Selective Cleaning of Wild Caenorhabditis Nematodes to Enrich for Intestinal Microbiome Bacteria
Summary
Wild Caenorhabditis nematodes are associated with many microbes, often in the gut lumen or infecting the intestine. This protocol details a method to enrich unculturable microbes colonizing the intestine, taking advantage of the resistance of the dauer cuticle.
Abstract
Caenorhabditis elegans (C. elegans) has proven to be an excellent model for studying host-microbe interactions and the microbiome, especially in the context of the intestines. Recently, ecological sampling of wild Caenorhabditis nematodes has discovered a diverse array of associated microbes, including bacteria, viruses, fungi, and microsporidia. Many of these microbes have interesting colonization or infection phenotypes that warrant further study, but they are often unculturable. This protocol presents a method to enrich the desired intestinal microbes in C. elegans and related nematodes and reduce the presence of the many contaminating microbes adhering to the cuticle. This protocol involves forcing animals into the dauer stage of development and using a series of antibiotic and detergent washes to remove external contamination. As dauer animals have physiological changes that protect nematodes from harsh environmental conditions, any intestinal microbes will be protected from these conditions. But, for enrichment to work, the microbe of interest must be maintained when animals develop into dauers. When the animals leave the dauer stage, they are singly propagated into individual lines. F1 populations are then selected for desired microbes or infection phenotypes and against visible contamination. These methods will allow researchers to enrich unculturable microbes in the intestinal lumen, which make up part of the natural microbiome of C. elegans and intracellular intestinal pathogens. These microbes can then be studied for colonization or infection phenotypes and their effects on the host fitness.
Introduction
The genetic model organism C. elegans is an excellent in vivo system to study host-microbe interactions1,2. They have relatively simple physiology compared to other animals, yet, much of their cell biology is fundamentally similar to mammals making them a good model for biological research1,3,4. Additionally, they are microscopic, easy to maintain, and remain transparent throughout their short lifespan. These properties enable rapid studies into the mechanisms governing host-microbe interactions and visualization of in vivo infection and colonization of the genetically pliable hosts5,6. Finally, C. elegans rapidly responds to bacterial, fungal, and viral infections, making them an excellent model to study host-microbe interactions and the gut microbiome7,8,9.
An increase in the sampling of wild C. elegans and other nematodes has allowed for research into the ecology of free-living nematodes and natural genetic variation10,11. Concurrently, sampling has also increased the discovery of naturally occurring biological pathogens and microbes that interact with C. elegans12,13,14,15, leading to the establishment of many host-microbe model systems that study interactions with viruses, bacteria, microsporidia, oomycetes, or fungi16,17,18,19,20. Typically, wild C. elegans is found in rotting stems and fruits, often in more temperate climates, and mostly they are self-reproducing21. When these samples are brought into the lab, wild nematodes are isolated into clonal populations, carrying an array of associated microbiota. When discovering new microbes of interest in Caenorhabditis nematodes, animals are often directly screened for infection or colonization by microscopy using easily visualized phenotypes. For example, viral infection can be visualized as a disintegration of intestinal structures, and microsporidian stages can be seen inside host cells as spores or meronts14,22. When a microbe of interest is discovered for future investigation, it must be separated from the other contaminating microbes found in the wild nematodes so that it can be studied in isolation. In many cases, the microbe of interest cannot be cultured in vitro, making it essential to enrich the microbe in the host nematode.
For example, this protocol describes a wild isolate of C. tropicalis containing a bacterium that colonizes within the intestinal lumen of nematodes, adhering to the intestinal epithelial cells in a directional manner. Phenotypically, the bacterium grows perpendicular along the intestinal lumen's inner sides, giving it a bristle-like appearance, visualized on a standard Normarski microscope in all stages of the animal, including the dauer stage. The nematode growth medium (NGM) plate on which this wild C. tropicalis strain was grown contained visible contamination with other microbes. This protocol was developed to reduce additional contaminating microbial growth on the plates for studying this unknown adhering bacterium. The nematodes were forced into the dauer stage to protect the bacteria in the lumen, and then cleaned using a series of washes. Afterward, the unknown bacterial species was identified by dissection of the intestines and PCR amplification of the 16S ribosomal DNA for sequencing.
Overall, this protocol can potentially enrich any microbe of interest associated with a wild-caught nematode. Afterward, researchers will identify the target microbe, visualize in vivo infection or colonization phenotypes via microscopy, and study effects on host fitness or other aspects of host-microbe interactions. The isolation and investigation of novel microbial species that interact with Caenorhabditis nematodes can uncover the genetic mechanisms of host immunity and novel paradigms of host-microbe interactions relevant to microbial pathogenesis and microbiome studies.
Protocol
1. Inducing dauer formation for wild nematodes on NGM plates
- Grow the wild Caenorhabditis strain on standard NGM plates with OP50-1 E. coli as a food source after obtaining the worms with an unculturable microbe of interest. Incubate the nematodes at 20 °C.
NOTE: The standard temperature to grow Caenorhabditis nematodes is between 15-25 °C, but should be determined empirically to prevent loss of the microbe of interest. - Starve the plate of animals at 20 °C for 4-5 days until all the OP50-1 is consumed and the majority have reached the dauer stage.
NOTE: Dauers are long-lived larvae that develop due to the absence of nutrition and have protective cuticles.
2. Washing the nematodes
- Add 5 mL of M9 minimal salts media (42 mM Na2HPO4, 22 mM KH2PO4, 8.6 mM NaCl, 19 mM NH4Cl) to a 6 cm plate of starved worms.
- Using a sterile glass pipette and bulb, pipette up the M9 and worms from the plate and transfer them to a sterile 15 mL centrifuge tube.
- Using a clinical centrifuge, spin down the worms in the tube at 1000 x g for 30 s at room temperature.
- Using a sterile 15 mL pipette, remove and discard the M9 supernatant above the pellet of worms. Do not disturb the live worms at the bottom of the tube by leaving approximately 50 µL of M9 above the worms.
- Add 10 mL of M9 + 0.05% of Triton X-100 to the same centrifuge tube and adequately tighten the tube's cap.
- Incubate the tube on a nutator for 20 min at room temperature to remove the external microbes. Remove the tube from the nutator and spin down the worms at 1000 x g for 30 s.
- Using a sterile pipette, remove and discard the M9 supernatant above the pellet of worms. Do not disturb the live worms at the bottom of the tube by leaving approximately 50 µL of M9 above the worms.
- Follow and repeat steps 2.5-2.8 three more times.
3. Disinfecting the nematodes
- Prepare an antibiotic and SDS solution in 10 mL of M9 buffer by adding 0.25% SDS (250 µL of 10% SDS), 50 µg/mL of carbenicillin, 25 µg/mL of kanamycin, 12.5 µg/mL of tetracycline, 100 µg/mL of gentamycin, 50 µg/mL of streptomycin, 37.5 µg/mL of chloramphenicol, and 200 µg/mL of cefotaxime (see Table of Materials).
- Incubate the tube containing nematodes in the antibiotic and SDS solution on a nutator at room temperature overnight.
NOTE: All non-dauer worms and embryos will die, but many of the dauer worms will survive this cleaning process.
4. Removing the antibiotic and SDS solution
- Spin down the worms in the tube at 1000 x g for 1 min at room temperature. Using a sterile pipette, remove the supernatant from the centrifuge tube without disturbing the worms at the bottom of the tube.
- Add 10 mL of M9 + 0.05% Triton X-100 and adequately tighten the cap of the tube. Incubate the tube on a nutator for 20 min at room temperature.
- Remove the tube from the nutator and spin down the worms at 1000 x g for 1 min. Follow and repeat steps 4.2-4.3 three times.
- After the last wash, leave the worm pellet undisturbed at the bottom in 100 µL of the solution and discard the rest.
5. Propagate a clean nematode strain
- Using a sterile glass pipette, transfer 100 µL of the supernatant and the pellet to the center of a 10 cm NGM plate seeded with OP50-1. Allow the plate to dry without disturbing the liquid at the center.
- Allow the dauers to crawl out of the center and through the OP50-1 lawn for 5-10 min.
- Carefully pick up one single dauer and plate it onto a 6 cm NGM seeded plate. In total, pick at least 10 dauers and plate them in individual 6 cm NGM plates seeded with OP50-1 (10 plates total).
- Incubate the plates for 4-5 days at 20 °C until dauers have grown and the following generation (F1) has reached the adult stage. Visually check for contamination on all the plates, seen as non-OP50-1 microbial growth.
- For each clean plate, verify the propagation of the microbe of interest via Normarski or fluorescent microscopy, such as fluorescent in situ hybridization (FISH)12, depending on the phenotype of interest.
6. Intestinal dissection and PCR identification of microbial species
- Grow animals at 20 °C for 3-4 days to allow them to starve and reduce the amount of OP50-1 bacteria. Add 5 mL of M9 media to the plate of starved worms.
- Using a sterile glass pipette and bulb, pipette up the M9 + worms from the plate and transfer them to a sterile 15 mL centrifuge tube.
- Using a clinical centrifuge, spin down the worms in the tube at 1000 x g for 30 s at room temperature.
- Using a sterile 15 mL pipette, remove the supernatant from the centrifuge tube without disturbing the live worms at the bottom of the tube.
- Add 10 mL of M9 + 0.05% Triton X-100 and incubate the tube on a nutator for 20 min to remove external microbes. Repeat four times to wash the worms.
- After the last wash, using a sterile pipette tip, remove the supernatant without disturbing the pellet at the bottom in 100 µL of the solution.
- Using a sterile pipette tip, transfer M9 and the worms to an unseeded NGM plate and let the plate dry while the worms crawl around for 20 min to help remove OP50-1 from the cuticle and the intestine.
- Add 250 µL of M9 to the dried plate and use a glass pipette to transfer M9 + worms to a new unseeded NGM plate. Again, let that plate dry and allow the worms to crawl around for 20 min.
- Add 250 µL of M9 to the plate and transfer 100 µL of the M9 + worms to a clean watch glass.
- Using two sterile 26 G syringe needles, decapitate the nematodes by holding the worm down with one needle and using the other needle to cut the worm. After decapitation, the intestine (granular) and the gonad (transparent) from the body will naturally come out of the body.
- Cut off a piece of the exposed intestine by holding down the intestine with one needle and cutting it with the other.
NOTE: If possible, verify that the microbe of interest is still present in everted intestines using Normarski microscopy, FISH, or immunohistochemistry23. - Transfer a single dissected intestine into a 0.5 mL PCR tube containing 10 µL of sterile water. Repeat for a total of at least 5 PCR tubes containing intestines from different animals.
- Freeze the PCR tubes at -80 °C for a minimum of 5 min. Remove the PCR tubes from the freezer and thaw out the samples.
- Pipette the liquid up and down a few times to separate the bacteria from the intestine.
- Conduct PCR using universal primer pairs against the DNA sequence of the small ribosomal subunit of bacteria, yeast, or microsporidia, depending on the suspected type of microbe.
NOTE: For example, a sample protocol using universal bacterial 16S primers is presented in Table 1. - Purify the amplicon using a filter-based DNA cleaning and concentration kit (see Table of Materials) and perform Sanger sequencing.
Representative Results
A wild C. tropicalis strain (JU1848) was isolated from rotten palm tree fruits in the Nouragues Forest of French Guiana (Figure 1A)24. This strain was found to have thin microbes that colonize the lumen of the intestine in a directional manner (Figure 1B). This microbe was easily transferred to C. elegans strain N2 via co-culture with strain JU1848, where it colonized the lumen of the intestine similarly.
Propagation of JU1848 on standard NGM plates seeded with E. coli OP50-1 over multiple generations continually resulted in visible contamination, seen as various dark, mucoid colonies on and off the OP50-1 lawn (Figure 2A). A plate of wild JU1848 was starved to force animals into dauer and cleaned as described. Single dauer animals that survived cleaning were plated onto individual 6 cm NGM plates seeded with OP50-1 and allowed to grow for 4 days at 20 °C. Multiple plates of F1 progeny were observed without visible microbial contamination (Figure 2B). The F1 progeny were verified to still contain adhering bacteria in the lumen of the intestine (see below).
Clean JU1848 animals were washed and decapitated to isolate intestinal pieces as described in the protocol (steps 6.1-6.12). Adhering bacteria in the lumen of the dissected intestine was verified via Nomarski microscopy (Figure 3). The microbe in the lumen of JU1848 was suspected to be a bacterium, so the dissected intestines were used as a template for PCR using universal bacterial 16S primers, 27F, and 1492R. From a total of six individual dissected intestines, the PCR products were sequenced via Sanger, and clean chromatographs showed that all the six sequences were identical. Based on these sequences, this bacterium was identified as a new species in the class Alphaproteobacteria but could not be classified into a known order or genus (Supplementary File 1).
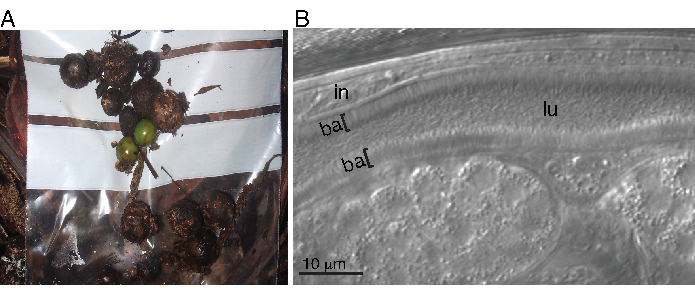
Figure 1: Adhering bacteria colonizing the lumen of a wild C. tropicalis. (A) Field sample image of rotten Euterpe sp. (Family: Arecaceae) palm tree fruits in the Nouragues forest of French Guiana. (B) Nomarski image of strain JU1848 seen with thousands of long, thin bacteria that form a brush-like appearance in the lumen (lu) of the host intestine (in). The bacterial (ba) layers coating the intestine are indicated with brackets ([). Please click here to view a larger version of this figure.
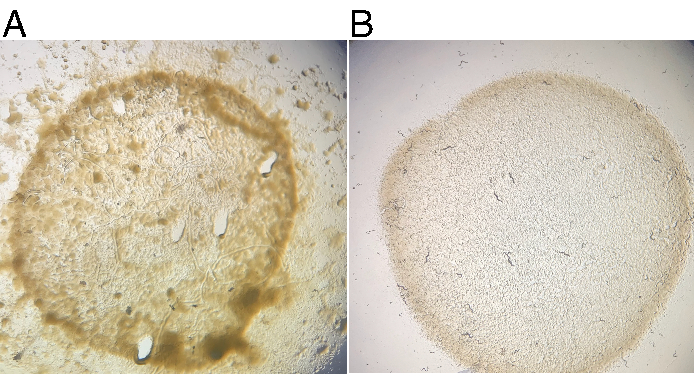
Figure 2: Contaminating microbial growth is lost after nematode cleaning. (A) Wild strain JU1848 propagates noticeable microbial growth on standard 6 cm NGM plates seeded with E. coli OP50-1 bacteria. (B) After cleaning, a plate of F1 progeny from a single dauer shows no visible microbial contamination after 4 days of incubation at 20 °C. Please click here to view a larger version of this figure.
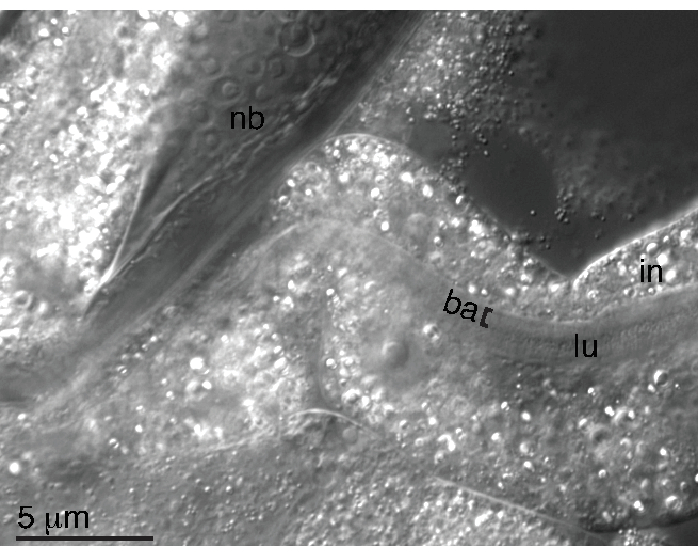
Figure 3: Adhering bacteria are seen in the lumen of the dissected intestine. Nomarski image of a clean JU1848 animal that was decapitated so that the intestines spill out. The colonizing bacteria (ba) are indicated with a bracket ([) and are seen in the lumen (lu) of a piece of the intestine (in) that is outside of the nematode body (nb). Please click here to view a larger version of this figure.
| Reagent | Concentration | Amount |
| 27F primer (5’-AGAGTTTGATCMTGGCTCAG-3’) | 20 mM | 2.5 µL |
| 1492R primer (5’-GGTTACCTTGTTACGACTT-3’) | 20 mM | 2.5 µL |
| Dissected intestine in water | N/A | 3 µL |
| 10x PCR buffer | 10x | 5 µL |
| dNTP | 10 mM | 1 µL |
| Taq Polymerase | 5 U/µL | 0.5 µL |
| Water | N/A | 35.5 µL |
Table 1: Sample PCR protocol using universal bacterial primers and dissected intestine.
Supplementary File 1: MUSCLE alignment of bacterial 16S rDNA sequences derived from PCR of six dissected JU1848 intestines. Please click here to download this File.
Discussion
This protocol describes the isolation and identification of microbes from wild-isolated Caenorhabditis nematodes using a series of cleaning procedures. Numerous microbes are associated with wild-isolated nematodes, and some of them have exciting phenotypes that can be used for future studies in host-microbe interactions and innate immunity. Many culturable microbiome and pathogenic bacteria have been isolated from wild Caenorhabditis nematodes using standard techniques for in vitro bacterial growth25,26. However, not all microbes can be cultured in vitro, and it becomes necessary to enrich them in wild nematodes. Some microbes have a resistant spore stage, such as microsporidia, and high concentrations of SDS can be used to kill most bacteria and fungi, allowing for specific enrichment of spores12. This protocol presents a method to enrich unculturable intestinal microbes that are not resistant to SDS and antibiotic treatment.
The technique presented here takes advantage of the environmental resistance seen in dauer animals due to physiological changes such as strengthening of the cuticle, suppressing pharyngeal pumping, and covering the mouth with a buccal plug27. A critical step in this protocol is the overnight incubation with various antibiotics and 0.25% SDS. This step is used to kill all external microbes while leaving internal microbes intact. While C. elegans dauers have been demonstrated to survive SDS concentrations as high at 10% for 30 min27, this protocol uses a moderate but prolonged incubation to not only kill microbes but further expose bacteria to antibiotics. Furthermore, a moderate concentration of SDS can help ensure that dauers from other Caenorhabditis species survive, as exposure of C. tropicalis to 1% SDS overnight resulted in the death of all dauer animals. If all of the dauers die, then the concentration of SDS and/or the length of exposure to SDS should be reduced. Conversely, if the F1 generation plates still have visible contamination after cleaning, the SDS concentration and incubation time should be increased.
Another critical step is the isolation of single dauer animals after cleaning. This step is crucial as not all animals are clean after SDS and antibiotic treatment. Therefore, the animals are placed in the center of a 10 cm NGM plate with OP50-1 and allowed to crawl radially outward. Often it is best to pick more distal animals, as extended crawling through OP50-1 appears to help remove any potential surviving microbes attached to the cuticle. However, this leads to a limitation of the protocol, as it will be more challenging to enrich for a microbe of interest if it is not present in the population at a high frequency. Here, the adhering Alphaproteobacteria was present in 90%-95% of the population; therefore, most clean plates had the microbiome bacterium. However, if a microbe of interest is present at a much lower frequency in the population, it may be necessary to screen many more F1 plates.
This protocol could likely be used to isolate any number of non-culturable microbes of interest found in wild nematodes. However, the microbe must be in a tissue protected by the dauer cuticle, capable of surviving in dauer animals, and have an observable phenotype in the host. As such, this technique can be used to enrich other microbiome bacteria in the intestinal lumen besides the Alphaproteobacteria species described here, including bacteria that do not adhere. Also, the protocol was used to enrich for a facultative intracellular bacterium, Bordetella atropi, which infects the nematode Oscheius tipulae28. After enrichment, B. atropi was found to form colonies on NGM plates, showing that a microbe of interest may be discovered to be culturable in vitro once faster-growing contaminants are removed. This technique would likely work for microsproidians and viruses, including the Orsay virus, given this capacity to enrich an intracellular bacterium. However, these microbes must be capable of surviving the transition into and out of dauer.
It is important to remember that while this protocol can be performed in a Biosafety Level 1 laboratory, a sterile technique must be maintained throughout to prevent further microbial contamination. The protocol can be changed according to the researcher's needs, including the types/concentrations of antibiotics, the percentage of SDS, and/or the addition of antifungals such as nystatin. Often, the number of contaminating microbes found in a wild-isolated nematode can vary dramatically. Here, the apparent loss of non-OP50-1 E. coli growth on NGM plates was used as a readout for a clean nematode strain. But, there may be non-culturable populations of contaminating microbes present, so it is essential to conduct a metagenomics method such as 16S rRNA amplicon sequencing to see the extent of contamination26. Once the worm strain is cleaned, it can be frozen and stored away for future studies. Overall, this protocol allows researchers to enrich unculturable microbes in wild nematodes, allowing them to study effects on host fitness, characterize phenotypes of colonization or infection, and take advantage of genetic tools to understand the mechanisms underlying host-microbe interactions.
Divulgazioni
The authors have nothing to disclose.
Acknowledgements
Thank you to Dr. Christian Braendle and the Centre Nationale de la Recherche Scientifique (CNRS) Nouragues Field Station.
Materials
| Agarose | Fisher Scientific | BP1356 | |
| 10% SDS | Invitrogen | AM9822 | |
| BD PrecisionGlide Needle – 26 G | Fisher Scientific | 305115 | |
| Carbenicillin | Millipore-Sigma | C1389-1G | |
| Cefotaxime | Millipore-Sigma | C7039-500mg | |
| Chloramphenicol | Millipore-Sigma | C0378-25G | |
| DNA Clean and Concentrator Kit | Zymo Research | 11-303C | |
| DreamTaq Polymerase | Fisher Scientific | EP0711 | |
| Gentamycin | Millipore-Sigma | G1264-250mg | |
| Kanamycin | Millipore-Sigma | K1876-1G | |
| KH2PO4 | Fisher Scientific | P-286 | |
| NaCl | Fisher Scientific | S-671 | |
| NH4Cl | Fisher Scientific | A-661 | |
| Streptomycin | Millipore-Sigma | S6501-50G | |
| Tetracyclin | Millipore-Sigma | T7660-5G | |
| Triton X-100 | Fisher Scientific | BP-151 | |
| Watch glasses | VWR | 470144-850 |
Riferimenti
- Balla, K. M., Troemel, E. R. Caenorhabditis elegans as a model for intracellular pathogen infection. Cellular Microbiology. 15, 1313-1322 (2013).
- Pukkila-Worley, R., Ausubel, F. M. Immune defense mechanisms in the Caenorhabditis elegans intestinal epithelium. Current Opinion in Immunology. 24, 3-9 (2012).
- Bossinger, O., Fukushige, T., Claeys, M., Borgonie, G., McGhee, J. D. The apical disposition of the Caenorhabditis elegans intestinal terminal web is maintained by LET-413. Biologia dello sviluppo. 268, 448-456 (2004).
- Dimov, I., Maduro, M. F. The C. elegans intestine: organogenesis, digestion, and physiology. Cell and Tissue Research. 377, 383-396 (2019).
- Zhang, F., et al. Caenorhabditis elegans as a model for microbiome research. Frontiers in Microbiology. 8, 485 (2017).
- Szumowski, S. C., Botts, M. R., Popovich, J. J., Smelkinson, M. G., Troemel, E. R. The small GTPase RAB-11 directs polarized exocytosis of the intracellular pathogen N. parisii for fecal-oral transmission from C. elegans. Proceedings of the National Academy of Sciences of the United States of America. 111, 8215-8220 (2014).
- Bakowski, M. A., et al. Ubiquitin-mediated response to microsporidia and virus infection in C. elegans. PLoS Pathogens. 10, 1004200 (2014).
- Sowa, J. N., et al. The Caenorhabditis elegans RIG-I Homolog DRH-1 Mediates the Intracellular Pathogen Response upon Viral Infection. Journal of Virology. 94, 01173 (2020).
- Zugasti, O., et al. Activation of a G protein-coupled receptor by its endogenous ligand triggers the innate immune response of Caenorhabditis elegans. Nature Immunology. 15, 833-838 (2014).
- Félix, M. -. A., Duveau, F. Population dynamics and habitat sharing of natural populations of Caenorhabditis elegans and C. briggsae. BMC Biology. 10, 59 (2012).
- Lee, D., et al. Balancing selection maintains hyper-divergent haplotypes in Caenorhabditis elegans. Nature Ecology and Evolution. 5, 794-807 (2021).
- Luallen, R. J., et al. Discovery of a natural microsporidian pathogen with a broad tissue tropism in Caenorhabditis elegans. PLOS Pathogens. 12, 1005724 (2016).
- Osman, G. A., et al. Natural infection of C. elegans by an oomycete reveals a new pathogen-specific immune response. Current Biology. 28, 640-648 (2018).
- Felix, M. A., et al. Natural and experimental infection of Caenorhabditis nematodes by novel viruses related to nodaviruses. PLoS Biology. 9, 1000586 (2011).
- Zhang, G., et al. A large collection of novel nematode-infecting microsporidia and their diverse interactions with Caenorhabditis elegans and other related nematodes. PLoS Pathogens. 12, 1006093 (2016).
- Félix, M. -. A., Wang, D. Natural viruses of Caenorhabditis nematodes. Annual Review Genetics. 53, 313-326 (2019).
- Grover, M., Barkoulas, M. C. elegans as a new tractable host to study infections by animal pathogenic oomycetes. PLoS Pathogens. 17, 1009316 (2021).
- Bakowski, M. A., Luallen, R. J., Troemel, E. R. Microsporidia Infections in Caenorhabditis Elegans and Other Nematodes. Microsporidia: Pathogens of Opportunity: First Edition. , (2014).
- Taffoni, C., Pujol, N. Mechanisms of innate immunity in C. elegans epidermis. Tissue Barriers. 3, 1078432 (2015).
- Dirksen, P., et al. CeMbio – The Caenorhabditis elegans microbiome resource. G3: Genes, Genomes, Genetics. 10, 3025-3039 (2020).
- Frézal, L., Félix, M. -. A. C. elegans outside the Petri dish. eLife. 4, 05849 (2015).
- Troemel, E. R., Félix, M. -. A., Whiteman, N. K., Barrière, A., Ausubel, F. M. Microsporidia are natural intracellular parasites of the nematode Caenorhabditis elegans. PLoS Biology. 6, 2736-2752 (2008).
- Luallen, R. J., Bakowski, M. A., Troemel, E. R. Characterization of microsporidia-induced developmental arrest and a transmembrane leucine-rich repeat protein in Caenorhabditis elegans. PLoS One. 10, 0124065 (2015).
- Félix, M. -. A., et al. Species richness, distribution and genetic diversity of Caenorhabditis nematodes in a remote tropical rainforest. BMC Evolutionary Biology. 13, 10 (2013).
- Samuel, B. S., Rowedder, H., Braendle, C., Félix, M. -. A., Ruvkun, G. Caenorhabditis elegans responses to bacteria from its natural habitats. Proceedings of the National Academy of Sciences of the United States of America. 113, 3941-3949 (2016).
- Berg, M., et al. Assembly of the Caenorhabditis elegans gut microbiota from diverse soil microbial environments. ISME Journal. 10, 1998-2009 (2016).
- Androwski, R. J., Flatt, K. M., Schroeder, N. E. Phenotypic plasticity and remodeling in the stress-induced C. elegans dauer. Wiley Interdisciplinary Reviews: Developmental Biology. 6, 278 (2017).
- Tran, T. D., Ali, M. A., Lee, D., Félix, M. -. A., Luallen, R. J. Bacterial filamentation is an in vivo mechanism for cell-to-cell spreading. bioRxiv. , (2021).

