches: a RIP-based method with m5C-specific antibodies and RNA bisulfite conversion. As RIP offers only limited resolution on the identification of the methylated residue, we used bisulfite conversion that can offer single nucleotide resolution. RNA exposure to bisulfite (BS) leads to cytosine deamination, thereby converting the cytosine residue into uracil. Thus, during the RNA bisulfite conversion reaction, every non-methylated cytosine is deaminated and converted to uracil, while the presence of a methyl group in position 5 of the cytosine has a protective effect, preventing the BS-induced deamination and preserving the cytosine residue. The BS-based approach allows for the detection of a m5C modified nucleotide at single base resolution and for assessment of the methylation frequency of each transcript, providing insights into m5C modification dynamics18. The main limitation of this technique however relies on the false positive rate of methylated residues. Indeed, BS conversion is effective on single-stranded RNA with accessible C residues. However, the presence of a tight RNA secondary structure could mask the N5C position and hamper BS conversion, resulting in non-methylated C residues that are not converted to U residues, and thus false positives. To circumvent this issue and minimize the false positive rate, we applied 3 rounds of denaturation and bisulfite conversion cycles19. We also introduced 2 controls in the samples to enable estimation of bisulfite conversion efficiency: we spike-in ERCC sequencing controls (non-methylated standardized and commercially available sequences)20 as well as poly-A-depleted RNAs to assess bisulfite conversion rate on one hand, and to verify by RT-PCR the presence of a known and well conserved methylated site, C4447, on 28S ribosomal RNA on the other hand21.
In the field of virology, coupling these two epitranscriptomic investigation methods with next generation sequencing and accurate bioinformatic analysis allows for the in-depth study of m6A and m5C dynamics (i.e., RNA modification temporal changes that could occur upon viral infection and could uncover an array of new therapeutically relevant targets for clinical use).
Protocol
1. Cell Preparation
NOTE: Depending on the cell type and its RNA content, the starting number of cells can vary.
- Have enough cells to obtain between 200-500 µg of total RNA or 5-7 µg of poly-A-selected RNA. For example, 50 x 106 SupT1 cells should yield around 500 µg of total RNA upon extraction with phenol-based reagents, and is thus required for each individual condition tested.
- Prepare the required number of cells according to the experimental design, and thus according to the number of conditions tested (infection, timepoints, treatment). If the experiment aims at obtaining non-infected cells and HIV-infected cells at 24 h post-infection, a total of 100 x 106 cells is needed, half for non0-infected condition and half for infected condition.
2. RNA Extraction
- From Cells: RNA Extraction with Phenol-Chloroform
- For each condition, collect cells (e.g.,50 x 106) by centrifugation and discard the supernatant.
- Add 5 mL of phenol-based reagent to each 50 x 106 cell pellet and mix by pipetting up and down several times.
- Incubate for 5 min at room temperature to allow complete lysis. Lysed cells can be stored at -80 °C or processed directly.
NOTE: If needed, cells can also be divided in aliquots of 10 x 106 cells per tube in 1.5 mL tubes and lysed in 1 mL of phenol-based reagent for more convenient storage. - Add 1 mL of chloroform and mix by inversion.
- Incubate for 3 min at room temperature.
- Centrifuge for 15 min at 2,000 x g and 4 °C.
- Pipette out the aqueous phase (upper phase) and transfer to a new tube. Finish transferring the aqueous phase by angling the tube at 45° and carefully pipetting the solution out.
NOTE: The amount of aqueous phase may vary among samples but should be close to the amount of chloroform added to the sample (i.e., 1 mL). Do not transfer any interphase or organic layer! The use of phase-lock or phase-maker tubes can facilitate this process. - Add 0.5 mL of 100% molecular grade isopropanol to the aqueous phase.
- Incubate for 1 h at -80 °C to allow RNA precipitation.
- Centrifuge for 10 min at 12,000 x g and 4 °C to pellet the precipitated RNA.
- Discard the supernatant and resuspend the RNA pellet in 1 mL of 75% molecular biology grade ethanol. Vortex briefly.
- Centrifuge for 5 min at 7,500 x g and 4 °C and discard the supernatant.
- Air-dry the pellet for 15 min.
- Resuspend the pellet in 20 µL of RNase-free water and transfer to a new tube.
- Wash the empty tube with an additional 20 µL of water to maximize RNA recovery, and pool with the first 20 µL volume.
- Quantify the total RNA with a spectrophotometer and assess the RNA quality with a fragment analyzer.
- From Viral Particles: RNA Extraction with Column-Based Viral RNA Extraction Kit
NOTE: RNA extraction from viral particles with phenol-based reagent results in low quality viral RNA and in lower quality libraries. A column-based RNA extraction should thus be favored. RNA extraction kits using carrier RNA for RNA elution and recovery are not appropriate for this procedure and should be avoided. Since HIV RNA is poly-Adenylated, direct RNA extraction without further mRNA isolation is sufficient to enter the MeRIP-Seq and BS-Seq pipelines. Normally 1-2 mL of viral supernatant from universally infected cells should provide enough RNA to perform the entire workflow.- Prepare the buffer by adding 150 µL of beta-mercaptoethanol to 30 mL of lysis buffer. Reconstitute the Viral Wash Buffer by adding 96 mL of 100% ethanol.
- Collect virus-containing supernatants and centrifuge to pellet cell debris to minimize cellular RNA contamination.
- Transfer 1 mL of viral supernatant to a 15 mL tube.
- Add 3 mL of Viral RNA Buffer to 1 mL of viral sample and mix by vortexing.
- Transfer 700 µL of sample in a column, inserted in a Collection Tube.
- Centrifuge for 2 min at 13,000 x g at room temperature.
- Discard the flowthrough.
- Repeat the 3 previous steps until the whole sample has been processed, and thus all RNA has been captured on the silica-based matrix column.
- Add 500 µL of Viral Wash Buffer to the column.
- Centrifuge for 1 min at 10,000 x g at room temperature. Discard the flowthrough.
- Add 200 µL of Viral Wash Buffer to the column.
- Centrifuge for 1 min at 10,000 x g at room temperature. Discard the flowthrough.
- Place the column into an empty collection tube.
- Centrifuge for 1 min at 10,000 x g at room temperature to further discard any remaining wash buffer contaminant.
- Carefully transfer the column into a 1.5 mL tube.
- Add 20 µL of DNase/RNase-free water directly to the center of the column matrix and centrifuge at 10,000 x g for 30 s at room temperature.
- Add an additional 10 µL of DNase/RNase-free water directly to the center of the column matrix and centrifuge again for 30 s.
- Quantify the total RNA with a spectrophotometer and assess the RNA quality with a fragment analyzer.
NOTE: RNA extraction can be carried out with any method, if the quality of the retrieved RNA is high, with an RNA integrity/quality number > 9. Total RNA can be stored at -80 °C until further processing.
3. mRNA Isolation by poly-A Selection with Oligo(dT)25
NOTE: Due to the presence of highly methylated ribosomal RNA in cellular extracts, it is highly recommended to isolate poly-A RNA either by rRNA depletion or preferentially by poly-A positive selection. This step is optional and should be performed for cellular RNA samples only, to obtain sequencing results at higher resolution. If analyzing methylation of non-poly-adenylated viral RNAs, favor rRNA depletion rather than poly-A selection or eventually perform the analysis on total RNA.
- Bead Preparation for poly-A Capture
- Resuspend the Oligo(dT)25 magnetic beads stock vial by vortexing for >30 s.
- Transfer 200 µL of magnetic beads to a 1.5 mL tube. Prepare the number of tubes with magnetic beads according to the total quantity of RNA samples to be processed.
NOTE: One tube with 200 µL of Dynabead stock solution corresponds to 1 mg of beads and can accommodate a sample of 75 µg of total RNA. - Place the tubes on a magnet for 1 min and discard the supernatant. Remove the tubes from the magnet.
- Add 1 mL of Binding Buffer (20 mM Tris-HCl, pH 7.5, 1.0 M LiCl, 2 mM EDTA), and resuspend by vortexing. Place the tubes on the magnet for 1 min and discard the supernatant. Remove the tubes from the magnet. Repeat.
- Resuspend the washed magnetic beads in 100 µL of Binding Buffer.
- Total RNA preparation
- Dilute the total RNA at a final concentration of 0.75 µg/µL with RNase-free water, which corresponds to 75 µg/100 µL.
NOTE: If RNA is at a lower concentration, proceed as described below without modifying the volumes. - Aliquot the total RNA in multiple tubes by dispensing 100 µL of RNA sample per tube.
- Add 100 µL of Binding buffer to each RNA sample.
- Heat the total RNA to 65 °C for 2 min to disrupt secondary structures.
- Place immediately on ice until ready to proceed to the next step.
NOTE: Incubation time may vary according to the number of samples to be processed but should not exceed 1 h to avoid any RNA degradation.
- Dilute the total RNA at a final concentration of 0.75 µg/µL with RNase-free water, which corresponds to 75 µg/100 µL.
- Poly-A Selection
- To each RNA tube (from step 3.2), add 100 µL of washed magnetic beads (from step 3.1).
- Mix thoroughly by pipetting up and down and allow binding on a rotating wheel at room temperature for 15 min.
- Open all tubes, place them on the magnet for 1 min, and carefully remove all the supernatant.
- Recover the supernatant in a new tube and keep aside for a second round of RNA capture (step 3.3.14), in order to improve the poly-A final recovery.
- Remove the tube from the magnet and add 200 µL of Washing Buffer (10 mM Tris-HCl, pH 7.5, 0.15 M LiCl, 1 mM EDTA). Mix by pipetting carefully 4 to 5 times.
- Place the tube on the magnet for 1 min and discard the supernatant.
- Repeat the washing step once (repeat steps 3.3.5 and 3.3.6).
- Add 20 µL of ice-cold 10 mM Tris-HCl to elute poly-A RNA from the beads.
- Incubate at 80 °C for 2 min.
- Place the tube on the magnet and quickly transfer the supernatant containing the poly-A RNA to a new RNase-free tube. Place the tube on ice.
- Repeat the elution step (steps 3.3.8 to 3.3.10) to increase the yield.
- Wash the same beads once with 200 µL of washing buffer. Mix by pipetting carefully 4 to 5 times.
- Place on the magnet for 1 min and discard washing buffer.
- Add the flowthrough from step 3.3.4 to the beads and repeat the procedure from binding to elution (steps 3.3.2 to 3.3.10). Keep the RNA eluates in separate tubes for now.
NOTE: Optionally, again keep the supernatant equivalent to step 3.3.4 in a new tube as it can be used as a control. At the end of the procedure, purify and concentrate the RNA by ethanol precipitation or with a column-based method of choice (i.e., RNA clean and concentrator). This sample corresponds to a poly-A-depleted RNA sample and can be used as a control for bisulfite conversion (step 8.2.2). - Quantify the eluted RNA with a spectrophotometer and keep a 2 µL aliquot to further assess the RNA quality with a fragment analyzer.
NOTE: Poly-A RNA can be stored at -80 °C until needed.
4. RNA workflow
- Divide the cellular poly-A RNA (mRNA) and viral RNA samples into 2 aliquots, dedicated to the respective epitranscriptomic analysis pipeline:
(i) 5 µg of cellular mRNA or 1 µg of viral RNA for MeRIP-Seq and input controls (go to steps 5 to 7, and step 9).
(ii) 1 µg of cellular mRNA or 500 ng of viral RNA for BS-Seq (go to steps 8 and 9).
5. RNA Fragmentation
NOTE: RNA fragmentation is carried out with the RNA fragmentation reagent and is intended for MeRIP-Seq and control RNA samples. This is a very important step that requires careful optimization in order to obtain fragments that range between 100-200 nt.
- Divide the total volume of mRNA into 0.2 mL PCR tubes with 18 µL of mRNA/tube.
NOTE: Work quickly. Do not work with more than 8 samples at a time to have reproducible results. Scaling up the volume will not guarantee a reproducible and uniform fragmentation. - Warm up a thermocycler at 70 °C.
- Add 2 µL of fragmentation reagent on the edge of each PCR tube.
- Close the tube and spin down (so that the reagent gets in contact with RNA at the same time for the 8 tubes).
- Incubate the samples 15 min at 70 °C in the preheated thermocycler.
- As soon as the incubation is over, add quickly 2 µL of Stop solution in each tube.
- Spin down and let sit on ice until ready to proceed to the next step.
NOTE: Incubation time may vary according to the number of samples to be processed but should not exceed 1 h to avoid any RNA degradation. - Repeat the procedure for all the samples (if there are more than 8 aliquots).
- Pool the tubes together and proceed to RNA purification with an RNA clean and concentrator Kit (step 6) or any customized column-based kit to get rid of the buffers and recover clean fragmented RNA in water.
6. RNA Purification
NOTE: This step can be carried out by ethanol precipitation or with any kind of column-based RNA purification and concentration method (i.e., RNA Clean and Concentrator).
- Elute or resuspend the purified RNA in a total volume of 50-75 µL of DNase/RNase-free water.
NOTE: If a column-based method is used, two rounds of elution are strongly recommended to ensure maximum recovery. - Quantify the purified fragmented mRNA with a spectrophotometer and assess the RNA quality with a fragment analyzer.
- Keep 100 ng of fragmented mRNA as input control for library preparation and sequencing (go to step 9). The remaining fragmented mRNA (minimum 2.5 µg) can be used for MeRIP (go to step 7.2).
7. MeRIP
NOTE: A minimum of 2.5 µg of fragmented mRNA is required for each immunoprecipitation (IP), either using a specific anti-m6A antibody (test condition) or using an anti-IgG antibody (negative control).
- Magnetic Bead Preparation for Immunoprecipitation
- For each sample, prepare 4 mL of 1x IP buffer in a new conical tube by diluting 800 µL of mRNA IP buffer 5x (50 mM Tris-HCl pH 7.4, 750 mM NaCl, 0.5% Igepal CA-630, and nuclease-free water) with 3.2 mL of nuclease-free water.
NOTE: At least 2 reactions are needed (one test and one IgG control). - Place the tube on ice.
- Label the appropriate number of 1.5 mL microcentrifuge tubes for the number of desired IP reactions:
n tubes (test) for anti-m6A antibody.
n tubes (negative control) for Normal Mouse IgG. - Resuspend the magnetic beads (e.g., Magna ChIP Protein A/G ) by inverting and vortexing. No clumps of beads should be visible.
- For each reaction planned, transfer 25 µL of magnetic beads to a microcentrifuge tube.
- Add ten times more 1x IP buffer (from step 7.1.1) with respect to the original volume of beads used (i.e., 250 µL of 1x IP buffer per 25 µL of magnetic beads).
- Mix the beads by gently pipetting up and down several times for complete resuspension.
- Place the tube on the magnetic separator for 1 min.
- Remove and discard the supernatant, making sure not to aspirate any magnetic beads. Remove the tube from the magnet.
- Repeat the washing step (steps 7.1.6 to 7.1.9).
- Resuspend the beads in 100 µL of 1x IP Buffer per 25 µL of original volume of magnetic beads.
- Add 5 µL of antibody (1 µg/µL) per 25 µL of original volume of magnetic beads.
n tubes (test) with anti-m6A antibody (clone 17-3-4-1) [1 µg/µL].
n tubes (negative control) with Normal Mouse IgG (1 µg/µL). - Incubate on the rotating wheel for 30 min at room temperature to allow conjugation of the antibodies with the magnetic beads.
- Place the tube on the magnetic separator for 1 min. Discard the supernatant. Remove the tube from the magnet and resuspend the antibody-bead mixture in 100 µL of 1x IP Buffer.
- For each sample, prepare 4 mL of 1x IP buffer in a new conical tube by diluting 800 µL of mRNA IP buffer 5x (50 mM Tris-HCl pH 7.4, 750 mM NaCl, 0.5% Igepal CA-630, and nuclease-free water) with 3.2 mL of nuclease-free water.
- RNA Immunoprecipitation (RIP)
- Prepare 500 µL of RIP reaction mixture for each 2.5 µg mRNA sample as follows: 2.5 µg in 100 µL of Fragmented RNA (from step 6.12); 295 µL of nuclease-free water; 5 µL of 40 U/µL RNase Inhibitor; and 100 µL of 5x IP buffer.
- Add 500 µL of RIP reaction mixture to each antibody-bead mixture (~100 µL from step 7.1.14). Mix by gently pipetting several times to completely resuspend the beads. Place on ice.
- Incubate all RIP tubes on a rotating wheel for 2 hours at 4 °C.
- Centrifuge the MeRIP reactions briefly to spin down liquid droplets from the cap and tube sides. Place the tubes on a magnetic separator for 1 min.
- Transfer the supernatant in a new centrifuge tube, being careful not to disturb the magnetic beads .
NOTE: Flowthrough can be kept as control to verify RIP efficiency (go to step 7.3.9). - Remove tubes from the magnet. Wash the beads by adding 500 µL of cold 1x IP buffer. Mix the beads by gently pipetting several times to completely resuspend the beads.
- Place the tubes on a magnetic separator for 1 min and discard supernatant.
- Repeat the washing procedure (steps 7.2.6-7.2.7) twice for a total of 3 washes.
- Place the tubes on ice and immediately proceed to elution.
- Elution
- Prepare 20 mM m6A solution by dissolving 10 mg of N6-Methyladenosine, 5′-monophosphate sodium salt (m6A) in 1.3 mL of nuclease-free water. Prepare 150 µL aliquots and store at -20 °C.
- For each sample (test and controls): Prepare 225 µL of elution buffer by mixing the following components: 45 µL of 5x IP Buffer, 75 µL of 20 mM m6A, 3.5 µL of 40U/µL RNase Inhibitor, and 101.5 µL of nuclease-free water.
- Add 100 µL of elution buffer (from step 7.3.2) to the beads (from step 7.2.9). Mix by gently pipetting several times to completely resuspend beads.
- Incubate all tubes for 1 h with continuous shaking on a rocker at 4 °C.
- Centrifuge the RIP reactions briefly to spin down liquid droplets from the cap and tube sides. Place the tubes on a magnetic separator for 1 min.
- Transfer the supernatant containing eluted RNA fragments to a new 1.5 mL microcentrifuge tube. Be careful not to aspirate the beads, as it will increase background noise.
- Repeat elution steps (7.3.3 to 7.3.6) by again adding 100 µL of elution buffer, incubating 1 h at 4 °C, and collecting the eluate after magnetic separation.
- Combine all eluates from the same sample (total elution volume should be 200 µL).
- Purify the eluted RNA and the flowthrough (optional, from step 7.2.5) by ethanol precipitation or by a column-based method of choice (i.e., RNA Clean and Concentrator).
- Assess the RNA quantity and quality of flowthrough and eluted samples with a fragment analyzer using a high sensitivity detection kit. If the quality of the RNA is satisfactory, proceed to library preparation and high-throughput sequencing (step 9).
NOTE: The amount of RNA retrieved upon MeRIP is very low, and imperatively requires high sensitivity detection kits to ensure quantification. If no bioanalyzer is available, it is possible to proceed blindly to library preparation.
8. RNA Bisulfite Conversion
- Control and Reagent Preparation
- ERCC mix spike-in control: Add ERCC mix following manufacturer's instructions, which recommend the addition of 0.5 µL of undiluted ERCC mix to 500 ng of mRNA. This control can help to assess the efficiency of bisulfite conversion.
- Spike Poly-A-depleted RNA (from step 3.3.14) at a ratio 1/1000 (i.e., 500 pg of poly-A-depleted RNA for 500 ng of mRNA). This sample is enriched in ribosomal RNA and should thus contain the 28S rRNA, a positive control for bisulfite conversion.
NOTE: Total RNA can also be used as positive control instead of poly-A-depleted RNA. - Perform bisulfite conversion with an RNA methylation kit (e.g., Zymo EZ).
- RNA Wash Buffer: Add 48 mL of 100% ethanol (or 52 mL of 95% ethanol) to 12 mL of RNA Wash Buffer concentrate before use.
- Bisulfite Conversion
NOTE: Bisulfite conversion was carried out with a commercially available RNA bisulfite conversion kit following manufacturer's procedure as stated below.- In 0.2 mL PCR tubes, add 1000 ng of mRNA (or between 300 and 1000 ng). Add spike-in controls: 1 µL of ERCC mix (step 8.1.1) and 1000 pg of poly-A-depleted RNA (step 8.1.2). Complete volume up to 20 µL with DNase/RNase-free water.
- Add 130 µL of RNA Conversion Reagent to each 20 µL RNA sample.
- Mix the sample by pipetting up and down.
- Spin down briefly to ensure there are no droplets in the cap or sides of the tube.
- Place the PCR tubes in a thermal cycler and perform the following steps: denaturation at 70 °C for 5 min; conversion at 54 °C for 45 min; repeat denaturation and conversion steps for a total of 3 cycles; and then hold at 4 °C indefinitely.
NOTE: Three cycles of denaturation and bisulfite conversion ensure complete bisulfite conversion of the sample. Samples can be stored at -80 °C or directly processed. - Proceed with in-column desulphonation. Place a column into an empty collection tube and add 250 µL of RNA Binding Buffer to the column.
- Load the sample (~150 µL from Step 8.2.5) into the column containing the RNA Binding Buffer and mix by pipetting up and down.
- Add 400 µL of 95-100% ethanol to the sample-RNA Binding Buffer mixture in the column. Close the cap and immediately mix by inverting the column several times.
- Centrifuge at full speed (≥ 10,000 x g) for 30 s. Discard the flowthrough.
- Add 200 µL of RNA Wash Buffer to the column and centrifuge at full speed for 30 s.
- Add 200 µL of RNA Desulphonation Buffer to the column and incubate at room temperature for 30 min. After the incubation, centrifuge at full speed for 30 s. Discard the flowthrough.
- Add 400 µL of RNA Wash Buffer to the column and centrifuge at full speed for 30 s. Repeat the wash step with an additional 400 µL of RNA Wash Buffer. Discard the flowthrough.
- Centrifuge the column in the emptied Collection Tube at full speed for 2 min. Transfer the column into an RNase-free tube.
- Add ≥ 10 µL of DNase/RNase-free water directly to the column matrix, and incubate for 1 min at room temperature. Centrifuge at full speed for 30 s.
NOTE: We usually elute in a volume of 20 µL. The eluted RNA can be used immediately or stored at -20 °C for up to 3 months. For long-term storage, keep at -80 °C. - Take out 2.5 µL for fragment analyzer assessment of RNA quality and quantity and proceed to library preparation and high-throughput sequencing (step 9).
- Take 4 µL of converted RNA for bisulfite conversion control of efficiency (step 8.3).
- Bisulfite Conversion Control by RT-PCR
NOTE: This step ensures that bisulfite conversion was successful before proceeding to sequencing . 28S ribosomal RNA from Homo sapiens will be used as positive control for RNA methylation analysis, as the C residue at position 4447 (GenBank accession # NR_003287) has been described as being 100% methylated.
Primer Sequences:
H 28SF primer: 5'-GGGGTTTTAYGATTTTTTTGATTTTTTGGG-3'
H 28SR primer: 5'-CCAACTCACRTTCCCTATTAATAAATAAAC-3'- Prepare Reverse Transcription (RT) reaction mix using a High-Capacity cDNA Reverse Transcription Kit. Thaw the kit components on ice and prepare the RT master mix on ice as follows:
4 µL of Bisulfite converted RNA (from step 8.2.14):
2 µL of 10xRT Buffer
0.8 µL of 25x dNTP Mix [100 mM]
2 µL of 10x RT Random Primers
1 µL of MultiScribe Reverse Transcriptase
1 µL of RNase Inhibitor
9.2 µL of nuclease-free H2O
NOTE: Each RT reaction should contain a 20 µL final volume in 0.2 mL PCR tubes. - Put the tubes in the thermal cycler with the following RT program: 25 °C for 10 min; 37 °C for 120 min; 85 °C for 5 min; then at 4 °C indefinitely.
- Prepare the PCR reaction to amplify specifically the 28S rRNA with a PCR proofreading enzyme. Thaw the kit components on ice, gently vortex and briefly centrifuge. Prepare the PCR master mix on ice or on an ice-cold metal plate holder as follows:
0.6 µL of 10 µM H 28SF primer
0.6 µL of 10 µM H 28SF primer
6.5 µL of Template cDNA
22.5 µL of DNA Polymerase master mix
NOTE: Each PCR reaction should contain a 20 µL final volume in 0.2 mL PCR tubes. - Put the tubes in the thermal cycler with the following PCR program: initial denaturation at 95 °C for 5 min; 45 cycles of denaturation (95 °C for 15 s), annealing (57 °C for 30 s), and elongation (72 °C for 15 s), final elongation at 72°C for 10 min, and then holding at 4 °C indefinitely.
- Run 10 µL of the reaction on a 2% agarose gel. The expected band size is 130 – 200 bp.
- Prepare Reverse Transcription (RT) reaction mix using a High-Capacity cDNA Reverse Transcription Kit. Thaw the kit components on ice and prepare the RT master mix on ice as follows:
- Sequencing of PCR products
- Purify the PCR products with a column based method of choice to remove enzymes and dNTP residues and elute the amplified DNA in at least 20 µL of DNase/RNase-free water.
- Quantify the purified DNA with spectrophotometer.
- Sequencing reaction
- Use 40 ng of PCR product/sequencing reaction.
- Sequence in both directions with the H 28SF and H 28SR primers.
- Align the sequences with the known non-converted sequence (28S ribosomal N5 (RNA28SN5). Check for the presence of a C residue at position C4447, and for T residues instead of C elsewhere.
9. Library Preparation and High-Throughput Sequencing
- Prepare libraries for sequencing using mRNA kits (e.g., Illumina TruSeq Stranded), starting the protocol at the Elute-Prime-Fragment step and following manufacturer instructions.
- However, for the input RNA-Seq and MeRIP-Seq samples, incubate the samples at 80 °C for 2 min to only prime but not further fragment them.
- Carry out sequencing using Illumina platforms. Sequencing reactions can be carried out according to preferences and experimental design, either single or paired ends, with a minimum of 100 nt length.
10. Bioinformatics Analyses
- m6A Data Processing
- Run FASTQC24 to assess read quality in m6A and input FASTQ files from sequencing.
- Run Atropos25 to trim low-quality end and adapter sequences from the reads. Set the following parameters in running Atropos.
- Remove the following adapter sequences: AGATCGGAAGAG, CTCTTCCGATCT, AACACTCTTTCCCT, AGATCGGAAGAGCG, AGGGAAAGAGTGTT, CGCTCTTCCGATCT.
- Use the following Phred quality cutoff: 5, for trimming low-quality ends as specified by the manufacturer (https://support.illumina.com/downloads/illumina-adapter-sequences-document-1000000002694.html).
- Use the following minimum read length after trimming: 25 base pairs.
- Merge the GRh38 human genome and HIV [Integrated linear pNL4-3Env-GFP] reference in FASTA format.
- Index the merged reference with HISAT226.
- Run HISAT2 on trimmed reads to align to the indexed reference. Use default HISAT parameters.
- Sort and index the aligned reads with SAMtools27.
- Run SAMtools stat and Qualimap 228, for post-alignment quality check of the sequenced libraries.
- Optionally, collect and summarize quality measures from the previous step with multiQC29.
- HIV genome has homologous 634 bp sequences in the 5' LTR and 3' LTR: Realign multimapping reads from 5' LTR to the corresponding 3' LTR region with SAMtools.
- In order to identify the m6A peaks, run the peak calling software MACS230 (v 2.1.2). Carefully select MACS2 running parameters, in order to ensure correct functioning on RNA-Seq data as peak calling can be affected by gene expression level, and short exons may be miscalled as peaks. Hence, input signal must be subtracted from m6A signal, without the smoothing routinely applied by MACS2 to DNA based data. Apply the following parameters to the 'callpeak' sub-command from MACS2:
-keep-dup auto (controls the MACS2 behavior towards duplicate reads, 'auto' allows MACS to calculate the maximum number of reads at the exact same location based on binomial distribution using 1e-5 as p-value cutoff)
-g 2.7e9 (size of human genome in bp)
-q 0.01 (minimum FDR cutoff to call significant peaks)
-nomodel (to bypass building the shifting model, which is tailored for ChIP-Seq experiments)
-slocal 0
-llocal 0 (setting this and the previous parameter to 0 allows MACS2 to directly subtract, without smoothing, the input reads from the m6A reads)
-extsize 100 (average length of fragments in bp)
-B - Run the differential peak calling sub-command of MACS2, 'bdgdiff' to compare infected vs non-infected samples. 'bdgdiff' takes as inputs the bedGraph files generated by 'callpeak' in the previous step. For each time point, run the comparison of infected versus non-infected samples with 'bdgdiff', subtracting the respective input signal from the m6A signal and providing the additional parameters: -g 60 -l 120.
- m5C Data Processing
- Run Cutadapt31 to trim adapter sequences from the raw reads, with the following parameters:
adapter "AGATCGGAAGAGCACACGTCTGAAC"
-minimum-length=25. - Reverse-complement the trimmed reads using seqkit32, as the sequencing protocol produces reads from the reverse strand.
- Run FastQC to examine read quality.
- Merge GRh38 human genome and HIV [Integrated linear pNL4-3Env-GFP] reference in FASTA format.
- Index the merged reference with the application meRanGh from the meRanTK package33.
- Align with meRanGh with the following parameters:
-UN enabling unmapped reads to be written to output files
-MM enabling multi-mapped reads to be written to output file
-bg for output in bedGraph
-mbgc 10 filter reported region by coverage (at least 10 reads of coverage) - HIV genome has homologous 634 bp sequences in the 5' LTR and 3' LTR: realign multimapping reads from 5' LTR to the corresponding 3' LTR region with SAMtools.
- Run methylation calling via the meRanCall tool, provided by meRanTK, with the following parameters:
-rl = 126, read length
-ei = 0.1, error interval for the methylation rate p-value calculation
-cr = 0.99, expected conversion - Run the MeRanTK's utility estimateSizeFactors.pl for estimating size factors of each sample. The size factors will be used as parameters in the next step.
- Run MeRanCompare for differential methylation analysis of non-infected vs infected across time points 12, 24, and 36h. The following parameters are applied: a significance value of .01 as the minimal threshold for reporting and size factors from previous step.
- Run Cutadapt31 to trim adapter sequences from the raw reads, with the following parameters:
Representative Results
This workflow has proven useful to investigate the role of m6A and m5C methylation in the context of HIV infection. For this, we used a CD4+ T cell line model (SupT1) that we either infect with HIV or left untreated. We started the workflow with 50 million cells per condition and obtained an average of 500 µg of total RNA with an RNA quality number of 10 (Figure 1A-B). Upon poly-A selection we retrieved between 10 and 12 µg of mRNA per condition (representing about 2% of total RNA) (Figure 1B). At this point, we used 5 µg of poly-A-selected RNA for the MeRIP-Seq pipeline and 1 µg for the BS-Seq pipeline. Since HIV RNA is poly-adenylated, no further action is needed and MeRIP-Seq and BS-Seq procedures can be directly applied.
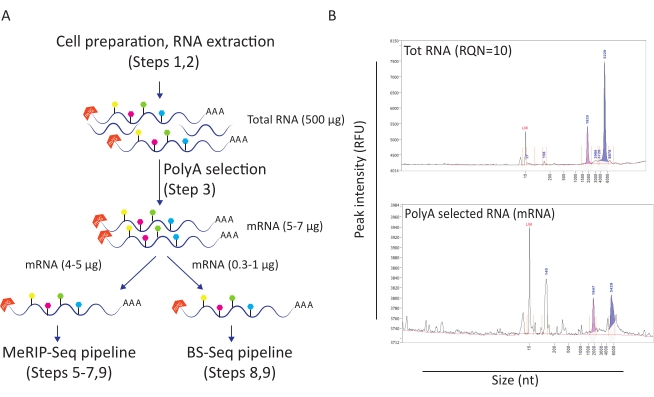
Figure 1: RNA preparation for downstream applications. A) Workflow depicting RNA preparation and distribution for simultaneous MeRIP-Seq and BS-Seq pipelines. Every filled hexagonal shape represents an RNA modification type, such as m6A (green) or m5C (pink). Amounts of RNA material needed to carry out the experiment are indicated. B) Representative results depicting expected RNA distribution profiles (size and amount) upon total RNA extraction (upper panel) and poly-A selection (lower panel). Samples were loaded on the fragment analyzer with standard sensitivity kit in order to assess RNA quality before entering specific MeRIP-Seq and BS-Seq procedures. RQN: RNA quality number; nt: nucleotides. Please click here to view a larger version of this figure.
MeRIP-Seq pipeline is an RNA immunoprecipitation-based technique that allows investigation of m6A modification along RNA molecules. For this, RNA is first fragmented and then incubated with m6A-specific antibodies coupled to magnetic beads for immunoprecipitation and capture. MeRIP-enriched RNA fragments and the untouched (input) fraction are then sequenced and compared to identify m6A-modified RNA regions and thus m6A-methylated transcripts (Figure 2A). The resolution of the technique relies on the efficiency of RNA fragmentation. Indeed, shorter fragments allow for a more precise localization of the m6A residue. Here, cellular poly-A-selected RNAs and viral RNAs were subjected to ion-based fragmentation with RNA fragmentation buffer during 15 min in a 20 µL final volume to obtain RNA fragments of 100-150 nt. Starting with 5 µg of mRNA, we recovered 4.5 µg of fragmented RNA, corresponding to a recovery rate of 90% (Figure 2B). We used 100 ng of fragmented, purified RNA as input control, subjected directly to library preparation and sequencing. The remaining RNA (~4.4 µg) was processed according to the MeRIP-Seq pipeline, which starts with incubation of fragmented RNA with beads bound either to anti-m6A specific antibodies or to anti-IgG antibodies as control. m6A-specific RIP (MeRIP) of 2.5 µg of fragmented RNA allowed retrieving around 15 ng of m6A-enriched material that underwent library preparation and sequencing (Figure 2B). RIP with anti-IgG control, as expected, did not yield enough RNA to allow further analysis (Figure 2B).
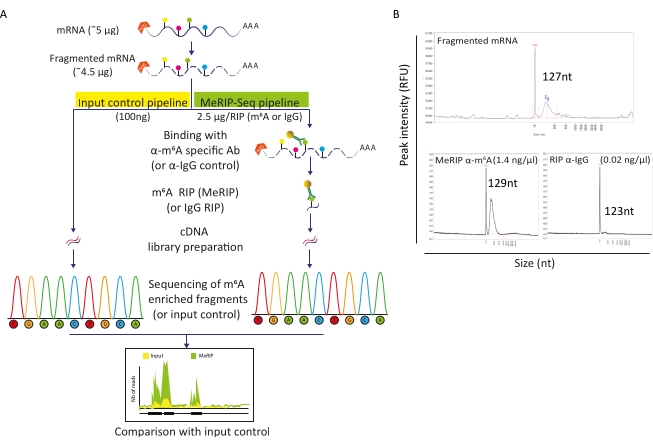
Figure 2: MeRIP-Seq pipeline. A) Schematic representation of MeRIP-Seq workflow and input control. Upon poly-A selection, samples were fragmented into 120-150 nt pieces and, either directly subjected to sequencing (100 ng, input control), or used for RNA immunoprecipitation (2.5 µg, RIP) with anti-m6A specific antibody or anti-IgG antibody as negative control prior to sequencing. B) Representative results showing expected RNA distribution profiles (size and amount) upon fragmentation (upper panel) and RIP (lower panels, MeRIP: left, IgG control: right). Samples were loaded on fragment analyzer to evaluate RNA quality and concentration before further processing to library preparation and sequencing. Fragmented RNA analysis was performed using the RNA standard sensitivity kit while immunoprecipitated RNA used the high sensitivity kit. Please click here to view a larger version of this figure.
BS-Seq pipeline allows exploration of m5C RNA modification at nucleotide resolution and leads to the identification of m5C-methylated transcripts. Upon bisulfite conversion, non-methylated cytosines are converted into uracil, while methylated cytosines remain unchanged (Figure 3A). Due to the harsh conditions of bisulfite conversion procedure (i.e., high temperature and low pH), converted mRNAs are highly degraded (Figure 3B), however this does not interfere with library preparation and sequencing. Bisulfite conversion is efficient only on single-stranded RNA and can thus potentially be hindered by secondary double-stranded RNA structures. To evaluate the efficiency of C-U conversion we introduced two controls. As a positive control, we took advantage of the previously described presence of a highly methylated cytosine in position C4447 of the 28S rRNA23. Upon RT-PCR amplification and sequencing of a 200 bp fragment surrounding the methylated site we could observe that all cytosines were successfully converted to uracils, thereby appearing as thymidines in the DNA sequence, except the cytosine in position 4447 that remained unchanged. As a control for bisulfite conversion rate, we used commercially available synthetic ERCC RNA sequences. This mixture consists in a pool of known, non-methylated and poly-adenylated RNA sequences, with a variety of secondary structures and lengths. Upon library preparation and sequencing, we focused on these ERCC sequences to calculate the conversion rate, which can be performed by counting the number of converted C among the total C residues in all the ERCC sequences and in each sample. We obtained a conversion rate of 99.5%, confirming the efficiency and the success of the bisulfite conversion reaction (Figure 3D).
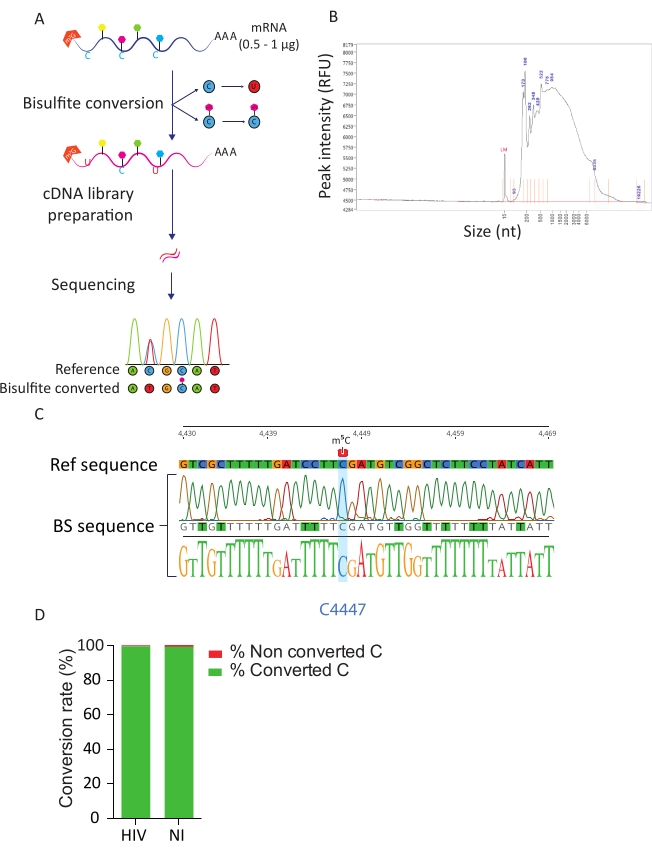
Figure 3: BS-Seq pipeline. A) Schematic representation of BS-Seq workflow. Upon poly-A selection, samples are exposed to bisulfite, resulting in C to U conversion (due to deamination) for non-methylated C residues. In contrast, methylated C residues (m5C) are not affected by bisulfite treatment and remain unchanged. B) Representative result of bisulfite converted RNA distribution profile (size and amount) upon analysis on fragment analyzer with a standard sensitivity kit. C) Electropherogram showing representative sequencing result of RT-PCR amplicon of the region surrounding the 100% methylated C at position 4447 in 28S rRNA (highlighted in blue). In contrast, C residues of the reference sequence were identified as T residues in the amplicon sequence due to bisulfite conversion success. D) Evaluation of C-U conversion rate by analysis of ERCC spike-in sequences in HIV-infected and noninfected cells. The average conversion rate is of 99.5%. Please click here to view a larger version of this figure.
M6A-enriched samples, bisulfite converted samples and input controls are further processed for library preparation, sequencing and bioinformatic analysis (Figure 4). According to the experimental design and biological question(s) addressed, multiple bioinformatic analyses can be applied. As proof of principle here, we show representative results from one potential application (i.e., differential methylation analysis), which focuses on the identification of differentially methylated transcripts induced upon HIV infection. Briefly, we investigated the m6A or m5C methylation level of transcripts, independently from their gene expression level, in both non-infected and HIV-infected cells, in order to further understand the role of RNA methylations during viral life cycle. Upon gene expression normalization, we identified that the ZNF469 transcript was differentially m6A-methylated according to the infection status, indeed this transcript was not methylated in non-infected cells while it displayed several methylated peaks upon HIV infection (Figure 5A). A similar differential methylation analysis on m5C revealed that the PHLPP1 transcript contained several methylated residues, which tend to be more frequently methylated in the HIV condition (Figure 5B). In this context, both analyses suggest that HIV infection impacts the cellular epitranscriptome.
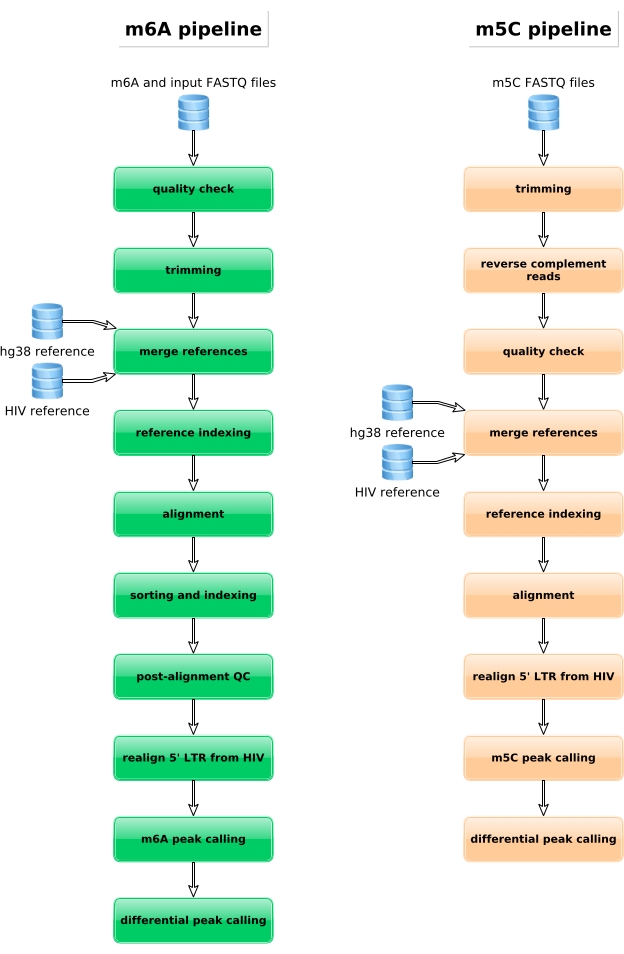
Figure 4: Schematic representation of the bioinformatic workflow for the analysis of m6A and m5C data. Please click here to view a larger version of this figure.
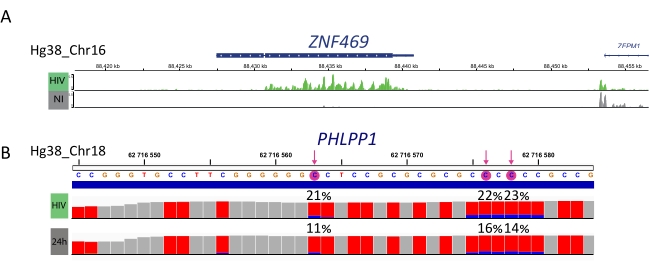
Figure 5: Example of differentially methylated transcripts upon infection. A) Representative result showing m6A methylation of ZNF459 transcript in HIV-infected (green) and non-infected (grey) cells. Peak intensity (upon input expression subtraction) is shown on the y-axis and position in the chromosome along the x-axis. Differential methylation analysis reveals that ZFN469 transcript is hypermethylated upon HIV infection. B) Representative result of m5C methylated gene in HIV-infected (upper lane) and non-infected (lower lane) cells. The height of each bar represents the number of reads per nucleotide and allows coverage assessment. Each C residue in represented in red, and the proportion of methylated C is represented in blue. The exact methylation rate (%) is reported above each C residue. Arrows highlight statistically significant differentially methylated C. Samples were visualized using IGV viewer. Please click here to view a larger version of this figure.
Discussion
The role of RNA modifications in viral infection is still largely unknown. A better understanding of the role of epitranscriptomic modifications in the context of viral infection could contribute to the quest for new antiviral treatment targets.
In this work, we provide a complete workflow that allows investigation of the m6A and m5C epitranscriptomes of infected cells. Depending on the biological question, we advise to use poly-A-selected RNA as starting material. Although optional, as the pipeline could be used with total RNA, it is important to keep in mind that rRNAs as well as small RNAs are highly modified and contain an important number of methylated residues. This could result in a decreased quality and quantity of meaningful sequencing data.
However, if the focus of the study is non-poly-adenylated RNA, the RNA extraction step should be adapted in order to avoid discarding small RNA (in case of column-based RNA extraction) and to privilege ribosome-depletion techniques rather than poly-A selection to enter the pipeline.
In order to ensure high quality RNA, correct fragmentation and suitable m6A-enriched and BS converted RNA quality for library preparation we strongly advise to use a fragment analyzer or a bioanalyzer. However, this equipment is not always available. As an alternative, quality of RNA, mRNA and size of fragmented RNA could also be assessed by visualization on agarose gel. Alternatively, library preparation can be performed without previous assessment of RNA quantity.
We used the antibody-based MeRIP-Seq16 technique to explore the m6A epitranscriptomic landscape. This technique is based on RNA immunoprecipitation and is successful; however, some steps need careful optimization and can be critical. Although m6A methylation has been described to occur mainly within the consensus sequence RRA*CH, this motif is highly frequent along mRNA molecules and does not allow precise identification of the methylated site. It is thus critical to achieve a reproducible and consistent RNA fragmentation, generating small RNA fragments, to improve the RIP-based resolution. In this protocol, we recommend an optimized procedure, providing reproducible and consistent results in our experimental setting; however, this fragmentation step may need further optimization according to specific sample features.
Recently a new technique allowing m6A direct sequencing was described. It is based on the use of specific reverse transcriptase variants that exhibit unique RT-signatures as a response to encountering m6A RNA modification24. This technology, upon careful optimization, could circumvent the major limitation faced with MeRIP-Seq (decreasing the amount of initial material and allowing a higher resolution). To explore the m5C modification we decided to use the bisulfite conversion technique in order to detect at nucleotide resolution the modified C residues. In order to reduce the false positive rate due to the presence of RNA secondary structures, we performed 3 cycles of denaturation/bisulfite conversion and further control bisulfite conversion rate performance thanks to the use of ERCC spike-in controls. One of the limitations linked to this technique is that bisulfite conversion is very harsh and three cycles of denaturation/bisulfite conversion could degrade some RNA and hence reduce resolution. However, in our setting, we chose to settle for a potentially slightly lower resolution in order to increase the quality of the dataset.
Thanks to these optimizations and controls, we were able to provide a reliable and sound workflow that can be exploited to investigate the epitranscriptomic landscape and its alteration in the context of viral infections, host-pathogen interactions, or any exposure to specific treatments.
Divulgazioni
The authors have nothing to disclose.
Acknowledgements
This work was supported by the Swiss National Science Foundation (grants 31003A_166412 and 314730_188877).
Materials
| AccuPrime Pfx SuperMix | Invitrogen | 12344-040 | |
| anti-m6A antibody _Clone 17-3-4-1 | Millipore | MABE1006 | |
| Chloroform | Merck | 67-66-3 | |
| ERCC | Invitrogen | 4456740 | |
| EZ RNA Methylation Kit | Zymo Research | EZR5001 | |
| Fragment analyzer RNA Kit – HS RNA Kit | Agilent | DNF-472-0500 | |
| Fragment analyzer RNA Kit – RNA Kit | Agilent | DNF-471-0500 | |
| High-Capacity cDNA Reverse Transcription Kit | Applied Biosystem | 4368814 | |
| Illumina TruSeq Stranded mRNA | Illumina | 20020594 | |
| Magnetic Beads A/G Blend | Merck | 16-663 | |
| N6-Methyladenosine, 5′-monophosphate sodium salt (m6A) | Sigma Aldrich | M2780-10MG | |
| Normal Mouse IgG | Merk | 12371 | |
| Oligo(dT)25 | Life Technologies | 61005, | |
| PCRapace | Stratec | 1020220300 | |
| Quick RNA Viral Kit | Zymo Research | 1034 | |
| RNA Clean & Concentrator | Zymo Research | R1015 | |
| RNA Fragmentation Reagent | Ambion | AM8740 | |
| RNase Inhibitor | Ambion | AM2684 | |
| Trizol | TRIzol Reagent | 15596026 |
Riferimenti
- Machnicka, M. A., et al. MODOMICS: a database of RNA modification pathways–2013 update. Nucleic Acids Research. 41, 262-267 (2013).
- Zaccara, S., Ries, R. J., Jaffrey, S. R. Reading, writing and erasing mRNA methylation. Nature Reviews Molecular Cell Biology. 20 (10), 608-624 (2019).
- Davalos, V., Blanco, S., Esteller, M. SnapShot: Messenger RNA Modifications. Cell. 174 (2), 498 (2018).
- Saletore, Y., et al. The birth of the Epitranscriptome: deciphering the function of RNA modifications. Genome Biology. 13 (10), 175 (2012).
- Zhao, B. S., Roundtree, I. A., He, C. Post-transcriptional gene regulation by mRNA modifications. Nature Reviews Molecular Cell Biology. 18 (1), 31-42 (2017).
- Netzband, R., Pager, C. T. Epitranscriptomic marks: Emerging modulators of RNA virus gene expression. Wiley Interdisciplinary Reviews: RNA. 11 (3), 1576 (2020).
- Pereira-Montecinos, C., Valiente-Echeverria, F., Soto-Rifo, R. Epitranscriptomic regulation of viral replication. Biochimica et Biophysica Acta. 1860 (4), 460-471 (2017).
- Lichinchi, G., et al. Dynamics of the human and viral m(6)A RNA methylomes during HIV-1 infection of T cells. Nature Microbiology. 1, 16011 (2016).
- Courtney, D. G., et al. Epitranscriptomic Addition of m(5)C to HIV-1 Transcripts Regulates Viral Gene Expression. Cell Host & Microbe. 26 (2), 217-227 (2019).
- Kennedy, E. M., et al. Posttranscriptional m(6)A Editing of HIV-1 mRNAs Enhances Viral Gene Expression. Cell Host & Microbe. 19 (5), 675-685 (2016).
- Tirumuru, N., Wu, L. HIV-1 envelope proteins up-regulate N (6)-methyladenosine levels of cellular RNA independently of viral replication. Journal of Biological Chemistry. 294 (9), 3249-3260 (2019).
- Tirumuru, N., et al. N(6)-methyladenosine of HIV-1 RNA regulates viral infection and HIV-1 Gag protein expression. Elife. 5, (2016).
- Cristinelli, S., Angelino, P., Janowczyk, A., Delorenzi, M., Ciuffi, A. HIV Modifies the m6A and m5C Epitranscriptomic Landscape of the Host Cell. Frontiers in Virology. 1 (11), (2021).
- Khoddami, V., Cairns, B. R. Transcriptome-wide target profiling of RNA cytosine methyltransferases using the mechanism-based enrichment procedure Aza-IP. Nature Protocols. 9 (2), 337-361 (2014).
- Hussain, S., Aleksic, J., Blanco, S., Dietmann, S., Frye, M. Characterizing 5-methylcytosine in the mammalian epitranscriptome. Genome Biology. 14 (11), 215 (2013).
- Dominissini, D., Moshitch-Moshkovitz, S., Salmon-Divon, M., Amariglio, N., Rechavi, G. Transcriptome-wide mapping of N6-methyladenosine by m6A-seq based on immunocapturing and massively parallel sequencing. Nature Protocols. 8 (1), 176-189 (2013).
- Dominissini, D., et al. Topology of the human and mouse m6A RNA methylomes revealed by m6A-seq. Nature. 485 (7397), 201-206 (2012).
- Shobbir Hussain, J. A., Blanco, S., Dietmann, S., Frye, M. Characterizing 5-methylcytosine in the mammalian epitranscriptome. Genome Biology. 14 (215), (2013).
- Amort, T., et al. Distinct 5-methylcytosine profiles in poly(A) RNA from mouse embryonic stem cells and brain. Genome Biology. 18 (1), 1 (2017).
- Endrullat, C., Glökler, J., Franke, P., Frohme, M. Standardization and quality management in next-generation sequencing. Applied & Translational Genomics. 10, 2-9 (2016).
- Schaefer, M., Pollex, T., Hanna, K., Lyko, F. RNA cytosine methylation analysis by bisulfite sequencing. Nucleic Acids Research. 37 (2), 12 (2009).
- Cristinelli, S., Angelino, P., Janowczyk, A., Delorenzi, M., Ciuffi, A. HIV Modifies the m6A and m5C Epitranscriptomic Landscape of the Host Cell. biorxiv. 1 (11), (2021).
- Squires, J. E., et al. Widespread occurrence of 5-methylcytosine in human coding and noncoding RNA. Nucleic Acids Research. 40 (11), 5023-5033 (2012).
- Aschenbrenner, J., et al. Engineering of a DNA Polymerase for Direct m(6) A Sequencing. Angewandte Chemie (International ed. in English). 57 (2), 417-421 (2018).
- Didion, J. P., Martin, M., Collins, F. S. Atropos: specific, sensitive, and speedy trimming of sequencing reads. PeerJ. 5, 3720 (2017).
- Kim, D., Langmead, B., Salzberg, S. L. HISAT: a fast spliced aligner with low memory requirements. Nature Methods. 12 (4), 357-360 (2015).
- Li, H., et al. The Sequence Alignment/Map format and SAMtools. Bioinformatics. 25 (16), 2078-2079 (2009).
- Okonechnikov, K., Conesa, A., García-Alcalde, F. Qualimap 2: advanced multi-sample quality control for high-throughput sequencing data. Bioinformatics. 32 (2), 292-294 (2016).
- Ewels, P., Magnusson, M., Lundin, S., Käller, M. MultiQC: summarize analysis results for multiple tools and samples in a single report. Bioinformatics. 32 (19), 3047-3048 (2016).
- Zhang, Y., et al. Model-based Analysis of ChIP-Seq (MACS). Genome Biology. 9 (9), 137 (2008).
- Martin, M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet Journal. 17 (1), (2011).
- Shen, W., Le, S., Li, Y., Hu, F. SeqKit: A Cross-Platform and Ultrafast Toolkit for FASTA/Q File Manipulation. PLOS ONE. 11 (10), 0163962 (2016).
- Rieder, D., Amort, T., Kugler, E., Lusser, A., Trajanoski, Z. meRanTK: methylated RNA analysis ToolKit. Bioinformatics. 32 (5), 782-785 (2015).

