Osmotic Pump-based Drug-delivery for In Vivo Remyelination Research on the Central Nervous System
Summary
Demyelination takes place in multiple central nervous system diseases. A reliable in vivo drug delivery technique is necessary for remyelinating drug testing. This protocol describes an osmotic pump-based method that allows long-term drug delivery directly into the brain parenchyma and improves the drug bioavailability, with broad application in remyelination research.
Abstract
Demyelination has been identified in not only multiple sclerosis (MS), but also other central nervous system diseases such as Alzheimer’s disease and autism. As evidence suggests that remyelination can effectively ameliorate the disease symptoms, there is an increasing focus on drug development to promote the myelin regeneration process. Thus, a region-selectable and result-reliable drug delivery technique is required to test the efficiency and specificity of these drugs in vivo. This protocol introduces the osmotic pump implant as a new drug delivery approach in the lysolecithin-induced demyelination mouse model. The osmotic pump is a small implantable device that can bypass the blood-brain barrier (BBB) and deliver drugs steadily and directly to specific areas of the mouse brain. It can also effectively improve the bioavailability of drugs such as peptides and proteins with a short half-life. Therefore, this method is of great value to the field of central nervous system myelin regeneration research.
Introduction
The osmotic pump is a small implantable solution-releasing device. It can be used for systemic delivery when implanted subcutaneously or in the abdominal cavity. The surface of the osmotic pump is a semi-permeable membrane, and its inner side is a permeable layer. The osmotic pump operates by using the osmotic pressure difference between the osmotic layer and the tissue environment where the pump is implanted. The high osmolality of the osmotic layer makes the water in the tissue flow into the osmotic layer through the semi-permeable membrane on the pump surface. The osmotic layer expands and compresses the flexible reservoir inside the pump, thereby displacing the solution from the flexible reservoir at a certain rate for a long duration1. The pump has three different reservoir volumes, 100 µL, 200 µL, and 2 mL, with their delivery rates varying from 0.11 µL/h to 10 µL/h. Depending on the selected pump type, the device can operate from 1 day to 6 weeks2. In this protocol, a 100 µL osmotic pump with a transfer rate of 0.25 µL/h that can operate for 14 days is used.
Back in the 1970s, the osmotic pump had been used in neuroscience research3,4. For instance, Wei et al. adopted the osmotic pump approach to inject opioid peptides into the ventricle in a study of drug addiction3. After continuous improvement, the osmotic pump has now been used in the study of the controlled delivery of thousands of drugs, including peptides, growth factors, addictive drugs, hormones, steroids, antibodies, and so on. In addition, with special catheters (Brain Infusion Kits) attached, it can be used for targeted infusion to specific tissues or organs, including the spinal cord, brain, spleen, and liver5,6,7.
In the study of remyelination, many drugs have been shown to promote myelin regeneration in vitro, but most of them have not achieved significant effects in vivo, possibly due to the lack of an appropriate administration method. Traditional administration methods such as intraperitoneal injection, subcutaneous injection, and intragastric administration have limitations in the bioavailability of the drugs. In addition, some drugs have poor blood-brain barrier permeability, which undermines their access to the brain parenchyma. Together, these limitations call for a novel efficient delivery method. In combination with the brain infusion kits, osmotic pumps can bypass the blood-brain barrier and deliver drugs directly to the corpus callosum, which effectively improves the bioavailability of drugs, especially for some polypeptide and protein drugs with a short half-life. Therefore, the osmotic pump as a new drug delivery technique is of great value to the field of central nervous system myelin regeneration research. The application of this technique will be introduced in detail below.
Protocol
All animal procedures were conducted under institutional guidelines and protocols approved by the animal welfare and ethics committee of the Third Military Medical University.
1. Establishment of the lysolecithin-induced demyelination mouse model
- Prepare 1% lysolecithin (also called L-α-Lysophosphatidylcholine) solution with sterile PBS.
- Sterilize scissors, forceps, curved hemostat, and other surgical instruments by autoclave sterilization. Sterilize the surgical area and lay down sterile sheets. All materials and reagents used for surgery should be prepared aseptically. It is important to keep the surgical area sterile throughout the procedure.
- Anesthetize a postnatal day 56 (P56) C57BL6 mouse as follows.
- Place the mouse in the isoflurane chamber of the small animal anesthesia machine. Adjust the O2 flow to 300-500 mL/min and isoflurane to 3%-4%. After sufficient anesthesia, when the mouse becomes immobile with a slow and stable breath, transfer the mouse to the stereotaxic apparatus with a heating pad.
- Switch the gas output from the chamber to the anesthesia mask and adjust isoflurane to 1% – 1.5% to maintain the mouse in the anesthesia state. Wait until the mouse is fully anesthetized, inject ketoprofen (3 – 5 mg/kg) intraperitoneally to relieve pain. Before the operation, pinch the toes of the mouse and check its reaction to confirm successful anesthesia8.
- When the mouse is anesthetized, it cannot regulate its body temperature. Therefore, monitor and regulate the body temperature of the mouse during surgery. To keep the mouse's eyeballs moist while under anesthesia, cover the surface of the eyeballs with erythromycin eye ointment.
- Secure the mouse head in the stereotaxic apparatus with tooth bar and ear bars. (Figure 1A).
- Use a razor to remove hair from the top of the head. Sanitize the head skin with three cycles of betadine and 75% ethanol. For ethical concerns, cover the animal body except for the surgery site. Using a scalpel, make a 1 cm long mid-sagittal incision of the skin from the base of the neck to in between the eyes to expose the skull (Figure 1B).
- Gently wipe the surface of the skull with a sterile cotton swab containing 30% hydrogen peroxide to visualize the cranial sutures (Figure 1C). Adjust the height of the tooth bar and ear bars to place the lambda point and bregma point at the same height (i.e., with the same z-axis coordinates when the needle tip touches the points), so that the sagittal suture is horizontal.
- Gently place the tip of the microliter syringe needle (10 µL, 33 G) at the bregma point and reset the x, y, and z coordinates to 0 (Figure 1D). Move the syringe to the injection site (x: 1.04; y: 1.0, i.e., 1.04 mm lateral to the midline and 1.0 mm posterior to the bregma point) according to the prompt of the digital readout (Figure 1E).
- Slowly drill a small burr hole through the skull at the injection site without penetrating the dura with a 1 mL syringe needle (26 G, 0.45 mm) (Figure 1F). Slowly insert the microliter syringe needle into the brain tissue through the hole until a certain depth is reached (z = -1.62 mm for most P56 mice) (Figure 1G).
NOTE: Empirically, the insertion depth of -1.62 mm allows the needle tip to reach the middle of the corpus callosum of most P56 mice so that the lysolecithin could be directly delivered into the corpus callosum to induce demyelination. - Inject 1.5 µL of 1% lysolecithin at a speed of 0.3 µL/min. After the injection, wait for 5 min before slowly pulling out the microliter syringe to prevent the leakage of liquid along the injection needle path.
- Stitch the skin with 5-0 surgical sutures (Figure 1H).
- Place the mouse on a heating pad to avoid a drop in body temperature. Administer a subcutaneous injection of 5 mg/kg carprofen every 24 h to relieve the pain. Apply erythromycin ointment to the incision every day to ensure that the wound heals properly. Place the mouse that has undergone surgery in a cage alone and feed it with moist food until fully recovered. Monitor the mouse daily after the operation.
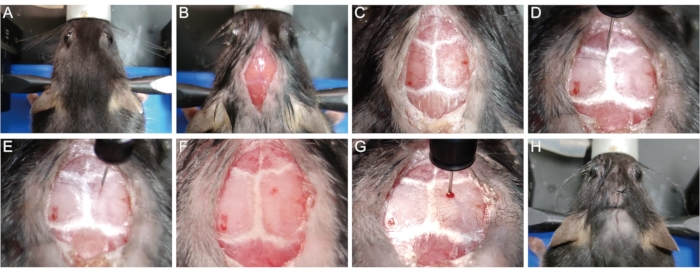
Figure 1: Establishment of the lysolecithin-induced demyelination mouse model. (A) Secure the mouse in the stereotaxic apparatus. (B) Open a 1 cm mid-sagittal incision to expose the skull. (C) Visualize the cranial sutures. (D) Reset the x, y, and z coordinates to 0 on the Bregma point. (E) Move the syringe to the injection site. (F) Drill a hole in the skull at the injection site. (G) Insert the needle into brain tissue slowly and inject lysolecithin. (H) Stitch the skin. Please click here to view a larger version of this figure.
2. Preparation of the osmotic pump
NOTE: Key components of the pump are shown in Figure 2A.
- Determine the depth of insertion of the brain infusion cannula into the brain. Ensure that the needle of the brain infusion cannula used is 3 mm long and each depth-adjustment spacer is 0.5 mm. To achieve an injection depth of 1.5 mm (close to the callosum), attach three depth-adjustment spacers to the needle of the brain infusion cannula with tissue adhesive (Figure 2B, C).
- To fill the osmotic pump, attach the syringe needle that comes with the pump package to a 1 mL syringe and aspirate the drug. Hold the pump upright, insert the syringe into the opening at the top of the pump, and slowly inject the drug, being careful not to create bubbles9 (see Figure 2D). When the liquid flows out of the opening, slowly pull out the syringe.
- Remove the white flange from the flow regulator with scissors or pliers being careful not to bend or crush the flow moderator. Then, insert the flow moderator into the pump (Figure 2E). To determine whether there are bubbles in the osmotic pump, weigh the osmotic pump separately before and after filling.
- Trim the catheter to a certain length according to the size of the animal (20-25 mm catheters for P56 mice that weigh about 25 g). Attach the catheter to the brain infusion cannula.
- Fill the catheter with drugs using the syringe without introducing air (Figure 2F).
- Connect the catheter to the flow moderator. After attachment, ensure that the catheter covers about 4 mm of the exposed flow moderator (Figure 2G).
- To ensure that the osmotic pump can work instantly after implantation, immerse the filled pumps in sterile 0.9% saline or PBS at 37 °C for at least 4 to 6 h (preferably extend to overnight) to pre-wet the semi-permeable membrane on the pump surface with solutions that have the same osmotic pressure as the tissue environment (Figure 2H).
- All solutions loaded into the pumps should be sterile. ALZET pumps are supplied sterile, having been exposed to a sterilizing dose of 60Co. However, If exterior contamination occurs, the surface of the pump can be cleaned by wiping it with isopropyl alcohol (70% in water).
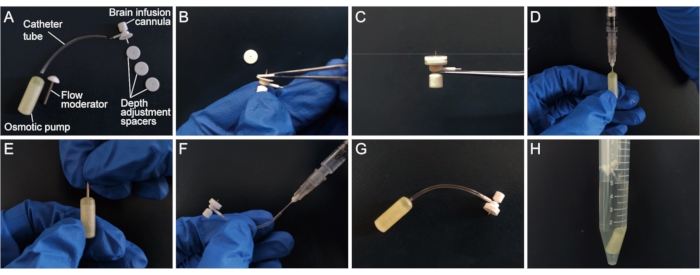
Figure 2: Preparation of the osmotic pump. (A) Key components of the osmotic pump. (B,C) Attach depth-adjustment spacers to the needle of the brain infusion cannula. (D) Fill the osmotic pump using a 1 mL syringe. (E) Insert the flow moderator into the pump. (F) Fill the catheter using the syringe. (G) Connect the catheter to the flow moderator. (H) Immerse the filled pumps in sterile 0.9% saline or PBS at 37 °C. Please click here to view a larger version of this figure.
3. Implantation of the osmotic pump
- Wait for 3 days after the establishment of the corpus callosum demyelination model. Turn on the small animal anesthesia system. Disinfect scissors, tweezers, and hemostatic pliers and soak them in 75% alcohol solution. Lay sterile sheets in the surgical area.
- Anesthetize and secure the mice on the stereotaxic apparatus again. Cover the surface of the eyeballs with an eye ointment to prevent dryness.
- Disinfect the original wound with 75% alcohol. Open the surgical incision that was previously stitched (Figure 3A) and expand the incision to the shoulder blades (Figure 3B).
- Separate the skin from the subcutaneous connective tissue with hemostatic pliers or tweezers at the scapula to open a cavity (Figure 3C). Place the osmotic pump into the cavity (Figure 3D,E).
- With a cotton swab, gently wipe and expose the pinhole on the surface of the skull created when establishing the demyelination model (see step 1.8). Insert the brain infusion cannula through this pinhole perpendicularly and secure it on the skull quickly with tissue adhesive (Figure 3F).
- Remove the removable tab above the brain infusion cannula with a pair of scissors (Figure 3G, H). Alternatively, remove the tab first before inserting the cannula to avoid shaking in this process.
- Stitch the incision or attach it with tissue adhesive (Figure 3I).
- After surgery, place the mouse on a heating pad to avoid a body temperature drop. Administer a subcutaneous injection of 5 mg/kg carprofen every 24 h to relieve the pain. Apply erythromycin ointment to the incision every day to ensure that the wound heals properly. Place the animal in a cage alone and feed with moist food until fully recovered. Monitor the mice every day and check whether the brain infusion cannula was firmly attached.
- Euthanize the mouse 11 days after the surgery by injecting 150-200 mg/kg Pentobarbital sodium intraperitoneally followed by perfusing transcardially with 4% formaldehyde.
- To verify that the solution is delivered normally, carefully remove the osmotic pump and measure the residual volume in the pump reservoir before brain dissection.
- To measure the residual volume, remove the brain infusion cannula, attach a 1 mL syringe to the catheter, and then aspirate the remaining solution to determine its volume. Compare the actual residual volume to the theoretical residual volume (initial volume – mean pumping rate * infusion duration).
NOTE: Excessive residual volume indicates unsuccessful infusion, which might be due to catheter occlusion or pump malfunction.
- To measure the residual volume, remove the brain infusion cannula, attach a 1 mL syringe to the catheter, and then aspirate the remaining solution to determine its volume. Compare the actual residual volume to the theoretical residual volume (initial volume – mean pumping rate * infusion duration).
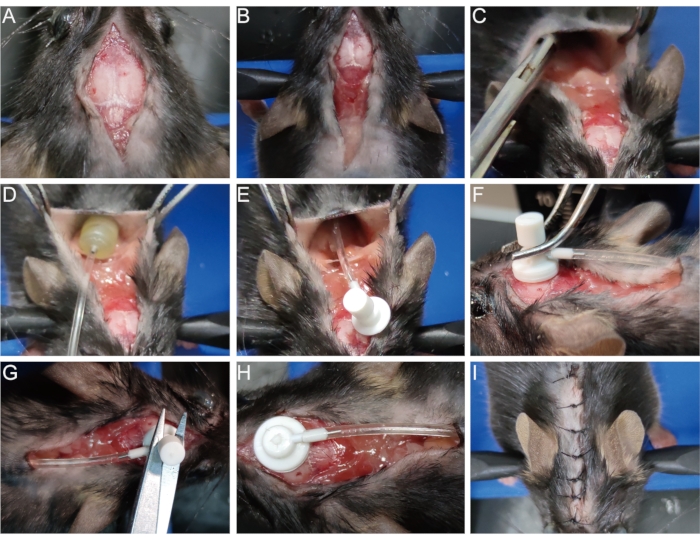
Figure 3: Implantation of the osmotic pump. (A) Open the surgical incision. (B) Expand the incision to the shoulder blades. (C) Separate the skin from subcutaneous connective tissue to make a cavity. (D,E) Place the osmotic pump into the cavity. (F) Insert the brain infusion cannula in the pinhole on the surface of the skull and firmly secure it on the skull. (G,H) Remove the removable tab from the cannula. (I) Stitch the incision. Please click here to view a larger version of this figure.
Representative Results
To verify the effect of the osmotic pump in myelin regeneration research, a lysolecithin-induced demyelination model was created in P56 mice, followed by implantation of osmotic pumps containing UM206 (1 mg in 1.5 mL 0.9% saline), a peptide with a short half-life and poor BBB permeability that has been recently reported to promote remyelination10. 0.9% saline was used as the control. Fourteen days after the model establishment, mice were transcardially perfused with 4% formaldehyde to isolate the brains for sectioning, followed by in situ hybridization and transmission electron microscopy to evaluate the remyelination level.
Staining of DAPI revealed the pinhole in the brain tissue just above the white matter, indicating successful implantation of the brain infusion cannula of the osmotic pump (Figure 4A). In the in-situ hybridization experiment, the mature oligodendrocyte marker MAG probe was used to label newly differentiated oligodendrocytes as shown in previous studies10,11,12. The results showed that the UM206 treatment yielded more MAG-positive cells in the demyelinated region than the control group (Figure 4B). Transmission electron microscopy of the demyelinated region also showed that the number of myelinated axons was increased in the UM206 treatment group compared to the control group (Figure 4C), suggesting that UM206 induced a higher level of remyelination. These results show that the osmotic pump can efficiently deliver drugs to the corpus callosum in the remyelination research.
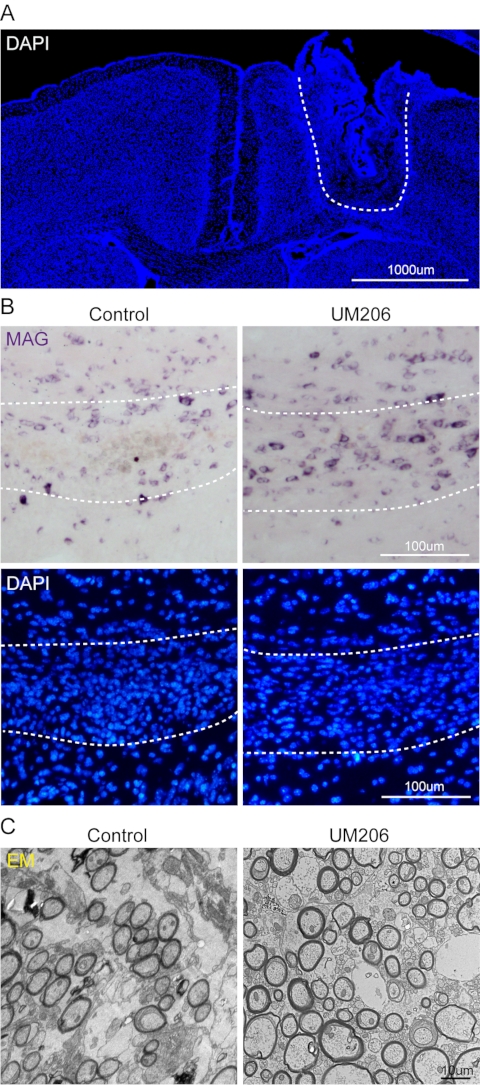
Figure 4: Representative results. (A) Representative image of DAPI-stained slice showing the pinhole in the brain tissue. Scale bar: 1,000 µm. (B) Representative images showing in situ hybridization of MAG in the demyelinated region as shown by DAPI staining. UM206 treatment increased the number of MAG-labeled oligodendrocytes. Scale bar: 100 µm. (C) Representative transmission electron microscopy images of the demyelinated region. UM206 treatment increased the number of myelinated axons. Scale bar: 10 µm. Please click here to view a larger version of this figure.
Discussion
This protocol describes the osmotic pump as a novel drug delivery technique for myelin regeneration research, which can deliver drugs directly to the treatment site and allow consistent drug delivery for a prolonged period, creating a stable drug concentration in the micro-environment of the central nervous system in the whole experimental duration. Compared with other drug delivery methods, the osmotic pump is more conducive to maintaining drug concentration in the demyelination lesion13. For example, for certain neurotrophic factors, systemic medication cannot achieve any effect because of the low concentration of the drug at the lesion site. But if the dosage is increased, the side effects will be more significant14. In such cases, administering to a specific site through an osmotic pump can reduce peripheral side effects effectively15. In addition, many myelin regeneration-related drugs have poor blood-brain barrier (BBB) permeability or display a short in vivo half-life due to susceptibility to proteolytic degradation. These problems could be well addressed by osmotic pumps.
However, the osmotic pump method is not without caveats and limitations. First, being an invasive drug delivery system, it inevitably causes brain tissue damage and neuroinflammation at the brain infusion cannula insertion site, which might obscure the drugs' effect. Thus, a proper solvent-only control group must be set up. Second, some drugs require solvents like dimethyl sulfoxide (DMSO), N-methyl-2-pyrrolidone (NMP) to dissolve, but these solvents are incompatible with the reservoir material and can cause a significant failure of the pumps. For example, high concentrations of dimethyl sulfoxide (DMSO) and PEG400 have been shown to adversely affect pump release and may not be suitable for use in osmotic pumps16,17,18. Third, drugs that are unstable at 37 °C might not be suitable for long-term infusion using the osmotic pump. All these issues are worthy of attention if planning to apply the osmotic pump.
Several steps in this protocol require extra attention during the experiments. For the normal operation of the osmotic pumps, researchers must ensure that the osmotic pump is assembled correctly and that no bubble is introduced into the pump, which will otherwise greatly undermine the infusion efficiency. In addition, catheter occlusion or osmotic pump malfunction may cause infusion failure19, which could be determined by the measurement of the residual volume in the pump reservoir after the experiment. For the application of the osmotic pump in younger mice with smaller brain sizes, a trial experiment is recommended to ensure a suitable depth of insertion. Furthermore, the brain infusion cannula must be firmly secured on the skull to minimize its movement during infusion.
At present, many in vitro studies have found a variety of drugs that can promote myelin regeneration, but due to poor BBB permeability, short half-life, and other problems, these drugs are difficult to be successfully validated in vivo. Therefore, the osmotic pump is of great value to the field of central nervous system myelin regeneration research, especially relevant for those drugs with a short half-life, poor BBB permeability, and obvious peripheral side effects.
Divulgazioni
The authors have nothing to disclose.
Acknowledgements
This work was supported by grants from the National Nature Science Foundation of China (NSFC 32070964, 31871045) to J.N. and the Shenzhen Basic Research Foundation (JCYJ20210324121214039) to Y.S.
Materials
| Anesthesia Air Pump | RWD | R510-29 | E05818-006 |
| Brain Infusion kit 3 | ALZET | 0008851 | 1-3 mm |
| Carprofen | Macklin | C830557-1g | 5 mg/kg every 24 h |
| Erythromycin eye ointment | Along technology | YCKJ-RJ-024780 | Cover the surface of the eyeballs during anesthesia |
| Erythromycin ointment | pythonbio | RG180 | |
| Gas Evacuation Apparatus | RWD | R546W | E05518-002 |
| L-α-Lysophosphatidylcholine | Sigma | L0906 | Dissolve at 1% with sterile PBS |
| Microliter Syringe | Hamilton | 65460-05 | Syringe Series:1700, 10 µL, 33 gauge |
| Micro-smotic pump model 1002 | ALZET | 0004317 | 0.25 µL per hour, 14 days |
| PBS (pH = 7.3) | ORIGENE | ZLI-9061 | |
| Pentobarbital sodium | Shanghai Civi | CAS NO: 57-33-0 | 150-200 mg/kg intraperitoneal injection for euthanasia |
| Small Animal Anesthesia Machine | RWD | R520IE | E05807-006 M |
| Stereotaxic Equipment | RWD | E06382 | |
| STERI 250 sterilizer | Keller | 31101 | Rapid sterilization of surgical instruments |
| Surgical sutures | Shanghai jinhuan | F504 | 5-0 |
| Syringe needle (1 mL) | Shanghai KDL | 6930197811018 | 26 gauge (0.45 mm x 16 mm) |
| Testing drug and solvent | Experiment dependent | N/A | |
| ThermoStar Homeothermic Monitoring System | RWD | 69026 | Maintain body temperature during anesthesia |
| Vetbond Tissue adhesive | 3M | 1469SB | Secure the brain infusion cannula , Adhere the skin incision |
Riferimenti
- Theeuwes, F., Yum, S. I. Principles of the design and operation of generic osmotic pumps for the delivery of semisolid or liquid drug formulations. Annals of Biomedical Engineering. 4 (4), 343-353 (1976).
- Herrlich, S., Spieth, S., Messner, S., Zengerle, R. Osmotic micropumps for drug delivery. Advanced Drug Delivery Reviews. 64 (14), 1617-1627 (2012).
- Wei, E., Loh, H. Physical dependence of opiate-like peptides. Science. 193 (4259), 1262-1263 (1976).
- Pettigrew, J. D., Kasamatsu, T. Local perfusion of noradrenaline maintains visual cortical plasticity. Nature. 271 (5647), 761-763 (1978).
- Wang, Y., et al. Reduced oligodendrocyte precursor cell impairs astrocytic development in early life stress. Advanced Science (Weinheim). 8 (16), 2101181 (2021).
- Tang, C., et al. Neural stem cells behave as a functional niche for the maturation of newborn neurons through the secretion of PTN. Neuron. 101 (1), 32-44 (2019).
- Watanabe, S., Komine, O., Endo, F., Wakasugi, K., Yamanaka, K. Intracerebroventricular administration of Cystatin C ameliorates disease in SOD1-linked amyotrophic lateral sclerosis mice. Journal of Neurochemistry. 145 (1), 80-89 (2018).
- DeVos, S. L., Miller, T. M. Direct intraventricular delivery of drugs to the rodent central nervous system. Journal of Visualized Experiments: JoVE. , e50326 (2013).
- Tang, C., Guo, W. Implantation of a mini-osmotic pump plus stereotactical injection of retrovirus to study newborn neuron development in adult mouse hippocampus. STAR Protocols. 2 (1), 100374 (2021).
- Niu, J., et al. Oligodendroglial ring finger protein Rnf43 is an essential injury-specific regulator of oligodendrocyte maturation. Neuron. 109 (19), 3104-3118 (2021).
- Breitschopf, H., Suchanek, G., Gould, R. M., Colman, D. R., Lassmann, H. In situ hybridization with digoxigenin-labeled probes: sensitive and reliable detection method applied to myelinating rat brain. Acta Neuropathologica. 84 (6), 581-587 (1992).
- Cree, B. A. C., et al. Clemastine rescues myelination defects and promotes functional recovery in hypoxic brain injury. Brain. 141 (1), 85-98 (2018).
- Eckenhoff, B., Yum, S. I. The osmotic pump: novel research tool for optimizing drug regimens. Biomaterials. 2 (2), 89-97 (1981).
- Thoenen, H., Sendtner, M. Neurotrophins: from enthusiastic expectations through sobering experiences to rational therapeutic approaches. Nature Neuroscience. 5, 1046-1050 (2002).
- Hagg, T. Intracerebral infusion of neurotrophic factors. Methods in Molecular Biology. 399, 167-180 (2007).
- Bittner, B., Thelly, T., Isel, H., Mountfield, R. J. The impact of co-solvents and the composition of experimental formulations on the pump rate of the ALZET osmotic pump. International Journal of Pharmaceutics. 205 (1-2), 195-198 (2000).
- Arnot, M. I., Bateson, A. N., Martin, I. L. Dimethyl sulfoxide/propylene glycol is a suitable solvent for the delivery of diazepam from osmotic minipumps. Journal of Pharmacological and Toxicological Methods. 36 (1), 29-31 (1996).
- Gullapalli, R., et al. Development of ALZET osmotic pump compatible solvent compositions to solubilize poorly soluble compounds for preclinical studies. Drug Delivery. 19 (5), 239-246 (2012).
- White, J. D., Schwartz, M. W. Using osmotic minipumps for intracranial delivery of amino acids and peptides. Methods in Neurosciences. 21, 187-200 (1994).

