Summary
The present protocol describes a moxibustion application method for mice, with the benefits of reducing mouse fearfulness, guaranteeing their welfare, and improving efficiency for researchers.
Abstract
The field of moxibustion research is expanding, with a rapid increase in publications in recent years. Moxibustion is a therapy that ignites moxa on the skin of humans, with an increase in peripheral skin temperature and localized redness. During this treatment, the recipient must remain still to prevent scalding and expose intervention sites for easy manipulation; however, maintaining a fixed posture during moxibustion is a big challenge for animals. Thus, manipulating moxibustion in small animals, such as mice, can lead to several difficulties for researchers. In addition, an uncomfortable posture for animals can lead to fear and resistance to moxibustion, increased risk of injury, diminished animal welfare, and less valid research data. An efficient, comfortable moxibustion method is needed to protect animal welfare and minimize the adverse effects on experimental results. However, moxibustion methods are highly variable and often have limited efficacy. More importantly, an uncomfortable moxibustion posture might cause a stress response, such as those observed with anxiety, fear, and anger, which could influence the research data. Therefore, strategies for animal moxibustion that inflict the least harm possible during the intervention are required. This protocol introduces a mouse tethering method for moxibustion intervention, minimizing mouse discomfort and improving study efficiency. Essential strategies for tethering mice and application of moxibustion are highlighted, and the structure of the tethering instrument is described.
Introduction
Moxibustion is an external treatment used in traditional Chinese medicine1. It has been widely applied for centuries as a means of keeping fit and preventing and curing diseases2,3. However, the mechanism of moxibustion remains to be elucidated; thus, this field of research has expanded in recent years as researchers explore its effects and biological mechanisms.
During moxibustion, the recipient must remain still for the best results to be achieved4. Moxibustion treatment typically lasts for 15-30 min5,6. Because of this, manipulation of moxibustion for animals, especially small animals such as mice and rats (favorable for use in animal experiments), has become a big challenge for researchers. Most moxibustion devices are suitable for clinical use and cannot be used for animals, especially for small animals such as mice7,8,9. Besides this, unlike human beings, animals can barely follow orders and remain steady over a while. A fixed and uncomfortable posture for animals can lead to passive emotions, including anxiety, fear, anger, nervousness, and resistance to treatment, increasing the risk of injury, diminishing animal welfare, and resulting in less valid research data10. To solve this problem, in previous studies, anesthetics have been applied for extended periods during animal experiments involving external traditional Chinese medicine therapies, including acupuncture, moxibustion, and massage research11,12,13. However, given that physical conditions change with anesthesia, and in most clinical cases, moxibustion is administered to people in conscious states, anesthesia might impact research data authenticity. Therefore, a method of tethering animals consciously and comfortably become a challenge. To solve these technical problems, specialized devices for mouse moxibustion have been introduced. This protocol provides a tethering method that will assist researchers in performing moxibustion treatment on animals, allowing the animals to remain steady, conscious, and comfortable.
Protocol
All experiments were carried out following the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Chengdu University of TCM Institutional Animal Care and Use Committee. The care and maintenance of the animals in the laboratory were performed following References14,15. Adult male C57BL/6J mice, weighing 20-25 g, were used for the test. All mice were maintained on a 12 h light/dark cycle at 24 °C and 40%-50% humidity, with free access to food and water. The following protocol depicts the moxibustion procedures under typical laboratory housing conditions.
1. Preparation of the equipment
- Mouse tethering
- Prepare a mouse tethering device consisting of a vertical plate, vertical plate base, and brackets made of wood.
- Construct the fixed body using a straight wooden splint (length: 30 cm x width: 3 cm x height: 3 cm) and attach the fixed velcro belt (which is made up of two sides: a hook side (4 cm x 3 cm) and a loop side (10 cm x 3 cm)) to allow tethering of the mouse body while maintaining flexibility (see Table of Materials).
- Use a glue gun to attach the hook side on the right side of the wooden splint and attach a 1/4 length of the loop side on the left side.
- Place the mouse on the fixed bed, and use the fixed velcro belt to tether the torso of the mouse.
NOTE: Ensure that the tethering belt is not tight enough to exert pressure on the heart, which could lead to the death of the mouse. Tether the mouse loosely but firmly. - Use tape to attach the lower/upper limbs to either side of the wooden splint and attach the tail to the surface of the wooden splint.
- Prepare a mouse tethering device consisting of a vertical plate, vertical plate base, and brackets made of wood.
- Preparation of the mouse-bed rack
NOTE: The rack comprises a vertical wood plate, wood baseplate, and wood brackets. The bracket consists of an L-shaped piece of stainless steel, which provides support for each mouse bed.- Attach the vertical plate onto the base plate, and create square grooves in the base plate to insert wooden sticks used as baffles (Figure 1).
- Prepare a transparent, plastic head cover if the mice are administered moxibustion above the head.
- Use a 1000 mL syringe, and remove the needle and core rod. Use a cutting tool or knife to cut the syringe jacket in half.
- Choose either side of the syringe jacket, cut off the head and tail, and set the middle section aside (length: 4 cm) as the headcover for the mouse.
- Drill a hole (1 cm) at the center of the syringe jacket with a drill machine and place a moxa stick in the hole. Place a headcover over the mouse's head to limit its head movement and protect it from the ignited moxa.
- Attach two parallel wooden strips (length: 7 cm x wide: 1 cm) and a horizontal cross-piece (length: 7.5 cm x width: 1 cm) to the head end of the mouse bed if mice are administered moxibustion on the forelimbs (Figure 2).
NOTE: The space between each parallel wooden strip and the mouse bed should be 1 cm. The number of brackets and height of the vertical plates can be determined based on experimental requirements. These steps should be performed gently to avoid terrifying the mice. Be sure to give mice adaptive training for 1 week before the experiment.
- Preparation of moxibustion stick holder
- Prepare the moxibustion stick holder comprising a magnetic metal rectangle (length: 30 cm), a bracket base (length: 5 cm x width: 5 cm x height: 2 cm), and wooden cubes (length: 3 cm x width: 1.5 cm x height: 1 cm), each with a hole (diameter: 0.55 cm) in the center into which a moxa stick may be placed (see Table of Materials).
- Attach a flat, thin magnet (length: 3 cm × width: 1.5 cm, thickness: 0.2 cm) to one side of each cube. The magnetic attraction between the cubes with moxa sticks and the holder allows adjusting the height of the moxa sticks.
NOTE: Ensure that the magnetic poles of the cube magnets are opposite the holder magnet's pole.
- To record the beginning and end of the moxibustion procedure, place a timing device (see Table of Materials) near the mouse tethering device where it can be easily read.
- Use a bare or thinly gloved hand when holding and tethering the mice. A thinly gloved hand is also effective during moxibustion treatment.
2. Moxibustion procedure
- Put on the laboratory gloves. Before moxibustion treatment, shave the fur via mouse shaver at the moxibustion site to expose the acupoint.
- Insert moxa sticks (0.5 cm in diameter and 10 cm in length) into the moxibustion stick holder (which comprises the magnetic metal rectangle, bracket base, and wooden cubes with holes in their centers for moxa stick insertion, and one stick holder can only accommodate one moxa stick, step 1.3).
- To ascertain the moxibustion temperature, attach a digital thermo-detector at the acupoint to monitor the temperature variation.
- Start the timer and ignite the moxa stick; fix or control the distance between the skin of the acupoint and the lighted end of the moxa stick depending on the temperature variation required for the experiment.
3. Simultaneous mouse moxibustion
NOTE: Mice can be treated with moxibustion as a group. A mouse-bed rack is used to position the mouse beds so that a group of mice can be treated simultaneously.
- Place each mouse bed on the rack and insert a wooden stick into the base plate to protect the mice from falling (Figure 3).
- Insert moxa sticks (0.5 cm in diameter and 12 cm in length) into the moxibustion stick holder at levels that coincide with each mouse bed. Adjust the distance between the mouse rack and stick holder, and then ignite the moxa sticks.
NOTE: This allows the mice to be treated with moxibustion in a relaxed, comfortable state. During moxibustion, ensure that the distance between the mouse rack and stick holder remains consistent for all mice. - Before modeling, conduct the mouse responses to the thermal withdrawal threshold (TWL) test to determine whether moxibustion would be effective in analgesia.
Representative Results
Table 1 summarizes the different factors that affect the responses to moxibustion. Positive outcomes of mouse moxibustion have been indicated by multiple measures of mouse welfare and positive effects.
Next, specific examples of results obtained when the moxibustion technique is paired with complete Freund's adjuvant (CFA)-induced injection procedures are outlined. The thermal withdrawal threshold (TWL) test was used to evaluate the efficacy of moxibustion in pain relief16.
After being adaptively fed for one week in a 12 h light/dark cycle at 24 °C and 40%-50% humidity, mice were either restrained and administered CFA injection (20 µL, including CFA-induced model group, and CFA-induced with moxibustion group, CFA+MOXI) or saline injection. CFA-induced inflammatory pain models were established17. The TWL test was applied before and 3 days after the establishment of each group to value the modeling. After the CFA-induced inflammatory pain model and after moxibustion, ST36 was applied (Figure 4).
The mice lay prone on the bed, and their trunks were fixed with the velcro belts. The hind limbs and tail of the mice were pasted on the sides of the bed. This posture also allowed mice to expose acupoints on the hind limbs, that is, the Zusanli (ST36, located 2 mm lateral to the anterior tubercle) acupoint16 (Figure 1). When the moxibustion acupoint was above the heads of the mice, that is, the Baihui (GV20, located at the intersection of the line connecting the apexes of the two auricles and the median line of the head) acupoint18, the headcovers were added, and the mice were tethered to the bed. When the moxibustion acupoints were at the forelimbs of the mice, that is, the Neiguan (PC6, located on the inside of the forelimb, between the ulna and radius, 3 mm from the front paw) acupoint19, the forelimbs were taped flat (as described in step 1.1.3., two parallel wooden strips with a horizontal piece across the wooden splint were used to stretch the mouse forelimbs) (Figure 2).
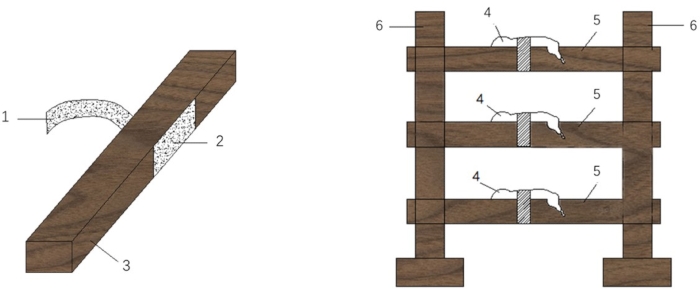
Figure 1: Safe and quick fixed bed. 1: hook side; 2: loop side of fixed velcro belt; 3: fixed body; 4: mice; 5: fixed beds; 6: mouse-bed rack. Please click here to view a larger version of this figure.
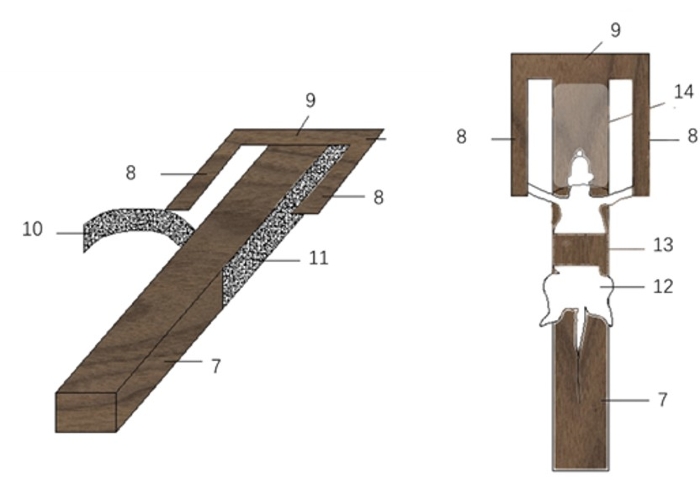
Figure 2: Mouse forelimb tether. 7: fixed body; 8: parallel wooden strips; 9: horizontal strip; 10: hook side; 11: loop side of fixed velcro belt; 12: mouse; 13: fixed velcro belt; 14: transparent headcover. Please click here to view a larger version of this figure.
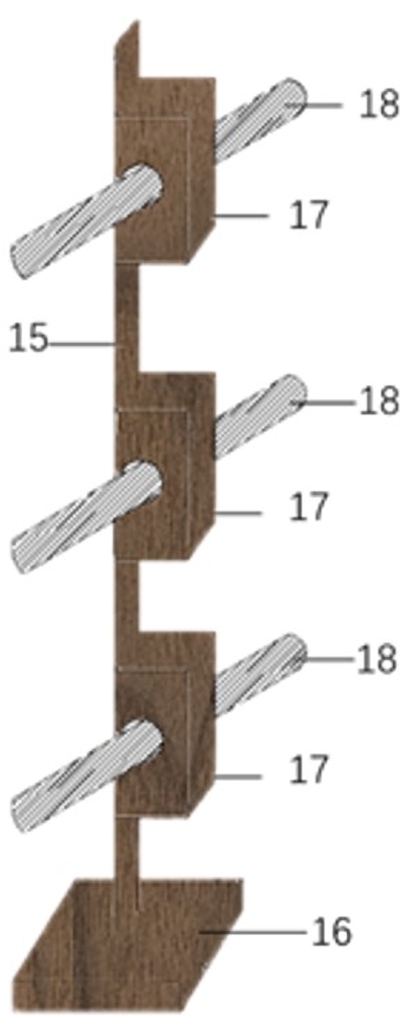
Figure 3: Mouse bed rack with moxa sticks. 15: magnetic metal rectangle; 16: base plate; 17: wooden cubes; 18: moxa sticks. Please click here to view a larger version of this figure.
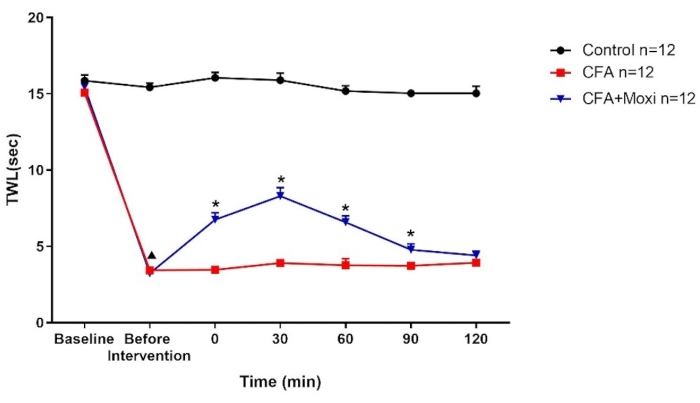
Figure 4: TWL test with moxibustion at ST36. Control group, CFA inflammatory pain model, CFA+Moxi inflammatory pain in the moxibustion group. Compared with the data in the control group, TWL scores dropped dramatically (p<0.01) with CFA intervention in the CFA (n=10) and CFA+Moxi (n=10) groups. After intervention in the CFA+Moxi group, the TWL scores gradually increased compared with those in the model group at 0, 30, 60, and 90 min time points (p<0.05). ▲p<0.01, compared with the control group; *p<0.05, compared with the CFA group. Please click here to view a larger version of this figure.
| Factor | Effect | Riferimenti | ||
| Age | There is no reported evidence that suggests that mice of different ages differ in their response to moxibustion. | |||
| Sex | No differences were found between the sexes. | |||
| Extrinsic | ||||
| Size | Triple moxibustion induced greater increases in skin temperature and blood perfusion than single moxibustion. | 17 | ||
| Temperature | Temperatures above 43 °C provided greater relief of disability and pain in patients with acute lower back pain. | 18 | ||
| Moxibustion at different temperatures had different analgesic effects on either chronic inflammatory pain (induced by injection of complete Freund's adjuvant) or neuropathic pain induced by spared nerve injury. | 19 | |||
| Sex of operator | Not reported | |||
| Moxa stick odor | Moxa smoke failed to generate analgesic effect and there was no big difference between Moxi and smoke-free Moxi groups. | 20 | ||
| The toxic compounds of moxa smoke may have some side effects on the heart, liver, and kidney in humans. | 21 | |||
| Both moxibustion and moxa smoke interventions were able to mitigate symptoms of Alzheimer’s disease in mice to some degree. | 22,23 | |||
| Ash cleaning and distance adjustment | Moxibustion with ash cleaning and distance adjustment can create a larger and longer lasting thermal effect on biological tissue | 24 | ||
| Noise | Not reported | |||
| Moxa stick composition | Moxa with leaf-moxa ratio of 5:1 can meet clinical needs; there is no need for an excessively high leaf-moxa ratio. | 25 | ||
Table 1: Factors affecting, or potentially affecting, responses to moxibustion.
Discussion
The moxibustion technique is effective for many disorders, such as fatigue, insomnia, diarrhea, and pain syndrome20,21,22,23,24,25,26. With the benefits of moxibustion treatment for many diseases, the mechanism of this technique has attracted the attention of researchers. The application of moxibustion in animals is necessary to study the molecular mechanisms of moxibustion. To analyze the mechanism of moxibustion, one of the biggest challenges for researchers is deciding how to tether animals in a suitable position, which will keep them steady without discomfort and allow treatment over some time. To overcome this problem, many studies have been conducted to anesthetize animals27,28. However, even a slight amount of anesthesia can reduce neurotransmission, which might interfere with the accuracy of the experimental data. In addition, administering anesthesia to mice makes the procedures more time-consuming, with each mouse receiving treatment individually.
In this protocol, a tethering technique for mouse moxibustion is introduced. After adjustment training, mice did not show any discomfort during the treatment process using this technique. The required equipment materials, such as wood splints, velcro, magnets, and syringes, are cheap and easy to acquire. This helps to reduce experimental costs. Another benefit is that the velcro can be flexibly controlled according to the body shape of the mouse on the mouse bed, avoiding suffocation and death caused by tightly tethering the mouse. This equipment also contains a fixed bed and moxa stick rack that enable researchers to treat a group of mice simultaneously, which significantly improves the efficiency of the treatment, releases the workload of operators, and reduces deviation among mice in the same group. The fixed bed rack optimizes space utilization, and the moxa stick rack uses an innovative magnetic system to adjust the distance between moxa sticks and mice. Given the vital role of temperature in moxibustion therapy, an appropriate fixed distance should be set with a digital thermo-detector to monitor the temperature variation if needed29. This rack can be further optimized with several holes from different directions for operators to perform moxibustion stimulation at acupoints in different positions. In addition, these designs can be modified and extended for experimental animals of various sizes, such as rats, guinea pigs, and rabbits.
The limitation of this method is that technical proficiency is required for researchers because researchers need to adjust the tightness of the binding based on the specific size of each mouse. Besides, the mice that received this treatment should be given adaptive training, given that some of the mice can experience mild discomfort when first exposed to the fixator.
Overall, this moxibustion process provides an efficient method of tethering the mice and providing moxibustion intervention with the fewest possible adverse effects on mouse welfare and behavior.
Divulgazioni
The authors have nothing to disclose.
Acknowledgements
This work has been supported by grants from the National Natural Science Foundation of China (81704187, 8210152562), Sichuan Science and Technology Program (2019YJ0587, 2018JY0482, 2019YJ0329), Sichuan Academy of Medical Sciences and Sichuan People's Hospital Research Fund (2018ZX05), the Xinglin Scholars Research Foundation of Chengdu University of Traditional Chinese Medicine (QNXZ2019034).
Materials
| Complete Freund's Adjuvant (CFA) | Sigma-Aldrich | SF588102 | |
| Glue gun | Deli Group Co., Ltd. | DL5041 | |
| Laboratory glove | Boci Co., Ltd | 53625130323 | |
| Magnetic metal rectangle (thickness: 0.2 cm) | Sitoo Stationery Co., Ltd | 1007908729 | |
| Moxa stick | Hanyi Airong factory | 5613 | |
| Steel sheet | Rizhan metal materials Co., Ltd | 1108 | |
| Syringe (1000 mL ) | Xinmin Fuda Co., Ltd | 796341 | |
| Timer | Bevoza Co., Ltd | KT003 | |
| Velcro belt | Minnesota Mining and Manufacturing Co., Ltd | 3MSJ3550 | |
| Wooden cube | Chuang Hing Wood Chip Co., Ltd | 2581457A | |
| Wooden splint | Chuang Hing Wood Chip Co., Ltd | 3410968M | |
| Wooden stick | Chuang Hing Wood Chip Co., Ltd | 2785476M | |
| Wooden strip | Chuang Hing Wood Chip Co., Ltd | 2374652S |
Riferimenti
- Ebrary, I. Guide for the care and use of laboratory animals. NIH Publication. 327 (3), 963-965 (2011).
- Wang, M., et al. Mechanism of traditional chinese medicine in treating knee osteoarthritis. Journal of Pain Research. 13, 1421-1429 (2020).
- Wang, T., Xu, C., Pan, K., Xiong, H. Acupuncture and moxibustion for chronic fatigue syndrome in traditional Chinese medicine: A systematic review and meta-analysis. BMC Complementary and Alternative Medicine. 17 (1), 163 (2017).
- Zimmermann, M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain. 16 (2), 109-110 (1983).
- Zhou, W., et al. Effect of moxibustion and acupuncture on gastric mucosal cell apoptosis and expression of NF-κB, Bcl-2 in chronic atrophic gastritis rats. Acupuncture Research. 46 (4), 284-288 (2021).
- Wang, Y., et al. βEffect of moxibustion on -EP and Dyn levels of pain-related indicators in patients with rheumatoid arthritis. Evidence-based Complementary and Alternative Medicine: eCAM. 2021, 6637554 (2021).
- Huang, P., et al. Design of moxibustion device for experimental rabbits. Chinese Acupuncture & Moxibustion. 39 (09), 1024-1026 (2019).
- Li, M., Sun, Z. An automatic moxibustion box with temperature control. Chinese Acupuncture & Moxibustion. 39 (06), 649-650 (2019).
- Luo, Z., Zhang, C., Zhou, M., Chen, C. Manufacture and clinical application of a medical warm moxibustion instrument. Shanghai J Acu-mox. 37 (05), 596-598 (2018).
- Liang, Y. L., Sun, Y. H., Sun, Y. H., Sun, L. H., Jiang, H. T. Design and use of rat box for moxibustion experiment. Acupuncture Research. 36 (03), 224 (2011).
- Meng, J., Fu, W., Zhu, G., Song, L. Effect of acupuncture on coronary collateral circulation function in dogs with experimental myocardial infarction. Acupuncture Research. 3 (1), 196-197 (1986).
- Tan, D. Experimental study on the effect of moxibustion at Neiguan point on the content of serum free fatty acid (FFA) in rabbits with acute myocardial ischemia. Liaoning Journal of Traditional Chinese Medicine. 6, 44-45 (1991).
- Seo, B., et al. Skeletal muscle regeneration with robotic actuation-mediated clearance of neutrophils. Science Translational Medicine. 13 (614), (2021).
- Bielitzki, J. T., Barbee, R. W., Garber, J. Guide for the care and use of laboratory animals. NIH Publication No 85-23(rev). 327 (3), 963-965 (2011).
- National Research Council (US), Institute for Laboratory Animal Research. Guide for the Care and Use of Laboratory Animals. National Research Council (US), Institute for Laboratory Animal Research. , (1996).
- Zuo, C., et al. Ipsi- and contralateral moxibustion generate similar analgesic effect on inflammatory pain. Evidence-based complementary and Alternative Medicine: eCAM. 2019, 1807287 (2019).
- Poulaki, S., Rassouli, O., Liapakis, G., Gravanis, A., Venihaki, M. Analgesic and anti-inflammatory effects of the synthetic neurosteroid analogue BNN27 during CFA-induced hyperalgesia. Biomedicines. 9 (9), 1185 (2021).
- Duan, L., et al. Metabolomics analysis on mice with depression ameliorated by acupoint catgut embedding. Frontiers in Psychiatry. 12, 703516 (2021).
- Wang, J., et al. Protection against doxorubicin-induced cardiotoxicity through modulating iNOS/ARG 2 balance by electroacupuncture at PC6. Oxidative Medicine and Cellular Longevity. 2021, 6628957 (2021).
- Chen, Y., Gao, X., Sun, C. Pricking and penetrating moxibustion therapy in patients with refractory insomnia: A randomized and controlled clinical trial. Journal of Traditional Chinese Medicine. 38 (5), 754-762 (2018).
- Liu, C., et al. Effectiveness and safety of fire-needle moxibustion on insomnia: Protocol for a systematic review and meta-analysis. Medicina. 98 (7), 14509 (2019).
- Han, K., et al. Moxibustion for treating cancer-related fatigue: A multicenter, assessor-blinded, randomized controlled clinical trial. Cancer Medicine. 10 (14), 4721-4733 (2021).
- Bao, C., et al. Effect of mild moxibustion on intestinal microbiota and NLRP6 inflammasome signaling in rats with post-inflammatory irritable bowel syndrome. World Journal of Gastroenterology. 25 (32), 4696-4714 (2019).
- Chen, F., et al. Efficacy and safety of moxibustion for chronic low back pain: A systematic review and meta-analysis of randomized controlled trials. Complementary Therapies in Clinical Practice. 39, 101130 (2020).
- Zhang, C., et al. The role of STIM1/ORAI1 channel in the analgesic effect of grain-sized moxibustion on inflammatory pain mice model. Life Sciences. 280, 119699 (2021).
- Zhao, L., et al. Effectiveness of moxibustion treatment as adjunctive therapy in osteoarthritis of the knee: A randomized, double-blinded, placebo-controlled clinical trial. Arthritis Research & Therapy. 16 (3), 133 (2014).
- Goldman, N., et al. Adenosine A1 receptors mediate local anti-nociceptive effects of acupuncture. Nature Neuroscience. 13 (7), 883-888 (2010).
- Torres-Rosas, R., et al. Dopamine mediates vagal modulation of the immune system by electroacupuncture. Nature Medicine. 20 (3), 291-295 (2014).
- Solovchuk, M., Deng, H., Sheu, T. Experimental and numerical study on the temperature elevation in tissue during moxibustion therapy. Evidence-based Complementary and Alternative Medicine:eCAM. 2020, 7514302 (2020).

.
