Individualized rTMS Treatment for Depression using an fMRI-Based Targeting Method
Summary
The present protocol describes the application of repetitive transcranial magnetic stimulation (rTMS), where a subregion of the dorsolateral prefrontal cortex (DLPFC) with the strongest functional anticorrelation with the subgenual anterior cingulate cortex (sgACC) was located as the stimulation target under the assistance of a fMRI-based neuronavigation system.
Abstract
To achieve greater clinical efficacy, a revolution in treatment for major depressive disorder (MDD) is highly anticipated. Repetitive transcranial magnetic stimulation (rTMS) is a non-invasive and safe neuromodulation technique that immediately changes brain activity. Despite its wide application in the treatment for MDD, the treatment response remains different among individuals, which may be attributable to the inaccurate positioning of the stimulation target. Our study aims to examine whether the functional magnetic resonance imaging (fMRI)-assisted positioning improves the efficacy of rTMS in treating depression. We intend to identify and stimulate the subregion of dorsolateral prefrontal cortex (DLPFC) in MDD with strongest anti-correlation with the subgenual anterior cingulate cortex (sgACC), and to conduct a comparative investigation of this novel method and the traditional 5-cm rule. To achieve more precise stimulation, both methods were applied under the guidance of neuronavigation system. We expected that the TMS treatment with individualized positioning based on resting state functional connectivity may show better clinical efficacy than the 5-cm method.
Introduction
Major depressive disorder (MDD) is characterized by significant and persistent depression, and in more severe cases, patients can encounter hallucinations and/or delusions1,2. Compared with the general population, the risk of suicide among MDD patients is approximately 20 times higher3. While medication is currently the most used treatment for MDD, 30% – 50% of the patients lack adequate response to antidepressants4. For the responders, the symptom improvement tends to appear after a relatively long latent period and is accompanied by side effects. Psychotherapy, although effective for some patients, is costly and time-consuming. A safer and more effective treatment for MDD is therefore urgently required.
Repetitive transcranial magnetic stimulation (rTMS)is a non-invasive and safe technique and has been approved for the treatment of various mental disorders5,6,7. Although its therapeutic mechanism remains unclear, rTMS was speculated to work by regulating the activity of the stimulated brain regions and the neural plasticity8,9,10, thus normalizing specific functional networks10,11,12. rTMS also causes network effect, which evokes changes in remote brain areas through connection pathways, leading to an amplified therapeutic effect13. Although rTMS changes brain activity immediately and robustly, its response rate in the treatment of MDD is only about 18%14. The main reason may be the inaccurate location of stimulation targets15.
The subgenual anterior cingulate cortex (sgACC) is mainly responsible for emotional processing and plays a role in regulating the response to stressful events, emotional response to internal and external stimuli, and emotional expression16,17,18. This subregion of ACC shares substantial structural and functional connectivity with the cerebral cortex and the limbic system19,20. Interestingly, studies have shown that the post-stimulation activity of this area is closely related to the clinical efficacy of TMS. For instance, the blood flow of sgACC decreased after a course of TMS targeted on the right dorsolateral prefrontal cortex (DLPFC), which was associated with the alleviation of depressive symptoms21. Vink et al.8 found that stimulation targeted on DLPFC was propagated to sgACC, and suggested that sgACC activity can be a biomarker of the treatment response of TMS. According to previous researches, Fox and colleagues22 proposed that targeting on a subregion of DLPFC that shows strongest functional anti-connectivity with sgACC (MNI coordinate: 6, 16, -10) enhances the antidepressant effect. Here, we demonstrate a study protocol aimed to examine this hypothesis.
Protocol
Inform all participants about the study and ask them to sign the informed consent form prior to the start of the study. The present protocol was approved by the Research Ethics Committee of the Affiliated Brain Hospital of Guangzhou Medical University.
NOTE: In this double-blind study, patients with depression were randomly divided into two groups. In the experimental group, stimulation targets are located by the DLPFC-sgACC-based individualized location method (Please see 3.3 for detailed description). The targets of the control group are obtained by the average 5-cm method (i.e. (-41, 16, 54))22.
1. Participants' selection
- Recruit patients with a diagnosis of MDD as confirmed by an expert psychiatrist.
NOTE: Confirm the diagnosis with the standardized MINI-International Neuropsychiatric Interview (M.I.N.I.)23 . The total score of Montgomery-Asberg Depression Rating Scale (MADRS)24 should be no less than 22. - Exclude patients who meet the exclusion criteria: (1) serious physical diseases such as malignant tumor, acute heart failure, multiple organ failure, or severe neurological conditions including but not limited to epilepsy, stroke, encephalitis, brain trauma; (2) comorbidity of other mental illness, or a history of substance use disorder; (3) having metallic implants, especially in brain or heart; (4) women during pregnancy or lactation; (5) had suicidal behaviors or attempted suicide in the past six months; and (6) a diagnosis of bipolar depression or psychotic depression.
NOTE: Recruit at least 36 subjects for each group to ensure statistical power. A balanced demographic profile between the two groups is recommended.
2. Preparation of Magnetic Resonance Imaging (MRI) and TMS
- Obtain fMRI images by a 3T MRI scanner before performing TMS.
- Reconfirm that the patient has no contraindications before an MRI scanning. Instruct the patient to try to lie still and think of nothing during the scan.
- Conduct a resting-state fMRI (rs-fMRI) scan using the FFE-EPI sequence with the following parameters: TR/TE = 2000/30 ms, FA = 90°, field-of-view = 220 x 220 x 256 mm3, matrix = 64 x 64, voxel size = 3.44 x 3.44 x 4 mm3, gap = 0.6 mm, number of signal averages = 1, volumes = 240, number of slices = 33.
- Conduct a structural MRI scan using the sagittal T1 weighted 3D turbo field echo (T1W 3D TFE) sequence with the following parameters: field of view = 256 x 256 mm2, TR/TE = 8.2/3.8 ms, view matrix= 256 x 256, slice thickness= 1 mm.
- Set TMS parameters.
NOTE: The protocol of TMS in our study is the intermittent theta-burst stimulation (iTBS). A daily treatment session includes 60 cycles of 10 bursts of 3 pulses at 50 Hz delivered at 100% RMT in 2-s trains, with an interval of 8 s. The whole treatment consists of 10 sessions carried out on weekdays of two consecutive weeks.
3. Treatment (Figure 1)
- Conduct MRI scans and clinical assessments of symptoms and cognitive performance one day before the treatment.
- Assign the patient randomly to one of the two groups, after scanning.
- For the experimental group, identify the subregion of DLPFC that shows strongest functional anti-connectivity with sgACC. For the control group, simply locate the target in the standard-space using the average 5-cm method, then convert it to the individual space coordinates.
- rs-fMRI data preprocessing
- Preprocess the rs-fMRI data using an MRI analysis software: (a) remove the first 10 volumes; (b) conduct the slice timing correction; (c) correct the head motion; (d) co-register EPI images to T1 images; (e) perform segmentation; (f) perform normalization using T1 images; (g) smooth the normalized images with a 6-mm Gaussian kernel of full-width half maximum (FWHM); (h) band-pass filter (0.01 – 0.08 Hz); and (i) perform nuisance regression (head motion effects, linear trends, white matter, cerebrospinal fluid, and global mean time course).
- Functional connectivity (FC) of the sgACC
- Select the sgACC (MNI coordinate: 6, 16, −10; Fox et al.) as the region of interest (ROI)25 with a 10-mm radius.
- Remove the white matter and cerebrospinal fluid in the ROI based on the Harvard-Oxford cortical atlas (http://www.cma.mgh.harvard.edu/), using a gray matter probability threshold of 0.25.
- Extract the average time course of the ROI.
- To generate FC map, compute Pearson's correlation coefficients between the ROI (sgACC) and DLPFC in a voxel-wise manner. Normalize each correlation coefficient using the Fisher's r-to-z Transformation.
NOTE: The DLPFC mask is a combination of 20mm radius spheres centered along the left hemisphere at BA9 (x=-36, y=39, z=43), BA46 (x=-44, y=40, z=29), the 5-cm approach site (x=-41, y=16, z=54), and the F3 Beam group-average stimulation site (x=-39, y=26, z=49)26. - According to the FC map, identify the peak coordinate in DLPFC that has the largest Pearson's anti-correlation coefficient with sgACC. This is the subregion of DLPFC with the strongest negative FC with sgACC, which will be later targeted in the TMS treatment for the experimental group.
- rs-fMRI data preprocessing
- Determine the resting motor threshold (RMT) for each subject and record the hotspot.
- Instruct the patient to sit back and relax, then put two recording electrodes on the thenar of the right hand and a reference electrode on the bony part of the wrist.
- Stimulate the motor hotspot with 10 consecutive stimulations with different intensities; in the meanwhile, record the times of thenar muscle contraction.
- Identify the minimum TMS intensity at which a motor evoked potential (MEP) ≥ 50 µV is recorded at least 5 times. Define it as the patient's RMT.
- Assess the severity of depression using clinical scales as described in Clinical Data Collection.
- Perform TMS treatment twice a day for 10 days.
NOTE: For a subject who did not receive treatments as planned, perform additional stimulations after the end of treatment course as needed. However, any subject who misses the treatment for more than four consecutive days should be excluded.- Create a new patient entry.
- Select the option Create New Patient. Input the patient's ID number or name in the textbox.
- Overlay the structural MRI images onto the navigation system.
- Select Import patient MRI, and then import the structural image of the patient and select the image type.
- Create individual head model and define the stimulation target.
- Press the button Specify MRI Fiducials.
- Place the crosshair on these spots in the MRI image: (1) Fiducial Markers: nasion, both left and right tragi; (2) AC-PC Markers for Talairach: anterior commissure, posterior commissure, inter-hemispheric point; (3) Talairach Markers: anterior point, posterior point, superior point, inferior point, left point, and right point.
NOTE: The "Talairach Markers" mark the borders of the brain. - Press Create Head Model. Select Manual Brain Segmentation and adjust the threshold of the scalp, the lower brain, and the upper brain.
- Click Define Target to proceed.
- Select Target Marker page. Click … to input the coordinate of the treatment target as identified in step 3.3, and then press Go to. Press Add Marker to name the point.
NOTE: The coordinates of the control group will be (-41, 16, 54).
- Coil calibration
- Click Proceed to Neuronavigation. In the textbox, select the right type of tools to be used in the treatment. Ensure all the tools of reference are in the view of the infrared camera.
- Press Validate Coil. Put the tip of pointer on the marked coil point. Press Validate (or the green button on the remote control) when the indicator of each tool turns green.
- Select patient and target.
- Select patient's name or ID on the Select Patient page. Click Select Targets on the next page.
- Choose Read target markers to browse the file of targets. Import the file and select the target as in step 3.6.3.5.
- Define the coordinate system.
- Click Define Coordinate System. Put a headband with a reference tool on the patient. Make sure the coil tracker and the reference tool are in the vision of the navigation system.
- Place pointer's tip on the nasion and both tragi in turn. Press the green button on the remote control each time when the indicator of a marker turns green.
- Head shape generation
- Continuously move the pointer's tip on the top of the head. Press the button on the remote control (or Fit) to continue.
NOTE: One can press Pause to stop the process, and resume by pressing the Start button again, once the pointer has been placed correctly.
- Continuously move the pointer's tip on the top of the head. Press the button on the remote control (or Fit) to continue.
- Neuronavigation and stimulation
- Press Neuronavigation. On the Active Coil page, set the stimulation intensity to 100% RMT. Choose Stimulate at targets to see the target on the head model online.
- When the coil matches the target crosshair, that is, when the indicator text turns green, stimulate.
- Prepare and start the treatment from step 3.6.4 directly if the patient's entry had been created before.
- Create a new patient entry.
- Conduct follow-up assessments on Day 1, Day 28, and Day 56 after the whole treatment course.
4. Clinic data collection (Figure 1b)
- Perform clinical assessments using MADRS24, Hamilton Depression Rating Scale (HAMD)27, Beck Depression Inventory-II (BDI-II)28, Hamilton Anxiety Scale (HAMA)29, Clinical Global Impression (CGI)30 and MATRICS Consensus Cognitive Battery (MCCB)31,32.
NOTE: MINI and MADRS are used for the screening. All the above scales are applied for pre- and post-treatment clinical assessment.
Representative Results
ROI-wise FC analysis should show that sgACC is significantly anti-correlated with DLPFC, in which the strongest negative correlation is the stimulus target to be chosen. Significant anti-correlation between the sgACC-DLPFC functional connectivity and the treatment response should be found in the correlation analysis33.
The current protocol is based on an innovative TMS targeting method that no previous studies have applied. Here we present results from an fMRI-guided TMS trial that applied the traditional 5-cm method. This study34 proposed a new treatment protocol, the Stanford Accelerated Intelligent Neuromodulation Therapy (SAINT), a high-dose iTBS regimen with fMRI-guided targeting. The response rate (a MADRS score was 50% lower from the baseline) among 23 MDD patients was 90.48%. 19 of 22 participants (86.4%) met the remission criteria in the intent-to-treat analysis34. Two participants dropped out due to therapeutic intolerances and high motor threshold. Table 1 presents the scores of clinical assessments post-TMS treatment. Therefore, we conjecture that the TMS treatment base on the FC can produce remarkable effectiveness.
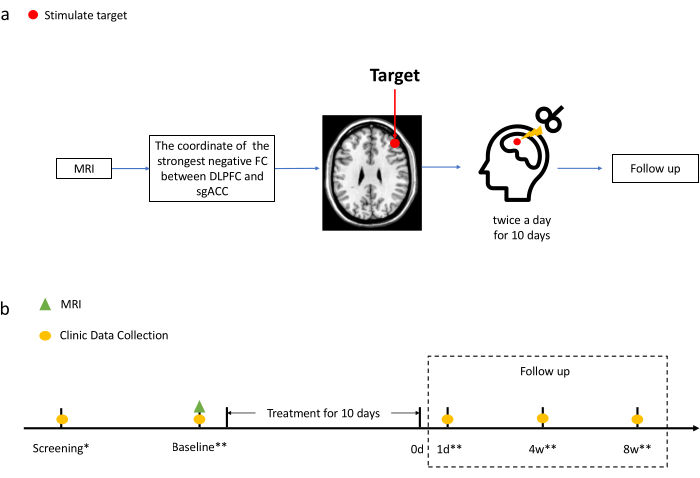
Figure 1. Treatment diagram. (a) Process of acquiring stimulation targets and the treatment. See 3.3 for the detailed description on obtaining the target coordinates for the experiment group. The target coordinate for the control group is defined as (-41, 16, 54). (b) Time points of MRI scan and clinical evaluation. Clinical data were collected on the screening, the baseline (i.e., before treatment), as well as Day 1, Day 28, and Day 56 after the treatment. The MRI scan was only performed on the baseline.
*Evaluate patients with M.I.N.I.23 and MADRS24.
**Evaluate patients with all the scales mentioned in Step 4. Please click here to view a larger version of this figure.
| Post-SAINT | One Month Post-SAINT | ||||||||||||||||||
| Measure | Mean | SD | N | Response(%) | N | Remission(%) | N | Mean | SD | N | Response(%) | N | Remission(%) | N | |||||
| MADRS | 5 | 6.37 | 21 | 90.48 | 21 | 90.48 | 21 | 10.95 | 11.76 | 20 | 70 | 20 | 60 | 20 | |||||
| HAM-D, 17-item | 4.29 | 4.43 | 21 | 90.48 | 21 | 80.95 | 21 | 8.05 | 8.31 | 20 | 75 | 20 | 65 | 20 | |||||
| HAM-D, 6-item | 2.24 | 3.1 | 21 | 85.71 | 21 | 85.71 | 21 | 4.4 | 4.72 | 20 | 75 | 20 | 70 | 20 | |||||
| BDI-II (N=18) | 4.47 | 5.76 | 15 | 100 | 12 | 93.33 | 15 | 12.25 | 13.06 | 16 | 57.14 | 14 | 62.5 | 16 | |||||
| Suicidal ideation | |||||||||||||||||||
| C-SSRS[b] | 0 | 0 | 18 | 100 | 14 | 100 | 18 | 0 | 0 | 19 | 100 | 14 | 100 | 19 | |||||
| HAM-D, item 3 | 0.05 | 0.22 | 21 | 100 | 19 | 95.24 | 21 | 0.1 | 0.31 | 20 | 100 | 18 | 90 | 20 | |||||
| MADRS, item 10 | 0.1 | 0.44 | 21 | 95.24 | 21 | 95.24 | 21 | 0.35 | 0.75 | 20 | 90 | 20 | 80 | 20 | |||||
Table 1. Clinical assessment scores immediately after and 1 month after the Stanford Accelerated Intelligent Neuromodulation Therapy (SAINT) for treatment-resistant depression[a] 34
[a] Treatment response was defined as a reduction on total MADRS score by ≥ 50%; remission was defined as a score of < 8 for the 17-item HAM-D, < 5 for the 6-item HAM-D, < 11 for the MADRS, < 13 for the BDI-II, and zero for the C-SSRS.
[b] Suicidal Ideation subscale.
Discussion
The sgACC is responsible for emotional processing and plays an important role in stress regulation16,17,18. A study suggests that targeting on a subregion of DLPFC that shows strongest functional anti-connectivity with sgACC (6, 16, -10) may enhance the antidepressant effect25. Therefore, precisely locating this target is the critical step of this protocol. Before the stimulation, the borders of the brain should be accurately marked out with the assistance of neuronavigation, and the head should be carefully registered to ensure the accuracy of a head model. Also, note that the 5-cm rule generally stimulates very posterior regions of the frontal brain, while our sgACC-DLPFC targeting protocol usually leads to a very anterior region35,36. Thus, the differential clinical efficacy among targeting methods may be associated with the orientation. Our method should be carefully evaluated by comparison with other approaches that define the stimulation target based on other functional connectivities.
Our protocol has some limitations. First of all, sgACC is located near the sphenoidal sinus, which causes severe signal loss due to the non-uniformity of the magnetic fields37. Besides, the accuracy of the neuronavigation largely depends on the quality of MRI images, which may lead to inaccurate stimulation targets. Improvement of the signal-to-noise ratio of MRI or a better replacement for sgACC may help address this problem. Another limitation is the time-consuming procedures that potentially affect patients' compliance for the treatment, since preparation such as establishing a head model takes a long time, not to mention the whole treatment course that lasts for about two weeks.
Despite these limitations, this method has its strength. Despite the fact that the 5-cm rule has been widely applied in clinical settings, it overlooks the individual differences on the anatomical features, which is considered an important reason for the heterogenous efficacy of TMS38. The neuronavigation system models the head individually by referring to structural MRI images, thus improving the accuracy of coil positioning. Research has proven that a neuronavigated TMS therapy is more effective than a traditional treatment using the 5-cm targeting method38, Furthermore, an operator can adjust the coil in real time under the guidance of the system39,40.
Traditional TMS therapy targets at DLPFC as a whole. In this protocol, the subregion of DLPFC with the strongest negative connectivity with sgACC was selected as the target. Baeken et al.41 found that sgACC is related to suicidal ideation and hopelessness. Patients with treatment-resistance depression show a stronger FC between sgACC and the right lateral frontotemporal cortex, which may be related to the refractory state of the patient42. In addition, strong connectivity between sgACC and DLPFC was found in MDD patients43, and the negative FC between sgACC and default mode network (DMN) was correlated with the clinical improvement. Therefore, we speculate that the connectivity of sgACC is closely related to the therapeutic effect of TMS, and that stimulating a specific region of DLPFC may change its FC with sgACC, which can improve the effectiveness of the treatment25,44.
In summary, the present TMS protocol is operated under the MRI-assisted neuronavigation system and targets the subregion of DLPFC that shows the strongest negative functional connectivity with sgACC. Although no previous studies have applied this targeting method, it may help enhance the accuracy of positioning and possibly improve the treatment response.
Divulgazioni
The authors have nothing to disclose.
Acknowledgements
The study was funded by China Postdoctoral Science Foundation funded project (2019M652854) and Natural Science Foundation of Guangdong, China (Grant No. 2020A1515010077).
Materials
| 3T Philips Achieva MRI scanner | Philips | ||
| Harvard/Oxford cortical template | http://www.cma.mgh.harva rd.edu/ | ||
| MATLAB | MathWorks | ||
| SPM12 | http://www.fil.ion.ucl.ac.uk/spm | ||
| The Visor2 system | ANT Neuro | The Visor2 software, the optical tracking system, tracking tools and calibration board are part of the visor2 system. | |
| TMS device | Magstim, Carmarthenshire, UK |
Riferimenti
- Schramm, E., Klein, D. N., Elsaesser, M., Furukawa, T. A., Domschke, K. Review of dysthymia and persistent depressive disorder: History, correlates, and clinical implications. Lancet Psychiatry. 7 (9), 801-812 (2020).
- Knight, M. J., Baune, B. T. Cognitive dysfunction in major depressive disorder. Current Opinion in Psychiatry. 31 (1), 26-31 (2018).
- Otte, C., et al. Major depressive disorder. Nature Reviews Disease Primers. 2 (1), 1-20 (2016).
- Rafeyan, R., Papakostas, G. I., Jackson, W. C., Trivedi, M. H. Inadequate response to treatment in major depressive disorder: Augmentation and adjunctive strategies. Journal of Clinical Psychiatry. 81 (3), (2020).
- Zhang, J. J., Fong, K. N., Ouyang, R. g., Siu, A. M., Kranz, G. S. J. A. Effects of repetitive transcranial magnetic stimulation (rTMS) on craving and substance consumption in patients with substance dependence: A systematic review and meta-analysis. Addiction. 114 (12), 2137-2149 (2019).
- Enokibara, M., Trevizol, A., Shiozawa, P., Cordeiro, Q. Establishing an effective TMS protocol for craving in substance addiction: Is it possible. American Journal on Addictions. 25 (1), 28-30 (2016).
- Diana, M., et al. Rehabilitating the addicted brain with transcranial magnetic stimulation. Nature Reviews Neuroscience. 18 (11), 685 (2017).
- Vink, J. J. T., et al. A novel concurrent TMS-fMRI method to reveal propagation patterns of prefrontal magnetic brain stimulation. Human Brain Mapping. 39 (11), 4580-4592 (2018).
- Baeken, C., De Raedt, R. Neurobiological mechanisms of repetitive transcranial magnetic stimulation on the underlying neurocircuitry in unipolar depression. Dialogues in Clinical Neuroscience. 13 (1), 139-145 (2011).
- Tik, M., et al. Towards understanding rTMS mechanism of action: Stimulation of the DLPFC causes network-specific increase in functional connectivity. Neuroimage. 162, 289-296 (2017).
- Castrén, E. Neuronal network plasticity and recovery from depression. JAMA Psychiatry. 70 (9), 983-989 (2013).
- Cantone, M., et al. Cortical plasticity in depression. ASN Neuro. 9 (3), 1759091417711512 (2017).
- Valero-Cabré, A., Amengual, J. L., Stengel, C., Pascual-Leone, A., Coubard, O. A. Transcranial magnetic stimulation: A comprehensive review of fundamental principles and novel insights. Neuroscience & Biobehavioral Reviews. 83, 381-404 (2017).
- Luber, B. M., et al. Using neuroimaging to individualize TMS treatment for depression: Toward a new paradigm for imaging-guided intervention. Neuroimage. 151, 65-71 (2017).
- Wassermann, E. M., Zimmermann, T. J. P. Transcranial magnetic brain stimulation: Therapeutic promises and scientific gaps. Pharmacology & Therapeutics. 133 (1), 98-107 (2012).
- Kim, H., et al. Hypometabolism and altered metabolic connectivity in patients with internet gaming disorder and alcohol use disorder. Progress in Neuro-Psychopharmacology & Biological Psychiatry. 95, 109680 (2019).
- Kim, J. Y., et al. The correlation between the frontostriatal network and impulsivity in internet gaming disorder. Scientific Reports. 9 (1), 1191 (2019).
- Wang, Y., et al. Impaired decision-making and impulse control in Internet gaming addicts: evidence from the comparison with recreational Internet game users. Addiction Biology. 22 (6), 1610-1621 (2017).
- Mayberg, H. S. Limbic-cortical dysregulation: A proposed model of depression. Journal of Neuropsychiatry and Clinical Neurosciences. 9 (3), 471-481 (1997).
- Rolls, E. T. The cingulate cortex and limbic systems for emotion, action, and memory. Brain Structure and Function. 224 (9), 3001-3018 (2019).
- Philip, N. S., et al. Network mechanisms of clinical response to transcranial magnetic stimulation in posttraumatic stress disorder and major depressive disorder. Biological Psychiatry. 83 (3), 263-272 (2018).
- Fox, M. D., Buckner, R. L., White, M. P., Greicius, M. D., Pascual-Leone, A. Efficacy of transcranial magnetic stimulation targets for depression is related to intrinsic functional connectivity with the subgenual cingulate. Biological Psychiatry. 72 (7), 595-603 (2012).
- Sheehan, D. V., et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): The development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. Journal of Clinical Psychiatry. 59, 22-33 (1998).
- Montgomery, S. A., Asberg, M. A new depression scale designed to be sensitive to change. British Journal of Psychiatry. 134, 382-389 (1979).
- Fox, M. D., Buckner, R. L., White, M. P., Greicius, M. D., Pascual-Leone, A. J. B. p. Efficacy of transcranial magnetic stimulation targets for depression is related to intrinsic functional connectivity with the subgenual cingulate. Biological Psychiatry. 72 (7), 595-603 (2012).
- Cash, R. F. H., et al. Personalized connectivity-guided DLPFC-TMS for depression: Advancing computational feasibility, precision and reproducibility. Human Brain Mapping. , (2021).
- Hamilton, M. A rating scale for depression. Journal of Neurology, Neurosurgery, and Psychiatry. 23 (1), 56-62 (1960).
- Beck, A. T., Steer, R. A., Brown, G. K. . Manual for the Beck depression inventory-II. , 1-82 (1996).
- Hamilton, M. The assessment of anxiety states by rating. British Journal of Medical Psychology. 32 (1), 50-55 (1959).
- Guy, W. ECDEU assessment manual for psychopharmacology, revised. U.S. Dept. of Health, Education, and Welfare, Public Health Service, Alcohol, Drug Abuse, and Mental Health Administration, National Institute of Mental Health, Psychopharmacology Research Branch, Division of Extramural Research Programs. , (1976).
- Kern, R. S., et al. The MATRICS consensus cognitive battery, part 2: Co-norming and standardization. American Journal of Psychiatry. 165 (2), 214-220 (2008).
- Nuechterlein, K. H., et al. The MATRICS consensus cognitive battery, part 1: Test selection, reliability, and validity. American Journal of Psychiatry. 165 (2), 203-213 (2008).
- Jing, Y., et al. Pregenual or subgenual anterior cingulate cortex as potential effective region for brain stimulation of depression. Brain and Behavior. 10 (4), 01591 (2020).
- Cole, E. J., et al. Stanford accelerated intelligent neuromodulation therapy for treatment-resistant depression. American Journal of Psychiatry. 177 (8), 716-726 (2020).
- Cash, R. F. H., et al. Subgenual functional connectivity predicts antidepressant treatment response to transcranial magnetic stimulation: Independent validation and evaluation of personalization. Biological Psychiatry. 86 (2), 5-7 (2019).
- Ge, R., Downar, J., Blumberger, D. M., Daskalakis, Z. J., Vila-Rodriguez, F. Functional connectivity of the anterior cingulate cortex predicts treatment outcome for rTMS in treatment-resistant depression at 3-month follow-up. Brain Stimulation. 13 (1), 206-214 (2020).
- Ojemann, J. G., et al. Anatomic localization and quantitative analysis of gradient refocused echo-planar fMRI susceptibility artifacts. Neuroimage. 6 (3), 156-167 (1997).
- Schonfeldt-Lecuona, C., et al. The value of neuronavigated rTMS for the treatment of depression. Clinical Neurophysiology. 40 (1), 37-43 (2010).
- Krieg, S. M., et al. Protocol for motor and language mapping by navigated TMS in patients and healthy volunteers; workshop report. Acta Neurochir (Wien). 159 (7), 1187-1195 (2017).
- Haddad, A. F., Young, J. S., Berger, M. S., Tarapore, P. E. Preoperative applications of navigated transcranial magnetic stimulation. Frontiers in Neurology. 11, 628903 (2020).
- Baeken, C., Duprat, R., Wu, G. R., De Raedt, R., van Heeringen, K. Subgenual anterior cingulate-medial orbitofrontal functional connectivity in medication-resistant major depression: A neurobiological marker for accelerated intermittent theta burst stimulation treatment. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging. 2 (7), 556-565 (2017).
- Wu, G. R., De Raedt, R., Van Schuerbeek, P., Baeken, C. Opposite subgenual cingulate cortical functional connectivity and metabolic activity patterns in refractory melancholic major depression. Brain Imaging and Behavior. 14 (2), 426-435 (2020).
- Salomons, T. V., et al. Resting-state cortico-thalamic-striatal connectivity predicts response to dorsomedial prefrontal rTMS in major depressive disorder. Neuropsychopharmacology. 39 (2), 488-498 (2014).
- Iseger, T. A., van Bueren, N. E. R., Kenemans, J. L., Gevirtz, R., Arns, M. A frontal-vagal network theory for major depressive disorder: Implications for optimizing neuromodulation techniques. Brain Stimulation. 13 (1), 1-9 (2020).

