2D and 3D Human Induced Pluripotent Stem Cell-Based Models to Dissect Primary Cilium Involvement during Neocortical Development
Summary
We present detailed protocols for the generation and characterization of 2D and 3D human induced pluripotent stem cell (hIPSC)-based models of neocortical development as well as complementary methodologies enabling qualitative and quantitative analysis of primary cilium (PC) biogenesis and function.
Abstract
Primary cilia (PC) are non-motile dynamic microtubule-based organelles that protrude from the surface of most mammalian cells. They emerge from the older centriole during the G1/G0 phase of the cell cycle, while they disassemble as the cells re-enter the cell cycle at the G2/M phase boundary. They function as signal hubs, by detecting and transducing extracellular signals crucial for many cell processes. Similar to most cell types, all neocortical neural stem and progenitor cells (NSPCs) have been shown harboring a PC allowing them to sense and transduce specific signals required for the normal cerebral cortical development. Here, we provide detailed protocols to generate and characterize two-dimensional (2D) and three-dimensional (3D) cell-based models from human induced pluripotent stem cells (hIPSCs) to further dissect the involvement of PC during neocortical development. In particular, we present protocols to study the PC biogenesis and function in 2D neural rosette-derived NSPCs including the transduction of the Sonic Hedgehog (SHH) pathway. To take advantage of the three-dimensional (3D) organization of cerebral organoids, we describe a simple method for 3D imaging of in toto immunostained cerebral organoids. After optical clearing, rapid acquisition of entire organoids allows detection of both centrosomes and PC on neocortical progenitors and neurons of the whole organoid. Finally, we detail the procedure for immunostaining and clearing of thick free-floating organoid sections preserving a significant degree of 3D spatial information and allowing for the high-resolution acquisition required for the detailed qualitative and quantitative analysis of PC biogenesis and function.
Introduction
Primary cilia (PC) are microtubule-based organelles that sense and transduce a plethora of chemical and mechanical cues from the extracellular environment. In particular, PC is the central organelle for the transduction of the Hedgehog signaling pathway in vertebrates1,2. While most neural cells have long been shown harboring a PC, the contribution of this organelle in shaping the central nervous system has long been undervalued. Studies on neocortical development have led to the discovery of multiple neural stem and progenitor cells (NSPCs), all harboring a PC, the location of which has been proposed to be crucial for progenitor fate determination3,4,5,6,7. PC has been shown crucial for cell mechanisms that are required for normal cerebral cortical development, including NSPC expansion and commitment8,9,10,11,12 as well as apicobasal polarity of radial glial scaffold supporting neuronal migration13. In addition, PC are required during interneurons tangential migration to the cortical plate14,15. Finally, a role for the PC has been proposed in the establishment of synaptic connections of neurons in the cerebral cortex16,17. Altogether, these findings argue for a crucial role of PC at major steps of cerebral cortical development18,19 and raise the need to investigate their involvement in the pathological mechanisms underlying anomalies of cerebral cortical development.
Recent studies have largely improved our understanding of important cellular and molecular differences between cortical development in human and animal models, emphasizing the need to develop human model systems. In this view, human induced pluripotent stem cells (hIPSCs) represent a promising approach to study disease pathogenesis in a relevant genetic and cellular context. Adherent two-dimensional (2D) cell-based models or neural rosettes contain NSPCs similar to those seen in the developing cerebral cortex, which become organized into rosette-shaped structures showing correct apicobasal polarity20,21,22. Furthermore, the three-dimensional (3D) culture system allows the generation of dorsal forebrain organoids that recapitulate many features of human cerebral cortical development23,24,25,26. Those two complementary cell-based modeling approaches offer exciting perspectives to dissect the involvement of PC during normal and pathological development of the cerebral cortex.
Here, we provide detailed protocols for the generation and characterization of neural rosettes and derived NSPCs as well as dorsal forebrain organoids. We also provide detailed protocols to analyze the biogenesis and function of PC present on NSPCs by testing the transduction of the Sonic Hedgehog pathway and analyzing the dynamics of crucial molecules involved in this pathway. To take advantage of the 3D organization of the cerebral organoids, we also set up a simple and cost-effective method for 3D imaging of in toto immunostained cerebral organoids allowing rapid acquisition, thanks to a light sheet microscope, of the entire organoid, with high resolution enabling to visualize PC on all types of neocortical progenitors and neurons of the whole organoid. Finally, we adapted immunohistochemistry on 150 µm free-floating sections with subsequent clearing and acquisition using resonant scanning confocal microscope allowing high-resolution image acquisition, which is required for the detailed analysis of PC biogenesis and function. Specifically, 3D-imaging software allows 3D-reconstruction of PC with subsequent analysis of morphological parameters including length, number, and orientation of PC as well as signal intensity measurement of ciliary components along the axoneme.
Protocol
1. Generation of 2D hIPS cell-based models of neocortical development
- Neural rosette formation
- Start with hIPSC cultures harboring large regular colonies, exhibiting less than 10% differentiation and no more than 80% confluency.
- Rinse the hIPSCs with 2 mL of PBS.
- Add 2 mL of NSPC induction medium supplemented with the Rock inhibitor (NIM + 10 µM of Y-27632).
- Manually dissect each hIPSC colony from one 35 mm dish using a needle to cut each colony precisely in horizontal and vertical directions to create a checker-board pattern dividing each colony into equal clusters.
- Detach the colony using a cell scraper and transfer them onto an ultra-low-attachment 35 mm dish.
- Let them float overnight (ON) in a humidified incubator at 37 °C and 5% CO2, so that they can form embryoid bodies (EBs).
NOTE: Embryoid bodies (EBs) are defined as floating spheroid clusters or aggregates of hIPSCs. - Transfer the EBs into poly-L-ornithine/laminin (PO/lam)-coated 35 mm dishes in 2 mL NIM + 10 µM of Y-27632.
- Daily refresh NSPC induction medium (NIM without Y-27632) until the formation of neural rosettes that takes approximately 12 to 14 days. Check under the microscope.
NOTE: After this step neural rosettes can be expanded, differentiated, and processed for immunostaining analysis or dissociated to get isolated neural stem and progenitor cells (NSPCs).
- Neural rosette expansion and early differentiation
- Cut neural rosettes in a grid-like pattern with a needle and dislodge the clusters using a cell scraper.
- Transfer the rosettes into a 4-well plate containing PO/lam-coated glass coverslip (4-5 rosettes/well) in 0.5 mL of NSPC maintenance medium (NMM).
- Incubate the plate in a humidified incubator at 37 °C and 5% CO2.
- Refresh NMM every other day until day 20.
- From Day 20, culture neural rosettes in 0.5 mL of cytokine depleted NMM to allow differentiation. Refresh the medium every 2-3 days.
- At Day 30 and Day 40 (10 or 20 days of differentiation), fix the rosettes with 0.5 mL of 4% PFA, 20 min, RT.
- PC biogenesis and function analysis on isolated NSPCs
- NSPC dissociation
- Cut neural rosettes from step 1.1.8 in a grid-like pattern with a needle and dislodge the clusters using a cell scraper. Transfer the cells into PO/lam-coated 35 mm dishes in 2 mL of NMM and incubate in a humidified incubator at 37 °C and 5% CO2.
- Refresh NMM every other day until confluency (approximately 5-7 days).
- After scraping away the large, clear cells surrounding neural rosettes, pick them manually and transfer them onto fresh PO/lam-coated 35 mm dishes to enrich for NSPCs and deplete non-neural cell types.
- After one or two manual passages (repeat step 1.3.1.3 if needed) required to remove all contaminating cell types, remove the medium and rinse with PBS.
- Add 300 µL of 0.05% trypsin and incubate for 5 min at 37 °C until most cells detach.
- Add 2 mL of the medium containing 10% FBS (DMEM + 10% FBS) to inactivate the trypsin. Collect the cell suspension in a 15 mL conical tube. Gently pipette the cell suspension up and down three times to break up the cell clumps.
- Centrifuge at 300 x g for 5 min.
- Carefully aspirate the supernatant and resuspend the cells in NMN.
- Seed the dissociated NSPCs as single cells (1 x 105 cells/cm2) in NMM onto PO/lam-coated dishes and incubate them in a humidified incubator at 37 °C and 5% CO2.
- Expand NSPCs in NMM by changing the medium every other day.
NOTE: NSPCs are seeded at high density to avoid differentiation.
- PC biogenesis analysis
- Seed dissociated NSPCs at 100,000 cells/cm2 and incubate them in a humidified incubator at 37 °C and 5% CO2 in NMM for 2 days.
- Aspirate the medium and starve NSPCs in cytokine depleted medium (NSPC starvation medium, Supplemental Table 1) for 48 h.
- Fix NSPCs with 4% PFA for 20 min at RT.
- Wash twice with PBS for 5 min at RT before immunostaining.
- PC function analysis: SHH signaling pathway
- Seed dissociated NSPCs at 100,000 cells/cm2 on PO/lam-coated 8-well Chamber Slides (300 µL) or T25 dishes (5 mL) and incubate in a humidified incubator at 37 °C and 5% CO2 in NMM for 2 days.
- Starve NSPCs in NSPC starvation medium (300 µL per well of 8-well chamber slide and 5 mL in T25 flask) for 48 h.
- To induce the SHH pathway, incubate the NSPCs with the starvation medium supplemented with recombinant SHH (100 ng/mL) or SAG (500 nM); (150 µL per well of an 8-well chamber slide and 2 mL in T25 flask) for 24 h.
- Fix NSPCs cultured on 8-well Chamber Slides in 250 µL of 4% PFA per well for 20 min at RT and wash them twice with 250 µL of PBS for 5 min at RT before immunostaining analysis.
- Remove the medium and wash NSPCs cultured in T25 dishes with PBS.
- Add 500 µL of 0.05% trypsin and incubate for 5 min at 37 °C until the cells detach.
- Add 2 mL of medium containing 10% FBS (DMEM + 10% FBS) to inactivate the trypsin and collect the cell suspension in a 15 mL conical tube.
- Centrifuge at 300 x g for 5 min and carefully aspirate the supernatant to obtain NSPC pellets that can be cryopreserved at -80 °C before RNA extraction and RT-PCR analysis.
- NSPC dissociation
2. Generation of dorsal forebrain organoids
- Single-cell culture of hIPSCs
- Start with hIPSC cultures harboring large regular colonies exhibiting less than 10% differentiation and that have been passaged almost once. Ensure that the cells are no more than 80% confluent.
- Wash the colonies with 2 mL of PBS.
- Add 500 µL of Gentle Cell Dissociation Reagent (GCDR) and incubate for 5 to 7 min at 37 °C.
- Aspirate the GCDR and add 2 mL of mTeRS1 medium supplemented with 5 µM of Y-27632.
- Pipette the cells gently up and down 10 times to dissociate all colonies into single cells.
- Transfer the cells on vitronectin-coated dishes and incubate in a humidified incubator at 37 °C and 5% CO2.
NOTE: Perform at least 1 passage of the hIPSCs using GCDR prior to the differentiation experiment to adapt the hIPSCs to single-cell culture conditions.
- On Day 0, allow hIPSCs to form embryonic bodies in EB medium containing a low concentration of FGF2 and a high concentration of rock inhibitor required for hIPSC survival. To do so follow the steps below.
- Wash the colonies with 2 mL of PBS.
- Add 500 µL of Gentle Cell Dissociation Reagent (GCDR) and incubate for 5 to 7 min at 37 °C.
- Aspirate the GCDR and add 1 mL of EB medium supplemented with 50 µM of Y-27632; Use a 1 mL pipette to gently detach the cells and transfer them to a 15 mL conical tube.
- Rinse once more with 2 mL of EB medium supplemented with 50 µM of Y-27632, transfer the remaining cells to the 15 mL conical tube. Immediately add 3 mL of EB medium supplemented with 50 µM of Y-27632 to the conical tube and gently homogenize the solution using a 5 mL pipette.
- Centrifuge the dissociated cells at 100 x g for 5 min.
- Gently aspirate the supernatant and resuspend the cells in 6 mL of EB medium supplemented with 50 µM of Y-27632.
- Centrifuge the cells at 100 x g for 5 min.
- Aspirate the supernatant and resuspend the cells in 1 mL of EB medium supplemented with 50 µM of Y-27632. Use a 1 mL pipette to gently dissociate the cells into a single-cell suspension.
- Immediately after resuspending the cells, dilute and count them.
- Dilute the proper volume of cell suspension (containing 900,000 cells) into 30 mL of EB medium supplemented with 50 µM of Y-27632 and 4 ng/mL of bFGF and gently mix the solution.
- Plate 9,000 cells/well into an ultra-low attachment 96U plate (300 µL/well). To avoid disturbing EB formation, incubate the plate in a humidified incubator at 37 °C and 5% CO2 until Day 3 without medium change.
- Neural induction (Dual SMAD inhibition)
- On Day 3, ensure EBs measure 300-400 µm and show regular borders. If both criteria are reached, replace half of the medium (150 µL) with induction medium-1 containing Dual SMAD inhibitors, leading to brightening of the contour of the EBs by Day 6 to Day 7 indicating neuroectodermal differentiation (Supplemental Table 2).
- On Day 4, replace 3/4 of the medium (225 µL) with induction medium-1.
- On Day 6 and Day 8: Refresh half of the induction medium-1 (150 µL).
- Organoid embedding into basement membrane matrix (Day 10)
- On Day 10, embed EBs in growth factor reduced basement membrane matrix (BMM).
NOTE: From this step, always use sterile scissors to cut the opening of the pipette tips to avoid damaging the organoids by repeated pipetting. - First transfer around 15-17 organoids in conical tubes. Let the EBs settle and remove the medium. Add 50 µL of induction medium-2 and transfer them into a microcentrifuge tube containing 100 µL of BMM.
- Spread the matrix-EB mixture onto the center of a well of an ultra-low-attachment plate and separate the EBs to prevent them from fusing.
- Let the BMM solidify in the incubator at 37 °C for 45 min.
- Add 3 mL of the induction medium-2 in each well and put the plate in a humidified incubator at 37 °C and 5% CO2.
NOTE: Ensure that this procedure is done as quickly as possible as BMM solidifies at room temperature.
- On Day 10, embed EBs in growth factor reduced basement membrane matrix (BMM).
- Stationary culture of organoids embedded into basement membrane matrix
- Day 12, 14: Refresh induction medium-2.
- Day 16: Refresh induction medium-2 and check under a microscope the expansion of neuroepithelial loops.
- Basement membrane matrix dissociation and culture on an orbital shaker
- On Day 17, dissociate organoids from BMM by pipetting up and down 10 times with a 5 mL pipette and transfer them into differentiation medium-1 (without vitamin A). Incubate onto an orbital shaker at 80 rpm in a humidified incubator at 37 °C and 5% CO2 to improve nutritional absorption.
NOTE: Use a different incubator for stationary culture and culture onto an orbital shaker to avoid any vibrations detrimental to adherent hIPS cell growth. - Day 19: Refresh differentiation medium-1.
- Day 21: Change differentiation medium-1 to differentiation medium-2 (with vitamin A).
- From Day 23 to Day 35: Refresh medium with differentiation medium-2 every 2-3 days.
- From Day 35 to Day 70: Refresh differentiation medium-2 with 1% BMM and refresh every 2-3 days.
- Collect the organoids after 28, 42, and 70 +/- 2 days of differentiation and fix them overnight at 4 °C, in 5 mL 4% PFA on a 15 mL conical tube.
- For further in toto or free-floating immunostaining analysis, organoids are then rinsed twice in PBS and conserved at 4 °C in PBS + 0.05% sodium azide.
- For immunostaining analysis on frozen sections, immerse 4% PFA fixed organoids in 10 mL 30% sucrose at +4 °C ON (or until organoids sink). Embed them into a frozen embedding matrix and store at -80°C until cryosectioning.
- On Day 17, dissociate organoids from BMM by pipetting up and down 10 times with a 5 mL pipette and transfer them into differentiation medium-1 (without vitamin A). Incubate onto an orbital shaker at 80 rpm in a humidified incubator at 37 °C and 5% CO2 to improve nutritional absorption.
3. In toto immunolabeling, clearing, and lightsheet acquisition of dorsal forebrain organoids
- Fixation
- Collect the organoids in 6-well plates, remove the medium, and fix them with 2 mL of 4% PFA, at 4 °C, overnight.
- Perform three washes in 2 mL of PBS at room temperature.
- Transfer the organoids into 2 mL tubes and store at 4 °C in 2 mL of PBS + 0.05% sodium azide.
- Permeabilization
- Incubate organoids in 1 mL of PBS containing 0.2% Triton X100 at RT for 1 h. Do this twice.
- Incubate in 1 mL of PBS containing 0.2% Triton X100 and 20% DMSO, at 37 °C, overnight.
- Incubate in 1 mL of PBS containing 0.1% Tween20, 0.1% Triton X100, 0.1% Deoxycholate, 0.1% NP40 and 20% DMSO, at 37 °C, overnight.
- Incubate in 1 mL of PBS containing 0.2% Triton-X100, at RT, for 1 h. Do this twice.
- Blocking and immunolabeling
- Block in 1 mL of blocking solution (PBS, 0.2% Triton X100, 6% Donkey Serum,10% DMSO), at 37 °C, ON.
- Incubate with primary antibodies diluted in 250 µL of PBS, 0.2% Tween20, 0.1 µg/mL of Heparin, 5% DMSO and 3% Donkey Serum, at 37 °C, for 2 days.
- Wash in 1 mL of PBS containing 0.2% Tween20 and 0.1 µg/mL of Heparin, for 1 h, at 37 °C. Do this four times.
- Wash in 1 mL of PBS containing 0.2% Tween20 and 0.1 µg/mL of Heparin, overnight, at 37 °C.
- Incubate with secondary antibodies diluted in 250 µL of PBS containing 0.2% Tween 20, 0.1 µg/mL of Heparin and 3% Donkey Serum, at 37 °C, for 2 days.
- Wash in 1 mL of PBS containing 0.2% Tween 20 and 0.1 µg/mL of Heparin, for 1 h, on a wheel, at RT. Do this four times.
- Wash in 1 mL of PBS containing 0.2% Tween 20 and 0.1 µg/mL of Heparin, overnight, on a wheel, at RT.
- Wash in 1 mL of PBS, for 24 h, at RT.
- Stock at +4 °C in 1 mL of PBS and 0.05% sodium azide until clearing.
- Clearing in TDE (2,2′ -Thiodiethanol)
- Incubate the organoids in 1 mL of 30% TDE (3 mL of TDE + 7 mL of PBS), for 24 h, at RT.
- Incubate in 1 mL of 60% TDE (6 mL of TDE + 4 mL of PBS), for 24 h, at RT.
- Incubate in 1 mL of 80% TDE (8 mL of TDE + 2 mL of PBS), for 24 h, at RT.
- Organoid embedding prior to light sheet acquisition
NOTE: Use a custom-made system; made with a 1 mL syringe with the tip cut with a scalpel.- Prepare 100 mL of 4% Low-Melting Agarose in 60% TDE solution and aliquot in 2 mL tubes. Conserve at +4 °C.
- Prior to the organoid embedding, preheat one tube containing 1.5 mL of 4% low-melting agarose in 60% TDE in a water bath at 37 °C until it liquefies.
- Meanwhile, prepare a custom-made molding system made from a 1 mL syringe the tip of which is cut off using a scalpel.
- Pump in the syringe 600 µL of the gel solution using the plunger.
- Position the sample using an enlarged pipette tip the opening of which has been cut using sterile scissors.
- Fill in the syringe with 400 µL of gel solution so that the sample is positioned within the lower third of the syringe.
- Let the gel polymerize and store in 80% TDE solution at 4 °C protected from light until acquisition.
- For Light sheet acquisition, use a 20x objective immersed in 80% TDE solution added in the sample chamber to allow to accurately adjust to the refractive index of the clearing method.
- Insert the syringe containing the agarose embedded organoid in the largest sample holder designed to accommodate a 1 mL syringe the plunger of which can be operated to position the organoid in front of the objective.
NOTE: Light sheet acquisition of an entire organoid takes approximately 5 to 10 min.
4. Immunostaining and clearing of free-floating sections of dorsal forebrain organoids
- Fixation, agarose embedding, and sectioning of the organoids
- Collect organoids after 28, 42, and 70 +/- 2 days of differentiation in a 6-well plate and fix overnight at 4 °C in 2 mL of 4% PFA.
- Rinse twice in 2 mL of PBS and conserve at 4 °C in 2 mL of PBS + 0.05% sodium azide.
- Embed the fixed organoids into 4% low-melting agarose in a plastic embedding mold (7 x 7 mm). Carefully remove the agarose block from the plastic mold and glue it to the vibratome stage.
- Section the embedded organoids using a vibratome to obtain 150 µm free-floating sections transferred in a well of a 24-well plate containing 500 µL of PBS using a paintbrush to avoid damaging the sections.
NOTE: Low-melting agarose is recommended as its melting temperature is only approximately 60 °C. Prepare 4% low-melting agarose in PBS solution and leave it to cool just above 37 °C in a water bath prior to organoid embedding.
- Permeabilization, blocking, and immunostaining
- Incubate the sections in 500 µL of PBS containing 0.3% Triton X100, at RT, under agitation, for 20 min. Do this three times.
- Incubate the sections in 500 µL of PBS containing 0.3% Triton X100 and 5% nonfat Milk, at RT, under agitation, for 2 h.
- Incubate the sections in 250 µL of primary antibody solution (PBS + 0.3% Triton X100 + 1% nonfat Milk), under agitation, at +4 °C, overnight.
- Wash the sections in 500 µL of PBS, at RT, under agitation, for 20 min. Do this four times.
- Incubate the sections in 250 µL of secondary antibody solution (PBS + 0.3% Triton X100 + 1% nonfat milk), at RT, under agitation, for 2 h.
- Wash the sections in 500 µL of PBS, at RT, under agitation, for 20 min. Do this four times.
- Store the sections in 500 µL of PBS, at +4° C until clearing.
- Clearing in TDE (2,2′ -Thiodiethanol)
- Incubate the sections in 500 µL of 30% and 60% TDE for 1 h each at RT, and then in 500 µL of 80% TDE overnight, at RT.
- Store the cleared sections in 500 µL of 80% TDE until acquisition at +4 °C.
- Mounting of free-floating sections prior to acquisition with resonant scanning confocal
- Mount a free-floating section in a sealed chamber enabling to maintain the sample in 80% TDE solution and designed to adapt on a motorized XY stage of confocal microscopes.
- Put one round coverslip in the chamber system.
- Carefully transfer the cleared immunostained section using a paintbrush.
- Fill the chamber with 80% TDE solution.
- Add two standard coverslips plus one second round coverslip and a silicone seal.
- Screw on the screwing ring of the chamber to perfectly seal the system.
5. Light sheet and resonant scanning confocal analysis
- Process light sheet and resonant scanning acquisitions using software that enable 3D visualization and analysis of the entire immunostained sample.
NOTE: Such software allows to quickly open huge data to easily make snapshots and animations. It allows to move the sample in different orientations and to generate 2D views thanks to a 2D slicer tool in different orientations, XY and YZ for example. - For automatic detection of both centrosomes and primary cilia, use a spot wizard enabling to quantify their number in pathological versus control conditions.
- For 3D reconstruction of PC enabling precise measurement of their length, use a filament wizard to manually fix the starting point of the PC and the software uses the fluorescence signal to reconstruct the PC precisely.
Representative Results
2D hIPS cell-based models to study primary cilium biogenesis and function
The protocol detailed here has been adapted from previously published studies20,21,22. This protocol allows the generation of neural rosette structures that contain neocortical progenitors and neurons similar to those seen in the developing neocortex. Detailed validation can be performed by conventional immunostaining analysis using specific makers3. For instance, apical progenitors (AP) should be double-stained with SOX2 and PAX6, intermediate progenitors (IP) are revealed by TBR2/EOMES staining, and early-born neocortical neurons are revealed by CTIP2 staining (Figure 1A–C). Such neural rosette-like structures model interkinetic nuclear migration (INM) of AP that can be visualized by immunostaining with antibodies raised against phospho-vimentin, which stains mitotic nuclei and TPX2 that stains the mitotic spindle. These markers, therefore, allow the analysis of several characteristics of AP, including the INM with cell division to take place apically around the central lumen (Figure 1D,D'), as well as the division mode determined by measuring the angle between the division plane and the apical surface. Finally, ciliogenesis can be analyzed by immunostaining with antibodies raised against PCNT, which stains the basal body of PC, and ARL13B that stains the axoneme. On such rosette structures, PC extend from the apical pole of APs into the central lumen of each ventricle-like region (Figure 1E,E'), while they are also protruding from CTIP2+ neurons (Figure 1E'').
Dissociation of these rosette structures allows obtaining isolated NSPCs cultured on poly-L-ornithine/laminin-coated culture plates in NMM at a high density to allow maintenance of a stable and expandable population of NSPCs for at least 15 passages without accumulating karyotype abnormalities21,27. To analyze PC biogenesis, dissociated NSPCs are cultured to confluence and starved for 48 h. Immunostaining analyses using antibodies raised against PC markers show that those NSPCs harbor PC (Figure 2A). By combining two complementary open-source tools, Ilastik, a machine-learning-based image analysis tool useful for PC segmentation28 and CiliaQ, a Fiji/ImageJ plugin package29, that enable 3D reconstruction of PC from 3D confocal image stacks, several structural parameters can be easily evaluated, including the number of PC and their length.
PC function of NSPCs can also be evaluated by testing the transduction of the Hedgehog signaling pathway. To assess the transduction of the SHH signaling pathway, NSPCs are starved for 48 h and treated with recombinant SHH (rSHH) or a smoothened agonist (SAG) for 24 h. Using antibodies raised against GPR161, SMO, and GLI2, immunofluorescent (IF) analysis allows testing the trafficking of these key SHH signaling actors along the PC, with GPR161 normally exiting the PC while GLI2 and SMO accumulate within the PC in response to SHH pathway activation (Figure 2C–D). Analysis of 3D confocal image stacks using CiliaQ tools allows quantifying GPR161 exit from the PC as well as GLI2 and SMO accumulation within the PC after SHH pathway activation (Figure 2F–G). In addition, semi-quantitative RT-PCR analysis on mRNA extracted from rSHH- or SAG-treated NSPCs shows the induction of two SHH target genes, GLI1 and PTCH1, attesting for normal SHH signaling transduction in NSPCs derived from control hIPSCs (Figure 2B).
Overall, 2D cell-based models of developing dorsal forebrain clearly reproduce several aspects of normal cerebral cortex development and represent promising tools to dissect PC-associated mechanisms underlying anomalies of neocortical development using control versus patient hIPSCs harboring mutations in genes responsible for human developmental anomalies of the cerebral cortex.
3D hIPS cell-based models to study primary cilium involvement during neocortical development
The protocol described here (Figure 3A) has been adapted from previously published protocols23,24,25,26,30 and successfully tested on five distinct control hIPSC lines. It allows the generation of hIPSC-derived dorsal forebrain organoids that increase in size over time while forming large neuroepithelial loops (Figure 3B). Immunolabeling analysis either on organoid cryosections (cryostat, 20 µm), free-floating sections (vibratome, 150 µm), or in toto, can be performed for quality controls. At day 28 ± 2, organoids consist of stratified neuroepithelial loops co-expressing the neural progenitor marker SOX2 and the dorsal forebrain marker PAX6, attesting for dorsal forebrain identity. At day 42 ± 2 (6 weeks of differentiation), those loops should display a more complex stratified organization. From the apical to the basal surface of these loop structures, a ventricular zone (VZ)-like region with SOX2/PAX6-positive apical radial glial progenitors (aRG) can be delineated as well as a subventricular zone (SVZ)-like region with TBR2-positive intermediate progenitors (IPs) and a cortical plate (CP)-like region containing CTIP2-positive early-born neocortical neurons (Figure 3C,E). The apical molecular polarity of aRG can be evaluated by the apical enrichment at the ventricular surface of ZO-1 and N-CADH (Figure 4A, Video 1). The aRG progenitor division properties can be evaluated using TP2X and P-VIM markers to analyze INM that normally leads to the alignment of aRG mitotic nuclei at the ventricular surface of the cortical loops (Figure 4B, Video 2). Such immunolabeling also allows measurement of the division angle to evaluate symmetric versus asymmetric division mode of aRG. P-Vim positive progenitors can also be observed in the SVZ-like region, harboring a unique basal process extending to the basal surface reminiscent of outer radial glia (oRG or basal radial glia, Figure 4C, Video 2). While they are absent from day 42 organoids, SATB2 positive late-born neurons should be detected from day 70 (10 weeks of differentiation) (Figure 3D,F), attesting for in vivo-like timing of neocortical neuronal differentiation.
Immunolabeling experiments using the basal body (gamma Tubulin) and axoneme (ARL13B) markers allow visualization of PC on 20 µm cryosections (Figure 3G,H). To take advantage of the 3D organization of dorsal forebrain organoids, whole-mount immunostaining can be performed. Light sheet acquisition of such in toto immunostained and cleared organoids allows detection of both centrosomes and PC on all NSPC types as well as on neocortical neurons (Video 3). In addition, to perform more accurate analyses of PC biogenesis, immunohistochemistry analysis on free-floating thick sections (150 µm) of dorsal forebrain organoids may be performed. After clearing, such sections may be acquired using a 40x (NA 1.3) or 63x (NA 1.4) oil objective of an inverted resonant white light confocal laser microscope, allowing to improve resolution while preserving the 3D spatial information of organoids. Such laser scanning confocal microscope enables rapid acquisition of thick sections, with a precise and flexible excitation and detection without any interference (Video 4). Several structural parameters of PC can be evaluated, including PC number, length, and orientation, using 3D-imaging software. Dedicated open-source, freely available tools such as Ilastik28, a machine-learning-based image analysis tool as well as the CiliaQ plugin package on Fiji/ImageJ29 are highly useful for automatic PC segmentation allowing subsequent qualitative and quantitative analysis of PC biogenesis and function in control versus pathological conditions.
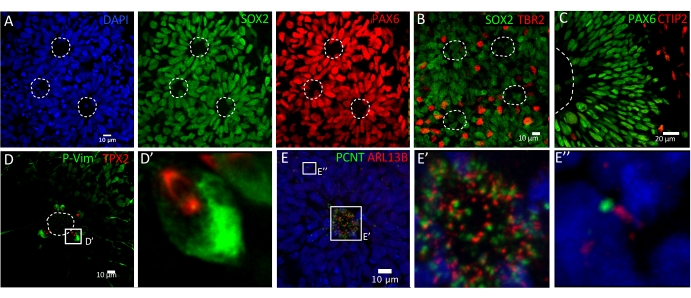
Figure 1: Generation and characterization of 2D neural rosettes. Immunohistochemical characterization of neural rosette structures using (A) SOX2 and PAX6 antibodies to detect AP, (B) TBR2/EOMES to reveal IP, and (C) CTIP2 to stain early-born neocortical neurons. Proliferating AP are detected with phospho-vimentin and TPX2 antibodies and are located at the apical surface (D,D'). PC are revealed by double immunostaining with PCNT and ARL13B antibodies. PC extend from each AP into the central lumen of each rosette, while they are also detected on IP and early-born neurons (E,E',E''). Please click here to view a larger version of this figure.
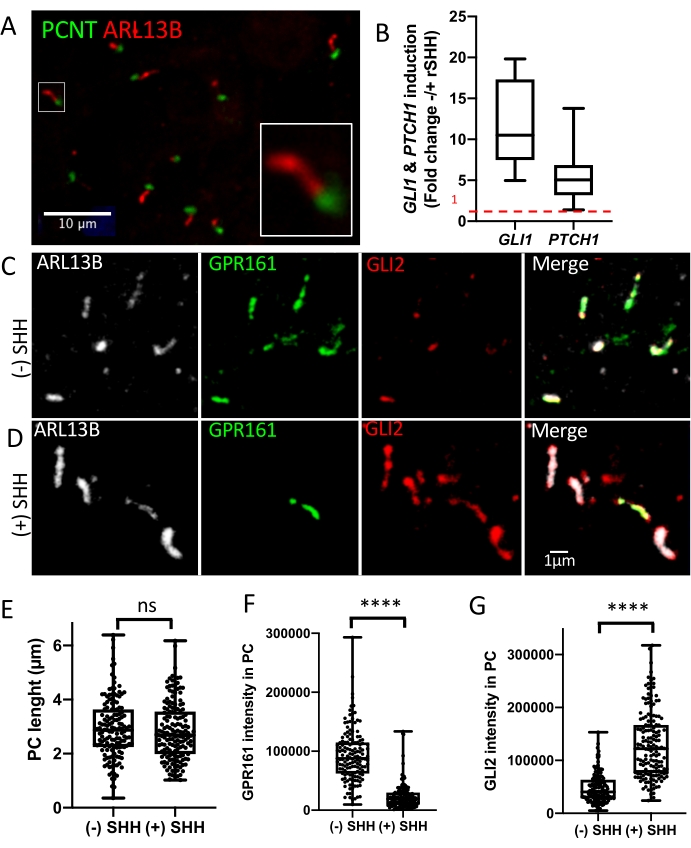
Figure 2: Isolated NSPCs derived from 2D neural rosettes harbor functional PC. Dissociation of 2D-neural rosettes gives rise to isolated NSPCs harboring PC after starvation for 48 h as revealed by ARL13B and PCNT immunostaining (A). NSPCs harbor functional PC as revealed by RT-PCR quantification of two SHH target genes GLI1 and PTCH1 after starvation for 48 h and subsequent activation of the pathway by treatment with recombinant SHH (rSHH) for 24 h. GLI1 and PTCH1 expression data were performed in triplicate and normalized to ACTB expression data. Data were analyzed with the 2−ΔΔCt method and presented as relative expression ± SEM (Mann Whitney test) (B). IF analysis on starved NSPCs with (D) or without (C) rSHH treatment to test the dynamics of two crucial actors of the SHH signaling pathway, GLI2, and GPR161, which respectively accumulate or exit the PC after SHH pathway activation. By combining Ilastik and CiliaQ tools for the analysis of confocal images, GPR161 (F) and GLI2 (G) signal intensity within PC was compared in +/- rSHH treated NSPCs. ARL13B staining was used for PC segmentation, and as expected PC length was unchanged in +/- rSHH treated NSPCs (E). Data are summarized in box and whisker plots (mean values ± SEM). ****p < 0.0001 (Mann Whitney test). Please click here to view a larger version of this figure.
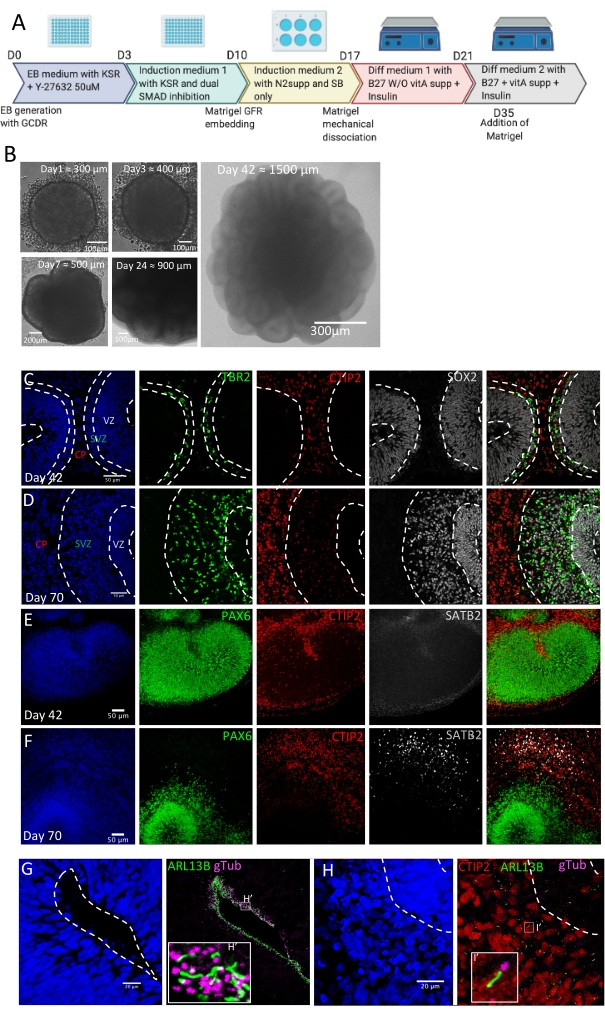
Figure 3: Generation and characterization of dorsal forebrain organoids. (A) Schematic overview of the protocol to generate dorsal forebrain organoids. (B) Representative bright-field images of organoids at days 1, 3, 7, 24, and 42 of differentiation. Confocal images of 20 µm cryosections of organoids at day 42 (C) and 70 (D) after immunostaining with SOX2, TBR2, and CTIP2 antibodies delineating, respectively, the ventricular zone (VZ), the subventricular zone (SVZ) containing TBR2 positive IP, and the preplate-like region (CP) containing CTIP2 positive early-born neocortical neurons. Confocal images of 20 µm cryosections of organoids at day 42 (E) and 70 (F) after immunostaining with PAX6, CTIP2, and SATB2 antibodies showing the appearance of SATB2 positive late-born neurons at Day 70. Confocal images of 20 µm cryosections of an organoid at day 42 after immunostaining with ARL13B and PCNT antibodies revealing PC at the apical pole of radial glial progenitors (G) while they are also present on CTIP2 positive neurons (H). Please click here to view a larger version of this figure.
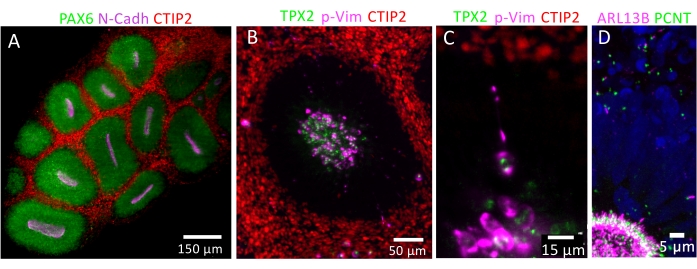
Figure 4: 3D imaging of dorsal forebrain organoids. (A) Light sheet acquisition of an entire dorsal forebrain organoid (Day 42) stained with PAX6, N-CADH, and CTIP2 antibodies showing the multiple neuroepithelial loops with PAX6 positive radial glial progenitors exhibiting correct apicobasal polarity as revealed by the accumulation of N-Cadherin at their apical side and CTIP2 positive early-born neocortical neurons delineating a preplate-like region. (B) Light sheet acquisition of an entire dorsal forebrain organoid (Day 42) stained with TPX2, P-Vim, and CTIP2 antibodies illustrating interkinetic nuclear migration of ventricular radial glial progenitors. (C) Zoom on the light sheet acquisition of an entire dorsal forebrain organoid (Day 42) stained with TPX2 and P-Vim antibodies showing a progenitor extending a unique basal process and localized in the subventricular zone, reminiscent of an outer radial glial progenitor. (D) Resonant Scanner acquisition of a 150 µm section of a dorsal forebrain organoid (Day 42) stained with PCNT and ARL13B antibodies showing PC in NSPCs and neocortical neurons. Please click here to view a larger version of this figure.
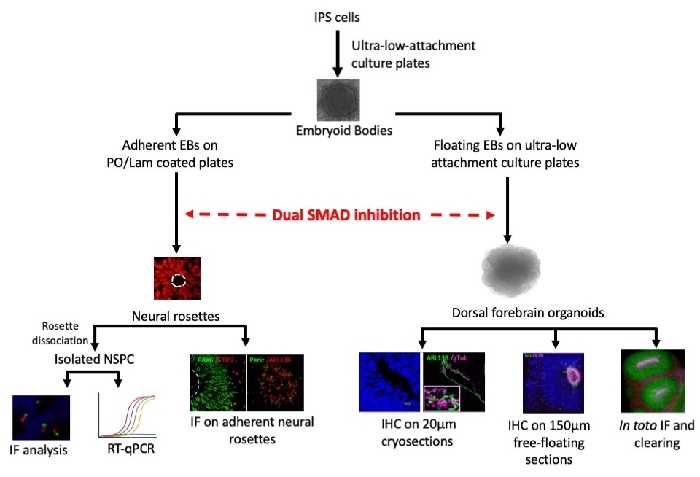
Figure 5: Generation and characterization of 2D and 3D hIPS cell-based models of dorsal forebrain development to dissect PC involvement to the pathophysiology of cerebral cortical anomalies. hIPS cells are allowed to form embryoid bodies (EBs) into low adhesion culture plates, and are then incubated into an induction medium containing dual SMAD inhibitors to induce neuroectodermal differentiation. Adhesion of EBs into PO/lam-coated dishes allows the generation of 2D neural rosette structures while the free-floating culture of EBs with subsequent embedding into BMM allows the generation of dorsal forebrain organoids. Withdrawal of mitogenic factors from adherent neural rosette medium promotes the onset of neurogenesis that can be tested by IF analysis. Dissociation of adherent neural rosettes allows the generation of isolated NSPC cultures useful for PC biogenesis and function analysis. Dorsal forebrain organoids are collected after 4, 6, or 10 weeks of differentiation and fixed for subsequent immunostaining analysis either in toto, on 150 µm thick sections, or 20 µm cryosections. Please click here to view a larger version of this figure.
Video 1: Light sheet acquisition of an entire dorsal forebrain organoid (Day 42) immunostained with PAX6, CTIP2, and NCADH antibodies. Please click here to download this Video.
Video 2: Light sheet acquisition of an entire dorsal forebrain organoid (Day 42) immunostained with TPX2, P-VIM, and CTIP2 antibodies. Please click here to download this Video.
Video 3: Light sheet acquisition of an entire dorsal forebrain organoid (Day 42) immunostained with PCNT and ARL13B antibodies. Please click here to download this Video.
Video 4: Resonant scanning confocal acquisition of a 150 µm section of a dorsal forebrain organoid (Day 42) immunostained with ARL13B and PCNT antibodies. Please click here to download this Video.
SUPPLEMENTARY FILES: Please click here to download the Tables.
Supplementary Table 1: Recipe of the media used for the generation of 2D-neural rosettes and NSPCs.
Supplementary Table 2: Recipe of the media used for the generation of dorsal forebrain organoids.
Discussion
PC are now regarded as key organelles regulating crucial steps during normal cerebral cortical development18,19,31 including NSPC expansion and commitment8,9,10,11,12 as well as neuronal migration13,14 and synaptogenesis16,17. In addition to analysis in animal models or human fetal cerebral tissues, the generation of highly innovative and relevant patient-derived hIPSC-based models of neocortical development is essential to dissect the role of PC during both normal and pathological cerebral cortical development.
The 2D hIPSC-based modeling protocol detailed here was adapted from three major publications20,21,22. Neuroepithelial differentiation is induced by dual SMAD inhibition using small molecule inhibitors of the activin/nodal (SB-431542) and BMP (LDN-193189) pathways22. Cells are organized in rosette-shaped structures containing NSPCs as well as neocortical deep layer neurons after 20 days of differentiation. In addition, these rosette-like structures show correct apicobasal polarity, interkinetic nuclear migration of apical progenitors as well as PC extending from all NSPCs and neurons. After dissociation of these rosette structures, a homogeneous and stable population of cortical progenitors is obtained21. When cultured to confluence and starved for 48 h, those cortical progenitors harbor PC for which the morphology, number, and length can be easily analyzed by combining Ilastik28 and CiliaQ28,29 plugin package on Fiji/ImageJ. Furthermore, by using cytokines to induce the SHH signaling pathway, PC function can also be used by IF to test the dynamics of crucial signaling pathway actors along the PC such as GLI2, SMO, and GPR161. In addition, semi-quantitative RT-PCR assays enable testing of the induction of SHH target gene expression, including GLI1 and PTCH1, in response to SHH pathway activation. Other signaling pathways dependent upon PC function should also be tested, including WNT and IGF pathways2,32,33. To conclude on this 2D modeling approach, which clearly reproduces several aspects of normal cerebral cortex development, it represents a useful and relevant tool for testing PC biogenesis and function in normal versus pathological conditions and should contribute to gain further insight into the involvement of PC during neocortical development.
Complementary to 2D hIPSC-based modeling approaches, dorsal forebrain organoids offer unprecedented opportunities to investigate normal and pathological cerebral development in vitro as they recapitulate many features and characteristics of the early developing human cerebral cortex. Two main types of protocols are currently used: the intrinsic and the guided methods. The intrinsic protocol developed by Lancaster and colleagues23 relies on the intrinsic ability of IPSCs to self-organized with minimal external factors and gives rise to cerebral organoids containing rudiments of distinct brain regions offering a unique opportunity to model the interactions between different brain regions. However, the high variability and heterogeneity inherent to such modeling strategy present significant challenges of reproducibility. Guided organoid differentiation protocols allow the generation of brain region-specific organoids with minimal heterogeneity24,25,26. This approach allowed us to successfully generate dorsal forebrain organoids with ventricle-like structures that recapitulate major processes of early human cerebral cortical development. The first critical issue is to start with high-quality hIPSC cultures harboring large regular colonies exhibiting less than 10% differentiation and which have been passaged almost once as a monolayer to adapt the hIPCs to single-cell culture conditions. Indeed, to limit organoid heterogeneity, one of the major challenges in the field, the formation of EBs with a homogeneous size is a prerequisite that implies the need for dissociation of hIPSC colonies into single-cell suspension allowing seeding at the defined cell density. Another critical step is BMM inclusion of EBs, needed to support 3D structure and neuroepithelial expansion. We favored the group inclusion of about fifteen organoids over individual inclusion even if it involves an additional step, the BMM dissociation. BMM dissociation prior to shaking culture has been shown to reduce variability within and between organoid batches, thus resulting in higher reproducibility34. In addition, it allows getting rid of cell processes extending into the BMM which are not detrimental for the following differentiation steps but which make it difficult to observe organoids for quality inspection during subsequent steps of the procedure. To improve nutritional absorption and oxygen exchange, we compared organoid maturation on orbital shakers and spinning bioreactors that led to similar results. We, therefore, chose the orbital shaker option, as it allows to significantly reduce the medium volumes and therefore the total cost of the experiments. Importantly, use a different incubator for stationary and shaking culture on an orbital shaker to avoid any vibrations detrimental to adherent hIPSC growth. We successfully applied this protocol on five distinct control hIPSC lines35 that give rise to homogeneous results ensuring the robustness of this procedure.
Characterization of such organoids can be achieved using several methods. To preserve the 3D spatial information within organoids, we set up a protocol allowing whole-mount immunostaining and clearing of entire organoids with subsequent light sheet acquisition. Different clearing methods have emerged with efficiency depending upon sample origin and thickness36,37. Here, we set up a simple, fast, and cost-effective clearing method that relies on TDE (2,2'-thiodiethanol), a glycol derivative previously used to clear mouse brain and intestinal organoids38,39. Acquisition of immunostained and cleared organoids was performed on a light sheet microscope using a 20x objective immersed in 80% TDE. In comparison to other 3D imaging acquisition methods, light sheet microscopy is of interest for several reasons: fast acquisition, good penetration, and reduced photobleaching. Optimization of the permeabilization step enables to reach efficient and homogeneous antibody penetration allowing to visualize basal bodies and axonemes of PC extending from all progenitor and neuronal cell types of the whole organoid. Furthermore, acquisition of free-floating 150 µm thick sections using an inverted resonant scanning confocal microscope with 40x (NA 1.3) or 63x (NA 1.4) oil objectives enables to gain further into resolution, while preserving a significant degree of 3D spatial information, and allowing qualitative and quantitative analysis of PC biogenesis and function.
Combining such 2D and 3D cell-based models and 3D imaging analysis (Figure 5) on hIPSCs generated either by reprogramming ciliopathy patient cells or by using CRISPR/CAS9 technology to specifically edit centrosomal or ciliary genes should allow significant progress in the understanding of the contribution of the PC during normal and pathological development of the cerebral cortex. Importantly, genome editing technology also allows to specifically rescue patient mutations to obtain isogenic control hIPSCs to overcome genetic heterogeneity that challenges the detection of disease mechanisms. In addition, single-cell genomic approaches are now widely used throughout the field and represent relevant and complementary approaches to immunostaining analysis. Thus, despite some limitations and difficulties inherent in all emerging technologies, and which are extensively being addressed, such 2D and 3D hIPSC-based models and the characterization methods we presented here offer powerful and relevant tools to dissect PC involvement into the pathological mechanisms underlying human developmental neocortical anomalies.
Divulgazioni
The authors have nothing to disclose.
Acknowledgements
This work was supported by grants from the Agence Nationale de la Recherche (ANR) to S.T. (ANR-17-CE16-0003-01) and N.B.B. (ANR-16-CE16-0011 and ANR-19-CE16-0002-01). LB is supported by the ANR under Investissements d'avenir program (ANR-10-IAHU-01) and the Fondation Bettencourt Schueller (MD-PhD program). The Imagine Institute is supported by state funding from the ANR under the Investissements d'avenir program (ANR-10-IAHU-01, CrossLab projects) and as part of the second Investissements d'Avenir program (ANR-17-RHUS-0002).
Materials
| 2-Mercaptoéthanol (50 mM) | ThermoFisher Scientific | 31350010 | |
| 6-well Clear Flat Bottom Ultra-Low Attachment Multiple Well Plates | Corning | 3471 | |
| 96-well Clear Round Bottom Ultra-Low Attachment Microplate | Corning | 7007 | |
| B-27 Supplement (50X), minus vitamin A | ThermoFisher Scientific | 12587010 | |
| B-27 Supplement (50X), serum free | ThermoFisher Scientific | 17504044 | |
| CellAdhere Dilution Buffer | StemCell Technologies | 7183 | |
| DMEM/F-12, Glutamax | ThermoFisher Scientific | 31331028 | |
| DMSO | ATCC | 4-X | |
| Dorsomorphin | StemCell Technologies | 72102 | |
| Easy Grip 35 10mm | Falcon | 353001 | |
| EDTA | ThermoFisher Scientific | 15575020 | |
| EGF , 25µg | Thermofischer | PHG0315 | |
| FGF2 , 25µg | Thermofischer | PHG0264 | |
| Gentle Cell Dissociation Reagent | StemCell Technologies | 7174 | |
| Insulin | ThermoFisher Scientific | 12585014 | |
| KnockOut Serum | ThermoFisher Scientific | 10828028 | |
| Laminin (1mg) | Thermofischer | 23017015 | |
| LDN193189 | StemCell Technologies | 72147 | |
| Matrigel Growth Factor Reduced | Corning | 354230 | |
| MEM Non-Essential Amino Acids Solution (100X) | ThermoFisher Scientific | 11140050 | |
| Mowiol 4-88 | Sigma Aldrich | 81381-250G | |
| mTeSR1 | StemCell Technologies | 85850 | |
| Neural Basal Medium | Thermofischer | 21103049 | |
| Orbital shaker | Dutscher | 995002 | |
| PBS | ThermoFisher Scientific | 14190094 | |
| Penicillin-Streptomycin (10,000 U/mL) | ThermoFisher Scientific | 15140122 | |
| PFA 32% | Electron Microscopy Sciences | 15714 | |
| Poly-L-Ornithine (PO) | Sigma | P4957 | |
| Recombinant human BDNF 10 µg | Stem Cell Technologies | 78005 | |
| Recombinant Human FGF-basic | Peprotech | 100-18B | |
| rSHH | R&D Systems | 8908-SH | |
| SAG | Santa Cruz | Sc-202814 | |
| SB431542 | StemCell Technologies | 72232 | |
| Stembeads FGF2 | StemCulture | SB500 | |
| Sucrose | Sigma Aldrich | S7903-250G | |
| Superfrost Plus Adhesion Slides | Thermo Scientific | J1800AMNZ | |
| Supplément N2- (100X) | ThermoFisher Scientific | 17502048 | |
| TDE 2,2’-Thiodiethanol | Sigma Aldrich | 166782-500G | |
| Vitronectin | StemCell Technologies | 7180 | |
| Y-27632 | StemCell Technologies | 72304 | |
| Primary Antibodies | |||
| ARL13B | Abcam | Ab136648 | 1/200e |
| ARL13B | Proteintech | 17711-1-AP | 1/500e |
| CTIP2 | Abcam | Ab18465 | 1/500e |
| GLI2 | R&D Systems | AF3526 | 1/100 |
| GPR161 | Proteintech | 13398-1-AP | 1/100 |
| N-Cadherin | BD Transduction Lab | 610921 | 1/500e |
| P-Vimentin | MBL | D076-3 | 1/500e |
| PAX6 | Biolegend | PRB-278P | 1/200e |
| PCNT | Abcam | Ab4448 | 1/1000e |
| S0X2 | R&D Systems | MAB2018 | 1/200e |
| SATB2 | Abcam | Ab51502 | 1/200e |
| TBR2 | Abcam | Ab216870 | 1/400e |
| TPX2 | NovusBio | NB500-179 | 1/500e |
| γTUBULIN | Sigma Aldrich | T6557 | 1/500e |
| Secondary Antibodies | |||
| Donkey anti-rabbit AF488 | ThermoFisher Scientific | A21206 | 1/500e |
| Goat anti-mouse AF555 | ThermoFisher Scientific | A21422 | 1/500e |
| Goat anti-mouse AF647 | ThermoFisher Scientific | A21236 | 1/500e |
| Goat anti-rat AF555 | ThermoFisher Scientific | A21434 | 1/500e |
Riferimenti
- Huangfu, D., et al. Hedgehog signalling in the mouse requires intraflagellar transport proteins. Nature. 426 (6962), 83-87 (2003).
- Goetz, S. C., Anderson, K. V. The primary cilium: a signalling centre during vertebrate development. Nature Reviews. Genetics. 11 (5), 331-344 (2010).
- Hansen, D. V., Lui, J. H., Parker, P. R. L., Kriegstein, A. R. Neurogenic radial glia in the outer subventricular zone of human neocortex. Nature. 464 (7288), 554-561 (2010).
- Lui, J. H., Hansen, D. V., Kriegstein, A. R. Development and evolution of the human neocortex. Cell. 146 (1), 18-36 (2011).
- Nonaka-Kinoshita, M., et al. Regulation of cerebral cortex size and folding by expansion of basal progenitors. The EMBO Journal. 32 (13), 1817-1828 (2013).
- Taverna, E., Götz, M., Huttner, W. B. The cell biology of neurogenesis: toward an understanding of the development and evolution of the neocortex. Annual Review of Cell and Developmental Biology. 30, 465-502 (2014).
- Fernández, V., Llinares-Benadero, C., Borrell, V. Cerebral cortex expansion and folding: what have we learned. The EMBO Journal. 35 (10), 1021-1044 (2016).
- Spear, P. C., Erickson, C. A. Apical movement during interkinetic nuclear migration is a two-step process. Biologia dello sviluppo. 370 (1), 33-41 (2012).
- Wilsch-Bräuninger, M., Florio, M., Huttner, W. B. Neocortex expansion in development and evolution – from cell biology to single genes. Current Opinion in Neurobiology. 39, 122-132 (2016).
- Anderson, C. T., Stearns, T. Centriole age underlies asynchronous primary cilium growth in mammalian cells. Current Biology: CB. 19 (17), 1498-1502 (2009).
- Paridaen, J. T. M. L., Wilsch-Bräuninger, M., Huttner, W. B. Asymmetric inheritance of centrosome-associated primary cilium membrane directs ciliogenesis after cell division. Cell. 155 (2), 333-344 (2013).
- Gabriel, E., et al. CPAP promotes timely cilium disassembly to maintain neural progenitor pool. The EMBO Journal. 35 (8), 803-819 (2016).
- Higginbotham, H., et al. Arl13b-regulated cilia activities are essential for polarized radial glial scaffold formation. Nature Neuroscience. 16 (8), 1000-1007 (2013).
- Baudoin, J. -. P., et al. Tangentially migrating neurons assemble a primary cilium that promotes their reorientation to the cortical plate. Neuron. 76 (6), 1108-1122 (2012).
- Higginbotham, H., et al. Arl13b in primary cilia regulates the migration and placement of interneurons in the developing cerebral cortex. Developmental Cell. 23 (5), 925-938 (2012).
- Kumamoto, N., et al. A role for primary cilia in glutamatergic synaptic integration of adult-born neurons. Nature Neuroscience. 15 (3), 399-405 (2012).
- Guadiana, S. M., et al. Arborization of dendrites by developing neocortical neurons is dependent on primary cilia and type 3 adenylyl cyclase. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 33 (6), 2626-2638 (2013).
- Thomas, S., Boutaud, L., Reilly, M. L., Benmerah, A. Cilia in hereditary cerebral anomalies. Biology of the Cell. 111 (9), 217-231 (2019).
- Hasenpusch-Theil, K., Theil, T. The multifaceted roles of primary cilia in the development of the cerebral cortex. Frontiers in Cell and Developmental Biology. 9, 630161 (2021).
- Shi, Y., Kirwan, P., Livesey, F. J. Directed differentiation of human pluripotent stem cells to cerebral cortex neurons and neural networks. Nature Protocols. 7 (10), 1836-1846 (2012).
- Boissart, C., et al. Differentiation from human pluripotent stem cells of cortical neurons of the superficial layers amenable to psychiatric disease modeling and high-throughput drug screening. Translational Psychiatry. 3, 294 (2013).
- Chambers, S. M., et al. Highly efficient neural conversion of human ES and iPS cells by dual inhibition of SMAD signaling. Nature Biotechnology. 27 (3), 275-280 (2009).
- Lancaster, M. A., Knoblich, J. A. Generation of cerebral organoids from human pluripotent stem cells. Nature Protocols. 9 (10), 2329-2340 (2014).
- Qian, X., et al. Generation of human brain region-specific organoids using a miniaturized spinning bioreactor. Nature Protocols. 13 (3), 565-580 (2018).
- Krefft, O., Jabali, A., Iefremova, V., Koch, P., Ladewig, J. Generation of standardized and reproducible forebrain-type cerebral organoids from human induced pluripotent stem cells. Journal of Visualized Experiments: JoVE. (131), (2018).
- Kadoshima, T., et al. Self-organization of axial polarity, inside-out layer pattern, and species-specific progenitor dynamics in human ES cell-derived neocortex. Proceedings of the National Academy of Sciences of the United States of America. 110 (50), 20284-20289 (2013).
- Topol, A., Tran, N. N., Brennand, K. J. A guide to generating and using hiPSC derived NPCs for the study of neurological diseases. Journal of Visualized Experiments: JoVE. (96), e52495 (2015).
- Berg, S., et al. ilastik: interactive machine learning for (bio)image analysis. Nature Methods. 16 (12), 1226-1232 (2019).
- Hansen, J. N., et al. Multifocal imaging for precise, label-free tracking of fast biological processes in 3D. bioRxiv. , (2020).
- Pașca, S. P. The rise of three-dimensional human brain cultures. Nature. 553 (7689), 437-445 (2018).
- Andreu-Cervera, A., Catala, M., Schneider-Maunoury, S. Cilia, ciliopathies and hedgehog-related forebrain developmental disorders. Neurobiology of Disease. 150, 105236 (2021).
- Christensen, S. T., Morthorst, S. K., Mogensen, J. B., Pedersen, L. B. Primary cilia and coordination of Receptor Tyrosine Kinase (RTK) and Transforming Growth Factor β (TGF-β) signaling. Cold Spring Harbor Perspectives in Biology. 9 (6), (2017).
- Wheway, G., Nazlamova, L., Hancock, J. T. Signaling through the primary cilium. Frontiers in Cell and Developmental Biology. 6, 8 (2018).
- Sivitilli, A. A., et al. Robust production of uniform human cerebral organoids from pluripotent stem cells. Life Science Alliance. 3 (5), (2020).
- Quelennec, E., et al. Generation of two induced pluripotent stem cell lines IMAGINi004-A and IMAGINi005-A from healthy donors. Stem Cell Research. 48, 101959 (2020).
- Belle, M., et al. Tridimensional visualization and analysis of early human development. Cell. 169 (1), 161-173 (2017).
- Vigouroux, R. J., Belle, M., Chédotal, A. Neuroscience in the third dimension: shedding new light on the brain with tissue clearing. Molecular Brain. 10 (1), 33 (2017).
- Lallemant, L., Lebreton, C., Garfa-Traoré, M. Comparison of different clearing and acquisition methods for 3D imaging of murine intestinal organoids. Journal of Biological Methods. 7 (4), 141 (2020).
- Aoyagi, Y., Kawakami, R., Osanai, H., Hibi, T., Nemoto, T. A rapid optical clearing protocol using 2,2′-thiodiethanol for microscopic observation of fixed mouse brain. PloS One. 10 (1), 0116280 (2015).

