Standing Neurophysiological Assessment of Lower Extremity Muscles Post-Stroke
Summary
This protocol describes the process for performing a neurophysiological assessment of the lower extremity muscles, tibialis anterior and soleus, in a standing position using TMS in people post-stroke. This position provides a greater probability of eliciting a post-stroke TMS response and allows for the use of reduced stimulator power during neurophysiological assessments.
Abstract
Transcranial magnetic stimulation (TMS) is a common tool used to measure the behavior of motor circuits in healthy and neurologically impaired populations. TMS is used extensively to study motor control and the response to neurorehabilitation of the upper extremities. However, TMS has been less utilized in the study of lower extremity postural and walking-specific motor control. The limited use and the additional methodological challenges of lower extremity TMS assessments have contributed to the lack of consistency in lower extremity TMS procedures within the literature. Inspired by the decreased ability to record lower extremity TMS motor evoked potentials (MEP), this methodological report details steps to enable post-stroke TMS assessments in a standing posture. The standing posture allows for the activation of the neuromuscular system, reflecting a state more akin to the system’s state during postural and walking tasks. Using dual-top force plates, we instructed participants to equally distribute their weight between their paretic and non-paretic legs. Visual feedback of the participants’ weight distribution was provided. Using image guidance software, we delivered single TMS pulses via a double-cone coil to the participants’ lesioned and non-lesioned hemispheres and measured the corticomotor response of the paretic and non-paretic tibialis anterior and soleus muscles. Performing assessments in the standing position increased the TMS response rate and allowed for the use of the lower stimulation intensities compared to the standard sitting/resting position. Utilization of this TMS protocol can provide a common approach to assess the lower extremity corticomotor response post-stroke when the neurorehabilitation of postural and gait impairments are of interest.
Introduction
Transcranial magnetic stimulation (TMS) is an instrument used to measure the behavior of neural circuits. The majority of TMS investigations focusing on the study of motor control/performance have been conducted in the upper extremities. The imbalance between the upper and lower extremity studies is in part due to the additional challenges in measuring the lower extremity corticomotor response (CMR). Some of these methodological obstacles include the smaller cortical representations of the lower extremity muscles within the motor cortex and the deeper location of the representations relative to the scalp1. In populations with neurological injury, additional hurdles are also present. For example, approximately half of the individuals post-stroke show no response to TMS at rest in lower extremity muscles2,3. The lack of post-stroke response to TMS is even seen when patients maintain some volitional control of the muscles, indicating at least a partially intact corticospinal tract.
The lack of measurable TMS responses with maintained motor function contributes to our decreased understanding of post-stroke postural and walking-specific motor control and the neurophysiological effects of neurorehabilitation. However, some of the challenges of lower extremity post-stroke neurophysiological assessments have been overcome. For example, a double-cone coil can be used to reliably activate the lower extremity motoneurons located deep in the interhemispheric fissure1. The double-cone coil produces a larger and stronger magnetic field that penetrates deeper into the brain than the more commonly used figure-of-eight coil4. Another methodological change that can be implemented to increase the responsiveness to TMS is measuring the CMR during a slight voluntary contraction5. Generally, this contraction is performed at a predetermined level of either maximal voluntary joint torque or maximal electromyographic (EMG) muscle activity. Peripheral nerve stimulation can also be used to elicit a maximal muscle response and the recorded EMG of this response can be used to set the targeted voluntary activation of the muscle.
Performing TMS assessment post-stroke during active muscle contraction is fairly common in the upper extremities where isometric tasks can mimic functional activities, for example, grasping/holding objects. In contrast, walking is accomplished through the bilateral activation of multiple muscle groups via cortical, subcortical, and spinal cord structures and requires postural muscle activation to resist the effects of gravity. This activation state is likely not reflected when measuring isolated muscles producing an isometric contraction. Several previous studies directed at understanding postural and walking-specific motor control have delivered TMS pulses while participants were walking6,7,8 and standing9,10,11,12,13,14,15. The measurement of the CMR in the upright position allows for the activation of postural muscles and subcortical components of the postural and gait motor-control networks. To date, there have not been any reports of performing standing TMS assessments in individuals post-stroke.
This study proposes a standardized methodology, built upon the existing body of literature of standing TMS methods6,7,8,9,10,11,12,13,14,15, for standing TMS assessment of the CMR post-stroke. This methodology can be utilized by research groups studying, but not limited to, postural deficits and walking-specific motor control post-stroke and establish greater consistency of TMS procedures. The purpose of this methodological investigation was to determine whether standing TMS assessments are feasible in individuals post-stroke with moderate gait impairments. We hypothesized that performing assessments in the standing position would 1) increase the likelihood of eliciting a measurable response (motor evoked potential, MEP) and 2) that the stimulator power/intensity used to perform standing TMS assessments would be lower than that of the usually performed sitting/resting assessments. We believe the successful completion and widespread use of this protocol may lead to a greater understanding of the neurophysiological aspects of post-stroke postural and walking-specific motor control and the effects of neurorehabilitation.
Protocol
All procedures were approved by the Institutional Review Board at the Medical University of South Carolina and conformed to the Declaration of Helsinki.
1. Participant recruitment
- Recruit individuals post-stroke from the local database. For this experiment, 16 individuals were recruited from a local electronic recruitment database. In some instances, participants were recruited specifically because they had failed to respond to TMS at rest in previous studies performed by our research group.
- Use the following inclusion criteria for this investigation: males and females between the ages of 18-85, at least 6 months post-stroke, residual paresis of the lower extremities, and able to stand 10 min without an assistive device.
- Exclude participants if they had a history of seizures, took prescription medications that lowered seizure thresholds, had a history of brain injury and/or other diseases of the central nervous system, had implanted devices or metal objects in their head, or had severe arthritis or orthopedic conditions limiting their passive range of motion.
NOTE: Participants' demographics are located in Table 1.
| Study ID | Age | Months post Stroke |
Sex | Race | Type of Stroke | Stroke Hemisphere |
Altezza (cm) |
Weight (kg) |
Self-Selected Walking Speed (m/s) | Walking Aid |
||
| 1 | 67 | 28.7 | M | C | Intracerebral Hemorrhage | Right | 180 | 74.8 | 0.61 | None | ||
| 2 | 84 | 55.8 | F | C | Ischemic | Right | 165 | 68.0 | 0.94 | None | ||
| 3 | 56 | 262.7 | F | C | Subarachnoid Hemorrhage | Left | 152 | 59.0 | 1.29 | None | ||
| 4 | 67 | 141.8 | M | C | Intracerebral Hemorrhage | Right | 180 | 72.6 | 0.27 | Cane / AFO | ||
| 6 | 48 | 21.6 | M | C | Intracerebral Hemorrhage | Right | 170 | 61.2 | 0.83 | None | ||
| 7 | 58 | 93.9 | M | C | Acute Ischemic | Left | 168 | 112.5 | 0.77 | Quad Cane / AFO | ||
| 8 | 71 | 55.3 | F | AA | Acute Ischemic | Left | 170 | 68.0 | 1.05 | None | ||
| 9* | 65 | 23.7 | M | C | Acute Ischemic | Right | 178 | 84.8 | – | Knee Brace | ||
| 10 | 70 | 26.6 | M | C | Acute Ischemic | Left | 173 | 78.9 | 0.81 | None | ||
| 12 | 70 | 10.0 | M | C | Acute Ischemic | Left | 170 | 86.2 | 1.11 | None | ||
| 13 | 65 | 80.6 | M | C | Acute Ischemic | Right | 185 | 139.7 | 0.93 | Cane / Crutch | ||
| 14 | 79 | 83.0 | M | C | Acute Ischemic | Right | 175 | 88.5 | 0.48 | Cane | ||
| 15 | 51 | 54.4 | M | AA | Acute Ischemic | Left | 178 | 90.7 | 1.35 | None | ||
| 17 | 65 | 18.5 | M | C | Acute Ischemic | Right | 170 | 74.8 | 0.28 | Cane | ||
| 18 | 63 | 48.8 | F | AA | Acute Ischemic | Right | 170 | 83.9 | 1.12 | None | ||
| 19 | 58 | 25.9 | M | C | Acute Ischemic | Both | 183 | 88.5 | 1.10 | None | ||
| * Participant removed from data analysis due to inability to complete required assessments | ||||||||||||
| AFO = ankle foot orthortic | ||||||||||||
Table 1: Participant demographics.
- Make initial contact with participants via phone and briefly explain the testing procedures. Invite interested individuals to the laboratory.
- Upon arrival to the research facility, have a member of the research staff fully explain the experimental protocol to the prospective participants.
- When a prospective participant confirms their willingness to participate in the study, obtain written informed consent approved by the local institutional review board.
2. Image guidance system and participant setup
- Utilize image guidance software to ensure consistent delivery of the TMS pulses during the assessment.
- Start a new project using the MNI head model native to the image guidance system. Open the software and select New MNI Head Project.
- In the pop-up window, click on the Targets tab and then click Configure Targets. Determine the scalp location directly superior to the pre-central gyrus and 0.5 cm lateral to the midsagittal line.
- Once the location is visually identified, add a new rectangular grid by clicking on the Nuovo, and then on the Rectangular Grid. The grid should appear on the screen, and the medial row should be 0.5 cm lateral to the midsagittal line.
- Resize the grid by typing 3 and 5 in the grid size boxes. Set the grid spacing to 10 by 10 mm by typing in the grid spacing boxes. Select the Cursor Tool, and then move the cursor to the scalp image.
- Press and hold the mouse button to rotate the scalp image to ensure all grid points are touching the skin. If the grid points are not on the scalp, adjust the curvature of the grid by moving the curvature slider.
- Repeat these procedures to place another 3 x 5 grid over the opposite hemisphere.
NOTE: This can be performed before a participant's enrollment into the study and arrival to the laboratory. Additionally, a participant's anatomical T1-weighted image can be used if available. Specific details on using anatomical MRIs for navigation can be found in the previously published article16.
- Start a new session within the image guidance software by selecting the Sessions tab once the software is open.
- Click on Nuovo, and then on Online Session. In the next window, select the two grids created in the previous section (section 2.1) by clicking on them, and then click on Add.
- In the IOBox tab, under TTL Trigger Options check the box next to Use Switch (Switch In) and input 0 ms into the Dead Time box. Click on the Next button at the top. Visually ensure the image guidance system's camera is active.
- Begin participant registration by placing the subject tracker, supplied with the image guidance system, around the participant's forehead.
- Manually adjust the camera to ensure the participant tracker is in the middle of the camera's field of view. Next, click on the Registration tab at the top of the software.
- Place the image guidance system's pointer/marker onto the registration landmarks: nasion and the right and left periauricular points. When the pointer is placed on the skin, click on the Next button to register the participant's skin locations to the image guidance software.
- After the registration landmarks have been captured, click on the Scaling tab at the top of the software window. Place the pointer on the rightmost, leftmost, topmost, frontmost, and backmost locations of the participant's scalp.
- Click on the Next button at each location to scale the image guidance system to the participant's head. After scaling is complete, click on the Perform tab at the top of the software. The image guidance system is now ready.
3. Surface electromyography preparation and setup
- Prepare the participants' tibialis anterior (TA) and soleus (SOL) muscles for surface electromyography (sEMG) electrodes. To prepare the skin for sEMG, clean the area using alcohol pads and, if necessary, remove any hair with a single-use safety razor. Place the sEMG disposable gel electrodes according to SENIAM guidelines17.
NOTE: Sensor placement for the TA is 1/3 of the way down on the line between the tip of the fibula and the tip of the medial malleolus. For the SOL, place the sensor 2/3 of the line between the medial condyle of the femur to the medial malleolus. - Once the electrodes are attached, visually inspect the signal for quality. Then, proceed to wrap the shanks with an elastic bandage to minimize any movement of the electrodes and the resultant artifact during testing.
NOTE: Record sEMG signals at 5000 Hz in a 0.5 s window starting 0.1 s before the delivery of the TMS pulses. The exact sampling frequency and amount of data collected will be dependent on the hardware and software used to record the sEMG response to TMS. For details on establishing EMG recordings and analyses see Tankisi et al.18.
4. Force plate and participant safety setup
- Open the data collection software and start a new trial to calibrate the dual-top force plate.
- Click Start and begin an FP Zero trial. Collect 3-5 s of data with no load on the force plate, and then click Stop.
- Once the force plate is calibrated, the participant has been registered to the image guidance system (section 2.2), and the sEMG electrodes have been placed and tested for the signal quality (section 3), instruct the participant to stand and fit them with a safety harness.
- Have the participant step onto the force plate and standardize their foot placement with masking tape pre-applied to the force plate to signify the foremost position of the foot and medial edges of the feet equal distances from the midline.
- Attach the participant's safety harness to the ceiling support. Place a rollator, or similar device, around the force plate to provide participants with something to steady themselves with during testing if needed.
NOTE: Ensure that during all standing TMS procedures the participants are secured to the ceiling via a safety harness to prevent a fall.
- Measure and collect the participant's weight as they stand on the force plate by clicking Start and selecting an FP Static trial. Record 2-5 s worth of data and click Stop to end the trial.
- When standing on the force plates, ensure that the data collection software displays two bar graphs representing the weight/force under each of the participant's feet (Figure 1A). When the participant shifts their weight to one side, the bar graphs will change in height (Figure 1B).
- If a participant unloads the weight on their legs to their arms, ensure the bar graph display changes color (Figure 1C). After a participant becomes comfortable standing with equal weight distributed between their legs, measuring the CMR can commence.
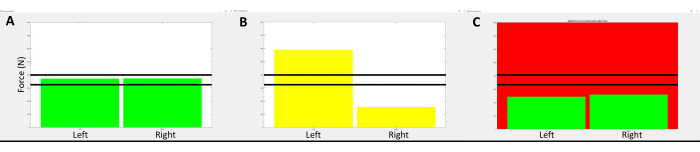
Figure 1: Representative image of the visual feedback provided to participants during the standing TMS assessment. (A) displays the visual feedback given to participants while they were standing with their weight equally distributed between the paretic and non-paretic legs. The vertical bars represent the amount of force measured by each of the areas of the force plate. The solid horizontal lines represent the range of vertical force measured to ensure loading of body weight on the lower extremities and not through the arms if participants needed to steady themselves with the provided hand support. If the participant's body weight was shifted to one side more than 5%, the vertical bars changed colors to inform the participant to lean toward the side that was unloaded, as shown in (B). If the participant loaded/unloaded more than +/- 5% of their body weight off their legs, the background screen color would change as shown in (C). Please click here to view a larger version of this figure.
5. Standing corticomotor response assessment
- Begin the neurophysiological assessments by identifying a stimulator intensity that produces consistent motor evoked potentials (MEP), i.e., EMG signal amplitude > 50 µV, and/or a visible corticosilent period in the active muscles, in the target TA, and SOL muscle.
NOTE: Use a double-cone coil to deliver all TMS pulses with the current moving through the coil in an anterior to posterior direction. Apply the TMS pulses only when the participant is maintaining equal weight distribution between their paretic and non-paretic legs, as indicated by the visual feedback/bar graphs mentioned in the previous section (section 4.2).- Test the paretic limb first by applying TMS pulses to the lesioned hemisphere. Begin by setting the TMS stimulator power level to 50% maximal stimulator output (%MSO) by turning the output control knob. Apply a single pulse at 50% MSO to the middle grid point located just lateral to the longitudinal fissure by pressing the trigger button on the stimulator. Apply 2-3 pulses with an interstimulus interval of 5-10 s.
NOTE: If a participant shows a response at 50% MSO, skip to section 5.2 and begin hotspot identification. - If responses are not seen in the TA and SOL, increase the stimulator power by 10% MSO by turning the output control knob and deliver 2-3 TMS pulses as in step 5.1.1.
- If no responses are seen after increasing the stimulator to 60% MSO, again increase the power by 10% MSO. If no MEPs are elicited at 70% MSO, randomly select several grid points and apply TMS pulses to determine whether there is a response at the current power setting.
- If no responses are recorded at any grid point at the current 70% MSO, return to the initial target grid point land, continue to increase the stimulator power by increments of 10% MSO and apply 2-3 stimulations as previously described.
NOTE: Repeat this process until reliable responses are recorded from the target muscles or until it is determined that the participant has no response to TMS. Not all participants will produce a measurable response to TMS.
- Test the paretic limb first by applying TMS pulses to the lesioned hemisphere. Begin by setting the TMS stimulator power level to 50% maximal stimulator output (%MSO) by turning the output control knob. Apply a single pulse at 50% MSO to the middle grid point located just lateral to the longitudinal fissure by pressing the trigger button on the stimulator. Apply 2-3 pulses with an interstimulus interval of 5-10 s.
- Once the stimulator power that produces a consistent response has been identified, begin identifying the hotspot, i.e., the scalp location that produces the largest response to the applied TMS pulses.
- Start a new hotspot trial by clicking Start and selecting Hotspot. Apply a single-pulse stimulation to each of the 15 grid points at the suprathreshold power level identified in the previous steps. Using the image guidance system, move the coil to the first grid point.
- Once the coil is in the proper position, apply the TMS pulse by pressing the trigger button on the stimulator unit. Next, move the coil to the next grid location and apply another single TMS pulse. Continue until a single stimulation has been applied to each grid point and click on Stop to end the trial.
- Examine the amplitudes of the sEMG signals recorded at each grid point. Visually identify the grid points with the largest MEP amplitude, recorded in the sEMG signals, for each of the targeted muscles. The grid locations with the largest MEP amplitudes are the hotspots and will be used to measure the corticomotor response in the following sections.
NOTE: On some occasions, a single grid location may provide the largest MEP amplitudes for both the TA and SOL. In these cases, determine the motor thresholds for each muscle separately.
- Next, determine the motor threshold of the targeted muscle using simple adaptive Parameter Estimation by Sequential Testing (PEST)19,20.
- Open the PEST program and set the initial stimulator intensity to the suprathreshold value used to identify the hotspot by typing the value into the box.
- Begin a new PEST trial by clicking the Start tab in the data collection software and select PEST.
- Apply a single TMS pulse to the identified target muscle's hotspot at the initial %MSO intensity displayed in the PEST program. Indicate in the PEST program that a response was observed in the muscle's sEMG signal by typing y or n. The PEST program will automatically calculate the next stimulation intensity.
- Adjust the stimulator's power level to match the PEST program and apply another single TMS pulse. Continue this process until the PEST program determines the motor threshold, indicated by a change in color of the stimulation intensity, and end the data collection trial by clicking on the Stop tab.
NOTE: The PEST procedure uses a freely available program that directs how much stimulator power to use with successive pulses. One of the PEST programs can be found here: (https://www.clinicalresearcher.org/software.htm).
- After the target muscle's hotspot and motor threshold have been identified, begin the CMR evaluation. Set the intensity of the stimulator to 120% of the determined motor threshold.
- Initiate a new trial in the data collection software by clicking on the Start tab and select an MEP trial. Place the coil on the muscle's hotspot and apply 10-20 single-pulse stimulations.
- Allow for 5-10 s between each stimulation. Record the evoked sEMG responses for off-line analysis. Allow the participant to rest ad libitum and for sufficient time between testing procedures to reduce the likelihood of the participant developing fatigue, which could affect the results.
- Click on the Stop tab after recording the MEPs to end the trial.
NOTE: The researcher handling the TMS coil should ensure that the participants have equal weight distribution under each leg immediately prior to applying any TMS pulse. If the investigator thinks the stimulation was applied while the participant's weight was not equally distributed, perform an additional stimulation and exclude the previous trial from future analysis. Test the non-paretic muscles immediately after the paretic muscles. Figure 2 displays the experimental setup during the standing TMS assessment.
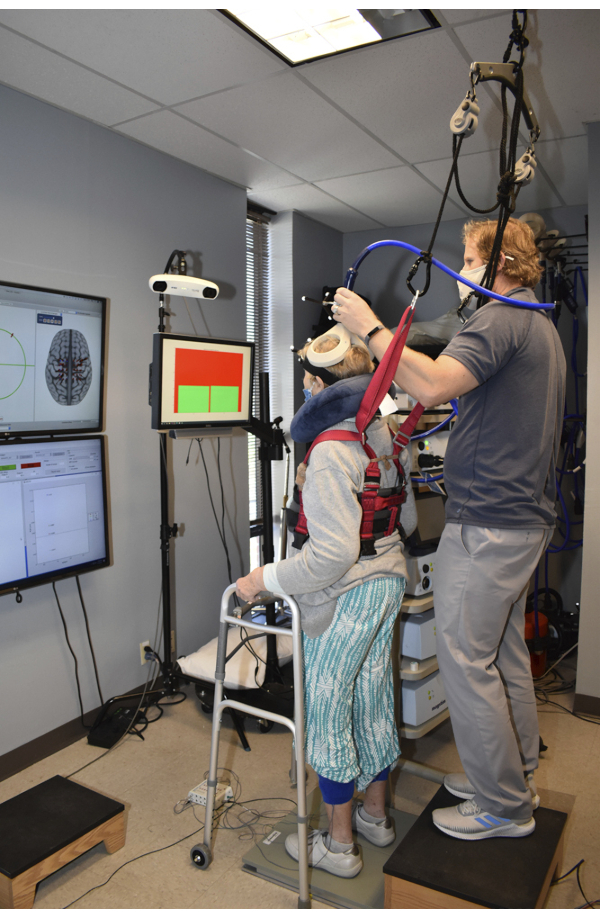
Figure 2: Image taken during the measurement of the corticomotor response (CMR) in the standing position. The image guidance system and the collected sEMG activity are displayed to research personnel during data collection as shown on the monitors located on the left side of the image. Visual feedback of the weight distribution was provided in front and slightly to the right of the participants. Participants wore a safety harness which was attached to the ceiling to prevent falls while standing on the dual-top force plate. Support for the participants' arms was provided to help participants steady themselves after TMS pulses were applied. Please click here to view a larger version of this figure.
6. Sitting corticomotor response assessment
- After the completion of the standing TMS assessment, remeasure the motor thresholds and the CMR in a resting/sitting position.
- Use the same procedures previously described (sections 5.2-5.4). The only change being the participant should be seated in a chair with their legs supported and muscles relaxed.
- Use the same hotspots identified during the standing assessment (section 5.2) in the sitting position. Perform the neurophysiological testing in the same manner as used in the standing position, except for using a stimulation intensity of 120% of the resting/sitting motor threshold.
NOTE: It may be necessary to perform additional testing using a previously determined stimulator power. For example, if comparisons between the amplitude of the MEP in different postural positions are performed, it may be necessary to use a similar absolute stimulator power. This will depend upon the research question at hand and should be identified during study design.
7. Statistical approach
- To test the hypothesis that standing would lead to an increased probability of evoking measurable responses construct a 2 x 2 table and test the proportions using McNemar's Test21.
- To compare the power levels of the motor thresholds, use a paired t-test on the participants who had measurable responses in both positions. Determine significance with an alpha = 0.05.
Representative Results
One participant was removed from the analysis due to the inability to tolerate the standing TMS procedure due to preexisting knee pain and a diabetic wound received before their arrival to the research laboratory, leaving a final sample size of 15. The diabetic wound was directly over the TA and precluded any sEMG measures of this muscle. There were no major adverse events reported to the investigators during either the sitting or standing TMS procedures. Several minor adverse events were reported, such as neck muscle pain and slight headaches. However, these minor events were reported at the end of the testing session, and it was not clear whether the sitting or standing procedures were more responsible for these side-effects. These minor adverse events are commonly seen after TMS evaluations and within the TMS literature22.
Total loading/unloading of body weight during TMS pulse application was +0.4% (SD 1.8%) of body weight. This signifies that the participants did not unload body weight from their legs to their arms when using the rollator as a means to support themselves during the TMS procedures. The average weight distribution of the participants' left leg was 50% (SD 6%). We attempted to measure motor thresholds in four separate muscles (paretic and non-paretic, TA and SOL), leading to a total of 60 motor thresholds in both the standing and sitting positions. In the standing position, we were able to elicit and measure a motor threshold 90.0% of the time compared to 65.0% in the sitting position. Within a single session, it was more likely that assessing the motor threshold in the standing position would result in a measurable response (McNemar Chi2, Yates correction, χ = 8.48, P = 0.004) (Table 2). This agrees with our first hypothesis that the standing position would result in an increased likelihood of evoking measurable responses. Our second hypothesis was that standing would result in motor thresholds requiring lower stimulator power. Our results show that when individuals presented with measurable motor thresholds in the sitting and standing positions, the measured thresholds in the standing position were lower (N = 38, Standing MT 45% MSO SD 9, Sitting MT 53% MSO SD 11, Paired t-statistic 4.99, P < 0.001). Figure 3 displays the measured motor thresholds for each muscle and condition for all participants.
| Sitting Response |
Standing Response | ||||
| Yes | No | Total | % | ||
| Yes | 38 | 1 | 39 | 65 | |
| No | 16 | 5 | 21 | 35 | |
| Total | 54 | 6 | 60 | ||
| % | 90 | 10 | 100 | ||
Table 2: The constructed 2 x 2 table shows the reported ability to successfully produce a response to TMS and the ability to measure a motor threshold in the sitting and standing conditions. The McNemar's test was used to compare the probability of eliciting a measurable response and it was found that the standing assessments were significantly more likely to evoke a measurable response compared to performing evaluations in a sitting position.
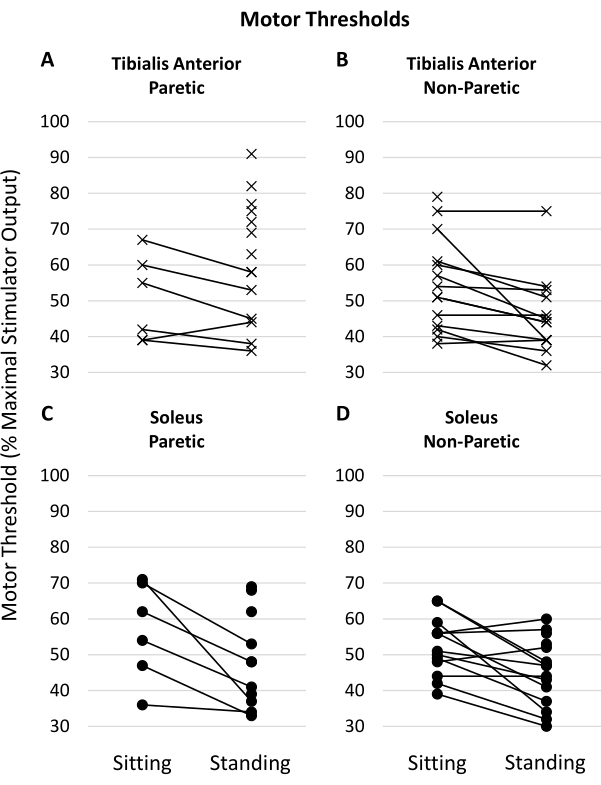
Figure 3: Measured motor thresholds in the muscles of interest. Lines connecting the left and right values indicate the individual had measurable motor thresholds for that muscle in both the sitting and standing positions. Motor thresholds are measured and reported as a percentage of maximal stimulator output (%MSO). (A,B) show motor thresholds measured in the paretic and non-paretic tibialis anterior muscles, respectively. (C, D) show the motor thresholds of the paretic and non-paretic soleus muscles, respectively. Please click here to view a larger version of this figure.
Discussion
The experimental protocol was well tolerated by most participants. One individual was unable to complete the standing TMS evaluation due to preexisting decubitus ulcers secondary to diabetic complications and orthopedic issues involving preexisting knee pain. The amount of loading/unloading of body weight from the legs was minimal. However, there was, on average, a slightly greater downward force measured during the application of the TMS pulses. This is likely due to the weight of the coil and the downward pressure applied by the investigators to ensure there was sufficient contact between the scalp/head and the TMS coil. The minimal changes in body weight captured during the TMS procedures compared to the static trials suggest that no significant effects of bodyweight loading or unloading contributed to our results. We also examined the weight distribution between the legs and found it to be symmetrical, with an average of 50% of the participants’ weight supported by their left legs. It is expected that post-stroke individuals who can stand for 10 min with little to no support can complete the described standing TMS assessments. The standing position allowed for a greater response rate to TMS compared to the resting/sitting position. The increase in TMS responsiveness in the standing position may allow individuals who were previously disqualified from neurophysiological studies due to lack of measurable TMS response to qualify for future studies investigating postural and walking-specific post-stroke motor control. Increasing the pool of eligible participants can lead to greater generalizability of research findings across the post-stroke population.
Motor thresholds assessed in the standing position were measured at a lower %MSO. Post-stroke motor thresholds are often increased23 and require stimulation at a high %MSO to measure the CMR. Applying high-power TMS pulses with a double-cone coil can lead to increased facial and upper extremity muscle contractions that can be uncomfortable for research participants. Performing neurophysiological evaluations at a lower intensity may increase the tolerability of TMS procedures in some post-stroke participants and increase participation in these types of studies.
This methodology describes the process for measuring the corticomotor response to single-pulse TMS. However, paired-pulse paradigms can also be collected in the standing position. Short-latency intracortical inhibition (SICI) and intracortical facilitation (ICF) use two TMS pulses delivered by the same coil with interstimulus intervals of 2 and 10 ms, respectively24. These intracortical measures can provide additional details on the neurophysiological state/behavior of the nervous system during standing compared to motor thresholds alone.
As with all scientific methods, there are limitations to the current protocol. An important item to consider is that individuals with post-stroke hemiparesis do not perform activities in the same manner as neurologically intact groups. People in the chronic phase post-stroke have usually developed compensatory strategies to perform physical tasks25,26, which extends into maintaining an upright posture. Even with equal/symmetrical weight-bearing between the paretic and non-paretic limbs, post-stroke participants may not be in a symmetrical upright posture. Standardizing foot positions on the force plate may help curb this limitation. Another limitation is that recent investigations have suggested recording more than 10 motor-evoked potentials27, due to the known variability in the CMR. In this investigation, we chose to record only 10 test pulses to reduce participant burden while standing. As mentioned previously, this protocol was well-tolerated/performed by individuals who have the ability to stand independently for at least 10 min. This fact may limit the use of this protocol in high/severe disability levels post-stroke or in individuals with orthopedic limitations.
Neurophysiological assessment methods of the lower extremities, and especially in neurologically impaired populations, have yet to receive much consistency within the literature. When posture and walking-specific impairments and/or lower extremity rehabilitation are the primary focus, there is no consensus on the best method to use. For instance, comparisons between resting, active, and standing measures and how these measures relate to clinical disability have not been fully investigated. Most researchers would agree that the double-cone coil is the most appropriate device to use to stimulate the lower extremity cortical representations. Outside of this parameter, much of the lower extremity TMS studies are done to individual research groups’ standards. The lack of consistency between research groups increases the difficulty in performing larger meta-analytical assessments needed to extend the generalizability of research findings. In this protocol, we provide a basis for lower extremity TMS procedures that can be used in studies investigating postural and walking-specific motor control and neurorehabilitation post-stroke.
Divulgazioni
The authors have nothing to disclose.
Acknowledgements
The authors would like to acknowledge Mr. Brian Cence and Mrs. Alyssa Chestnut for their contributions to participant recruitment and data collection.
Funding for this project was provided in part by a Technical Development Award from the NIH National Center for Neuromodulation for Rehabilitation (NM4R) (HD086844) and by Veteran's Affairs Rehabilitation Research and Development Career Development Award 1 (RX003126) and Merit award (RX002665).
The contents of this report do not represent the views of the U.S. Department of Veterans Affairs, U.S. National Institutes of Health, or the United States Government.
Materials
| Data Acquisition Software | MathWorks | MatLab | The custom data collection program was written in Matlab. However, other software/hardware providers can be used (e.g. National Instruments, AD Instruments, CED Spike2 or Signal) |
| Double-cone coil | Magstim | D110 | Double-cone coil for TMS pulse delivery |
| Dual force plate | Advanced Mechanical Technology Inc (AMTI) | Dual-top Accusway | Force plate used to measure force/weight distrobution under each leg independently. |
| Dual-pulse TMS | Magstim | Bistim 200 | Connects two Magstim 200 units together for dual-pulse applications |
| EMG pre-amplifiers | Motion Labs Inc | MA-422 | Preamplifiers for disposable surface EMG electrodes |
| EMG system | Motion Labs Inc | MA400 | EMG system for data collection |
| Neuronavigation System | Rogue Research | Brainsight | Software and hardware used to ensure consistent placement/delivery of magnetic stimulations. Marking the stimulation location on a participant's head or on a place showercap can also be used in the absence of neuronavigational software. |
| Recruitment Database | N/A | N/A | Electronic database including names of possible individuals who are eligble for your studies. |
| TMS unit (x2) | Magstim | Magstim 200 | Delivers TMS pulses |
Riferimenti
- Kesar, T. M., Stinear, J. W., Wolf, S. L. The use of transcranial magnetic stimulation to evaluate cortical excitability of lower limb musculature: Challenges and opportunities. Restorative Neurology and Neuroscience. 36 (3), 333-348 (2018).
- Sivaramakrishnan, A., Madhavan, S. Absence of a transcranial magnetic stimulation-induced lower limb corticomotor response does not affect walking speed in chronic stroke survivors. Stroke. 49 (8), 2004-2007 (2018).
- Kindred, J. H., et al. Individualized responses to ipsilesional high-frequency and contralesional low-frequency rTMS in chronic stroke: A pilot study to support the individualization of neuromodulation for rehabilitation. Frontiers in Human Neuroscience. 14, 578127 (2020).
- Lu, M., Ueno, S. Comparison of the induced fields using different coil configurations during deep transcranial magnetic stimulation. PLoS One. 12 (6), 0178422 (2017).
- Hess, C. W., Mills, K. R., Murray, N. M. Responses in small hand muscles from magnetic stimulation of the human brain. The Journal of Physiology. 388, 397-419 (1987).
- Petersen, N., Christensen, L. O., Nielsen, J. The effect of transcranial magnetic stimulation on the soleus H reflex during human walking. The Journal of Physiology. 513, 599-610 (1998).
- Capaday, C., Lavoie, B. A., Barbeau, H., Schneider, C., Bonnard, M. Studies on the corticospinal control of human walking. I. Responses to focal transcranial magnetic stimulation of the motor cortex. Journal of Neurophysiology. 81 (1), 129-139 (1999).
- Schubert, M., Curt, A., Colombo, G., Berger, W., Dietz, V. Voluntary control of human gait: conditioning of magnetically evoked motor responses in a precision stepping task. Experimental Brain Research. 126 (4), 583-588 (1999).
- Ackermann, H., Scholz, E., Koehler, W., Dichgans, J. Influence of posture and voluntary background contraction upon compound muscle action potentials from anterior tibial and soleus muscle following transcranial magnetic stimulation. Electroencephalography and Clinical Neurophysiology. 81 (1), 71-80 (1991).
- Lavoie, B. A., Cody, F. W., Capaday, C. Cortical control of human soleus muscle during volitional and postural activities studied using focal magnetic stimulation. Experimental Brain Research. 103 (1), 97-107 (1995).
- Soto, O., Valls-Solé, J., Shanahan, P., Rothwell, J. Reduction of intracortical inhibition in soleus muscle during postural activity. Journal of Neurophysiology. 96 (4), 1711-1717 (2006).
- Kesar, T. M., Eicholtz, S., Lin, B. J., Wolf, S. L., Borich, M. R. Effects of posture and coactivation on corticomotor excitability of ankle muscles. Restorative Neurology and Neuroscience. 36 (1), 131-146 (2018).
- Nandi, T., et al. In standing, corticospinal excitability is proportional to COP velocity whereas M1 excitability is participant-specific. Frontiers in Human Neuroscience. 12, 303 (2018).
- Tokuno, C. D., Keller, M., Carpenter, M. G., Márquez, G., Taube, W. Alterations in the cortical control of standing posture during varying levels of postural threat and task difficulty. Journal of Neurophysiology. 120 (3), 1010-1016 (2018).
- Mouthon, A., Taube, W. Intracortical inhibition increases during postural task execution in response to balance training. Neuroscienze. 401, 35-42 (2019).
- Charalambous, C. C., Liang, J. N., Kautz, S. A., George, M. S., Bowden, M. G. Bilateral assessment of the corticospinal pathways of the ankle muscles using navigated transcranial magnetic stimulation. Journal of Visualized Experiments: JoVE. (144), (2019).
- Hermens, H. J., Freriks, B., Disselhorst-Klug, C., Rau, G. Development of recommendations for SEMG sensors and sensor placement procedures. Journal of Electromyography and Kinesiology. 10 (5), 361-374 (2000).
- Tankisi, H., et al. Standards of instrumentation of EMG. Clinical Neurophysiology. 131 (1), 243-258 (2020).
- Mishory, A., et al. The maximum-likelihood strategy for determining transcranial magnetic stimulation motor threshold, using parameter estimation by sequential testing is faster than conventional methods with similar precision. The Journal of ECT. 20 (3), 160-165 (2004).
- Borckardt, J. J., Nahas, Z., Koola, J., George, M. S. Estimating resting motor thresholds in transcranial magnetic stimulation research and practice: a computer simulation evaluation of best methods. The Journal of ECT. 22 (3), 169-175 (2006).
- McNemar, Q. Note on the sampling error of the difference between correlated proportions or percentages. Psychometrika. 12 (2), 153-157 (1947).
- Rossi, S., et al. Safety and recommendations for TMS use in healthy subjects and patient populations, with updates on training, ethical and regulatory issues: Expert Guidelines. Clinical Neurophysiology. 132 (1), 269-306 (2021).
- McDonnell, M. N., Stinear, C. M. TMS measures of motor cortex function after stroke: A meta-analysis. Brain Stimulation. 10 (4), 721-734 (2017).
- Reis, J., et al. Contribution of transcranial magnetic stimulation to the understanding of cortical mechanisms involved in motor control. The Journal of Physiology. 586 (2), 325-351 (2008).
- Chen, G., Patten, C., Kothari, D. H., Zajac, F. E. Gait differences between individuals with post-stroke hemiparesis and non-disabled controls at matched speeds. Gait & Posture. 22 (1), 51-56 (2005).
- Knarr, B. A., Reisman, D. S., Binder-Macleod, S. A., Higginson, J. S. Understanding compensatory strategies for muscle weakness during gait by simulating activation deficits seen post-stroke. Gait & Posture. 38 (2), 270-275 (2013).
- Ammann, C., et al. A framework to assess the impact of number of trials on the amplitude of motor evoked potentials. Scientific Reports. 10 (1), 21422 (2020).

