High-frequency Ultrasonography of the Digital and Palmar Nerve Branches of the Hand in Peripheral Nerve Diseases
Summary
The present protocol describes high-frequency neuromuscular ultrasonography of the digital and palmar branches of the median and ulnar nerve, which can aid in localizing peripheral nerve diseases and be adapted to evaluate digital nerve injuries.
Abstract
Peripheral nerve ultrasound is a well-established imaging technique to evaluate certain peripheral nerve pathologies. However, there is a poor correlation between ultrasound abnormalities of peripheral nerves and electrodiagnostic or clinical evidence of axonal loss. This is a significant limitation of peripheral nerve ultrasound, as many peripheral nerve diseases encountered in clinical settings are related to axonal loss. Furthermore, clinical and electrodiagnostic evidence of axonal loss directly correlates with disability in all peripheral nerve diseases. However, due to the floor effects often encountered in electrodiagnostic studies, these correlations, as well as definitive diagnoses, are often challenging. Thus, imaging techniques that correlate with axonal loss are essential for expanding the utility of peripheral nerve ultrasound as a potential biomarker for peripheral nerve diseases. With new technological advancements and the ever-increasing imaging capabilities of high-frequency ultrasound, the palmar and digital nerve branches of the hand can be imaged with exceptionally high resolution even using point-of-care ultrasound devices. Their superficial and distal-most anatomic locations are ideal for evaluating polyneuropathies, as these branches degenerate earliest during axonal loss. However, no studies have systematically evaluated these nerve branches to determine if they can be reproducibly measured with ultrasound. The current protocol was adapted for the systematic assessment of cross-sectional areas of the median and ulnar nerves in the palmar surface and digits of the hand. This protocol provides reference data for a subset of nerves that demonstrate high intraclass correlation coefficients between three separate ultrasonographers. Finally, as a proof of concept and to demonstrate the clinical applications of this protocol, representative data from individuals with genetically confirmed inherited polyneuropathies are compared with established normative data to examine cross-sectional area differences.
Introduction
The expansion of clinical ultrasound to evaluate peripheral nerves and muscles has substantially improved the ability to diagnose neuromuscular disorders1. Over the past 2 decades, ultrasound has emerged as a tool to directly image anatomical changes in the neuromuscular system, which correlate with pathological processes. Ultrasound is most commonly combined with clinical history and examination to provide further detail or support electrodiagnostic studies, which are considered a gold standard equivalent for diagnosing peripheral nerve disease2. In some cases of focal neuropathies such as carpal tunnel syndrome, ultrasound can be used in lieu of electrodiagnostic results with high sensitivity and specificity3. Due to its low cost, its ability to be performed at the bedside, and its non-invasive properties, ultrasound is the preferred imaging modality for the neuromuscular system for many clinicians4,5.
Peripheral nerve ultrasound has been explicitly proven invaluable for the localization of peripheral nerve diseases caused by abnormalities in myelin, such as chronic immune demyelinating polyneuropathies (CIDP)6,7 and Charcot-Marie-Tooth disease type 1A (CMT1A)7,8. In these diseases, the focal or diffuse cross-sectional area enlargements of nerves in the upper and lower extremities are well described. However, cross-sectional area enlargement is not specific to demyelinating diseases, as it has also been described in axonal polyneuropathies, albeit sparsely8. However, the cross-sectional enlargement in axonal diseases is significantly less robust and is non-uniform throughout the nerve. Due to these challenges, the utility of ultrasound in axonal neuropathies is limited.
Most peripheral nerve ultrasound studies have focused on imaging relatively proximal nerve locations primarily due to the larger nerve size, which makes identification more straightforward. However, the distal-most branches of peripheral nerves degenerate earliest in a Wallerian-like fashion during axonal loss in polyneuropathies9,10. Due to their smaller diameters, the imaging resolution has been a limiting factor for the reproducible imaging of these nerve branches. Recently, transducer resolution has improved sustainably due to more rapid and seamless image compounding techniques. Now, structures of approximately 500 µm can be routinely imaged with point-of-care ultrasound, and structures with sizes as low as 30 µm can be imaged using ultra-high frequency systems11. Thus, it is conceivable that the distal nerve branches in the feet and hands could be reliably evaluated with point-of-care ultrasound.
The palmar and digital nerve branches of the hand are the distal-most branches of the median, radial, and ulnar nerves. The palmar branches carry motor nerves (median and ulnar only) to the interossei muscles, in addition to the digital sensory nerves12. In cadaver studies, the palmar and digital branches measure between 0.8 mm and 2.1 mm in diameter13, which is well within the detection range for high-frequency ultrasound transducers. In addition, their superficial location allows for high- and ultra-high frequency imaging because of the minimal frequency loss through connective tissues or muscles. However, no studies have established normative cross-sectional areas of the digital or palmar nerve branches of the hand using ultrasound, which are necessary to allow for clinical and research studies. Therefore, the present protocol evaluates the palmar and digital nerve branches in hand.
Technical considerations
The principles of neuromuscular-focused ultrasound must be reviewed as a foundation before starting this protocol14. In addition, several specific considerations were made for the current protocol. A transducer with a small footprint and frequency above 15 MHz is recommended, given the hand's natural contours. A 10-22 MHz transducer, with a footprint of 8 mm x 13 mm (see Table of Materials), was used with a compatible digital ultrasound system.
The next considerations are imaging depth and focal zones. In all the present imaging studies, the palmar and digital nerves were less than 0.35 cm in depth. Thus, using a consistent depth of 1 cm is recommended for reproducibility. Furthermore, improved imaging can be achieved at this depth by placing two focal zones at the device's maximal heights.
Consistent image adjustments (frequency, gain, and grey maps) are strongly recommended. With the minimal superficial tissues overlying and surrounding the nerves, adjustments in these parameters during imaging will not improve the imaging resolution or quality. Given the small diameters of these nerves, utilizing secondary image analysis software such as ImageJ15,16 for cross-sectional area measurements is recommended.
Protocol
All experiments in this study were performed in compliance with the Wayne State University and Detroit Medical Center Institutional Review Boards (IRB) under an approved protocol for the natural history of individuals with peripheral neuropathies. Informed consent was obtained from all human participants.
1. Instrumental setup
- Enter the patient's name or other identifiers as needed to organize the captured images.
- Sanitize the ultrasound probe (see Table of Materials) and apply ample ultrasound gel to the head of the transducer before imaging.
- Set the capture frequency to the highest frequency allowed by the ultrasound machine that does not require further image compounding or contrast.
NOTE: For the present study, 20 MHz was used. - Set the total imaging or screen depth to 1 cm, with two focal positions as superficial as possible.
NOTE: These depth and focal locations allow imaging of all the palmar and digital divisions of the median and ulnar nerves in the hand. Once determined by the examiner's preference, a consistent gain and grey map is recommended for the reproducibility of the measurements.
2. Patient preparation
NOTE: The patient inclusion criterion for inherited peripheral neuropathy was a confirmed mutation in a gene known to cause inherited peripheral neuropathy (please see the representative results section), with no exclusion criteria. For controls, the inclusion criterion was a normal result from electrodiagnostic testing of the upper extremities. Control exclusion criteria included a history or current diagnosis of diabetes mellitus, thyroid dysfunction, any known vitamin abnormalities, previous bariatric surgery, metabolic syndrome, a body mass index greater than 29, a history of traumatic nerve injury, or a history of large or small fiber peripheral neuropathy.
- Place the patient in a supine position with the elbow extended and the wrist supinated such that the dorsal surface of the forearm is resting comfortably on the table.
NOTE: Before positioning, examine the hand and arm that will be evaluated as any wounds, rashes, or skin irritation near or over the areas to be imaged are relative contraindications and may preclude ultrasound imaging. - Extend the patient's wrist and metacarpals so that the fingernails contact the table's surface. Allow the thumb to be slightly adducted and flexed for comfort.
3. Ultrasound examination
NOTE: There are four common palmar branches of the median nerve and two common palmar branches of the ulnar nerve13. Each digit has a medial and lateral digital branch, with digits 1-3 being purely median innervated and digit 5 being purely ulnar innervated. Digit 4 is dually innervated by the median nerve on the lateral surface and the ulnar nerve on the medial surface. This protocol concentrates on imaging the median common palmar nerve to digit 2, the lateral digital branch to digit 2, the ulnar common palmar branch to digit 5, and the medial branch to digit 5.
- Start by identifying the median common palmar branch to digit 2 using the lateral transverse flexor palmar crease as the landmark (Figure 1).
NOTE: The median common palmar branch to digit 2 and the ulnar common palmar branch to digit 5 are significantly larger than the accompanying blood vessels when compared to the digital nerves. Thus, identifying these nerves first can assist with identification in more distal branches, which are more similar in cross-sectional area to the blood vessels. - Place the transducer proximal to the radial flexor palmar crease (Figure 1).
NOTE: In the present study, individuals without a radial flexor palmar crease were not encountered. However, in the case of an absent palmar crease, place the transducer 2 cm proximal to the base of the second metacarpal. - Hold the transducer perpendicular to the expected course of the nerve with as little pressure as possible while still ensuring complete contact with the transducer and skin.
NOTE: Given the superficial location of the palmar and digital branches, they are susceptible to pressure distortions from the transducer. - Adjust the angle of the transducer such that the smallest cross-sectional area with a uniform epineurium is identified. This technique lessens the likelihood of out-of-plane imaging, which can alter results.
- Optimize the image by making gentle back and forth movements of the transducer to minimize anisotropy of the nerve.
NOTE: Anistrophy refers to the variance in the reflected ultrasound waveforms based on the angle of the transducer17. Adjusting the transmitted wave angle creates reflective waveforms that are out of plane and not received by the ultrasound transducer, leading to hypoechogenicity (or an increase in the black signal) of a structure. Nerves have relatively low anisotropy, meaning significant angle changes are required to create hypoechogenicity of the nerve. In comparison, muscles and tendons have relatively high anisotropy. By performing small back and forth motions with the transducer, the angular limits in which anisotropy arises can help identify the proper plane for nerve imaging18. - If possible, utilize Power Doppler imaging (PDI, see Table of Materials) to identify any blood vessels nearby (perform at all locations).
- Once optimized, capture the image at this location. Mark the nerve within the ultrasound system prior to saving so that the nerve can be located for further measurement. Label the image with the nerve name, location, and side.
- Next, image the lateral digital branch of digit 2 at the metacarpophalangeal joint by advancing the transducer distally toward the end of the digit 2. Then, stop the transducer just distal and lateral to the center of the flexor crease of the second metacarpophalangeal joint.
- Once optimized, capture the image at this location. Mark the nerve within the ultrasound system before saving so that the nerve can be located for further measurement. Label the image with the nerve name, location, and side.
- To evaluate for focal pathologies or non-uniform cross-sectional enlargements of the palmar or digital branches to digit 2, advance the transducer distally toward the end of the digit 2.
NOTE: Digital nerves can be adequately visualized within 1.5-2 cm proximal to the distal interphalangeal joint (DIP). - Then, from the most distal point from which the branch can be visualized, follow the nerve proximally to the common median nerve just distal to the carpal tunnel.
- Next, image the ulnar common palmar branch to digit 5 by identifying the ulnar transverse palmar crease (Figure 1).
- Place the transducer perpendicular to the expected course of the nerve and adjust the imaging as described in steps 3.3.-3.6.
- Once optimized, capture the image at this location. Mark the nerve within the ultrasound system prior to saving so that the nerve can be located for further measurement. Label the image with the nerve name, location, and side.
- Next, image the medial branch at the metacarpophalangeal (MCP) joint by advancing the transducer toward the end of the digit 5, stopping just distal to the flexor crease of the MCP.
- Optimize the image as previously mentioned in steps 3.3.-3.6.
- Once optimized, capture the image at this location. Mark the nerve within the ultrasound system prior to saving so that the nerve can be located for further measurement. Label the image with the nerve name, location, and side.
- Evaluate for focal or segmental cross-sectional abnormalities along digit 5. By identifying the distal-most location, visualize the nerve and scan proximally back to Guyon's canal.
NOTE: Guyon's canal, also known as the ulnar canal or tunnel, is located on the medial aspect of the hand/wrist where the ulnar nerve and artery pass. The borders of Guyon's canal include the superficial carpal ligament superiorly, the flexor retinaculum and hypothenar muscles inferiorly, the pisiform and pisohamate ligament medially, and the hook of hamate laterally19. - Perform all measurements on both sides.
NOTE: For the present study, for individuals with a BMI of less than 33, imaging of the entire course of the median and ulnar nerve back into the brachial plexus was possible with the transducer used here. Although this protocol only focuses on a limited number of nerves, in the case of focal or traumatic neuropathies (for which the evaluation of nerves is not shown in this protocol), it is recommended to use the distal heads of the metacarpals as a starting point for tracing and evaluating other digital and palmar nerves. - Save all the images and export them to a mass storage device. If using ImageJ, export the images as .jpg files.
4. Measuring cross-sectional area
NOTE: ImageJ, an open-source image processing software (see Table of Materials), was utilized for the present study, and the steps below are adapted for this software.
- Open ImageJ software.
- Select File in the program interface and click on Open. Navigate to the directory where the ultrasound images are stored.
- Select the .jpg associated with the nerve to be measured.
- Set the scale for the image by selecting the line tool and using the scale bar on the original ultrasound image to draw a 1 cm straight line. Click on Analyze and choose Set Scale.
NOTE: The distance in pixels is automatically calculated from the 1 cm line and is auto-populated in the first box. To output measurements in millimeters squared (mm2), change the known distance to 10. Close the set scale box. - Use the freehand selection tool to outline the nerve at the border of the epineurium and surrounding tissues (Figure 2, yellow line).
NOTE: The zoom function can be used in ImageJ to determine the exact pixels separating the epineurium from the surrounding structures for more precise measurements. - Measure the cross-sectional area by clicking on Analyze and choosing Measure.
Representative Results
For the normative data, 20 individuals were selected with normal electrophysiology results, no neurological complaints, past medical history of or current diabetes mellitus, thyroid dysfunction, vitamin abnormalities, metabolic syndrome, carpal or cubital tunnel syndrome, exposure to chemotherapeutics, or severe hand trauma, and who had not been pregnant within the last 1 year (Table 1). Given the small subset, we did not stratify our data by age, gender, weight, or height, all of which are known to affect nerve cross-sectional area14,20. Thus, this data can best be applied as a reference point for other laboratories to compare with. The upper limits of normal nerve cross-sectional area were calculated as the mean cross-sectional area plus two standard deviations.
Cross-sectional nerve enlargement in proximal portions of the peripheral nerves has been described as an ultrasound feature of demyelinating polyneuropathies21,22,23. Cross-sectional area enlargement greater than the upper limit of normal nerve cross-sectional area was found in an individual with Charcot-Marie-Tooth disease 1A (CMT1A), an inherited demyelinating disease (Figure 3B, Figure 4B, Figure 5B, Figure 6B). Similarly, enlargement in proximal nerve regions was seen uniformly throughout the median and ulnar nerves up to the axilla in this individual, as described by others7,8.
In an individual with CMT2A (Figure 3C, Figure 4C, Figure 5C, Figure 6C), an inherited axonal polyneuropathy, bilateral enlargement of the common medial branch to digit 2 was found. However, no other areas of cross-sectional enlargement were found along the course of the median or ulnar nerves. Electrophysiology studies confirmed mild axonal loss in both the median and ulnar nerves. This subtle nerve cross-sectional enlargement phenomenon was described previously in certain axonal peripheral nerve pathologies but is poorly understood24.
One individual with hereditary neuropathy with liability to pressure palsies (HNPP) also had only enlargement of the median lateral palmar branch to digit 2 (Figure 3D) and was not found to have enlargement at any other site in the bilateral median or ulnar nerves. Additionally, median and ulnar nerve electrophysiology showed no abnormalities despite the second digit's symptomatic and clinical evidence of sensory loss. Given the suspicious clinical history, a family history of similar symptoms combined with focal nerve enlargement on ultrasound genetic testing was requested, which demonstrated a deletion of a single copy of PMP2225, confirming the diagnosis of HNPP.
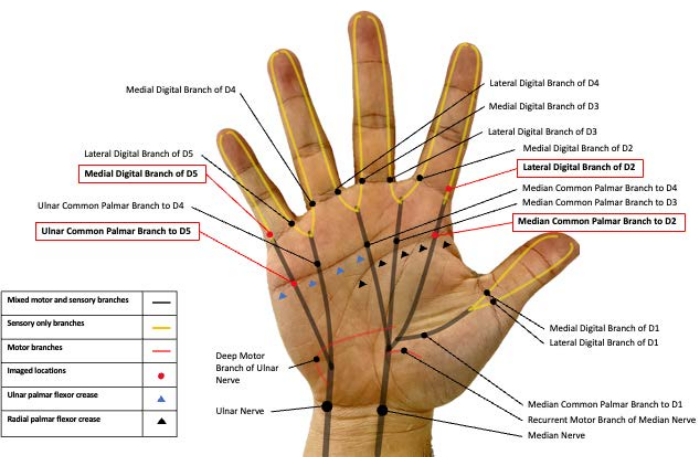
Figure 1: Peripheral nerve branches in the palmar surface of the hand. Representative image of the median and ulnar nerve branches in hand. The nerves imaged in the present study are highlighted in red boxes. D2: Digit 2, D5: Digit 5. Please click here to view a larger version of this figure.
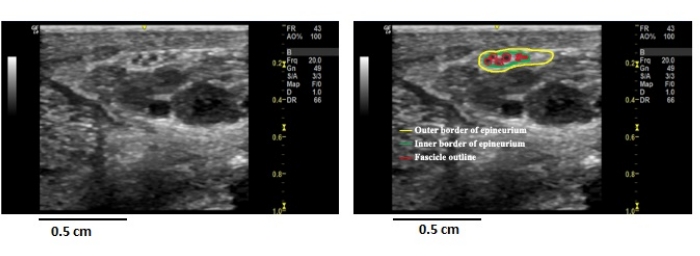
Figure 2: Ultrasound anatomy of the peripheral nerve. Image from a healthy individual's left common palmar nerve to digit 2 (left image) compared to an outlined image showing the epineurium and fascicles (right image). One can ensure the transducer is perpendicular (as close to 90° as possible) to the nerve being imaged by ensuring uniformity in these structures. Please click here to view a larger version of this figure.
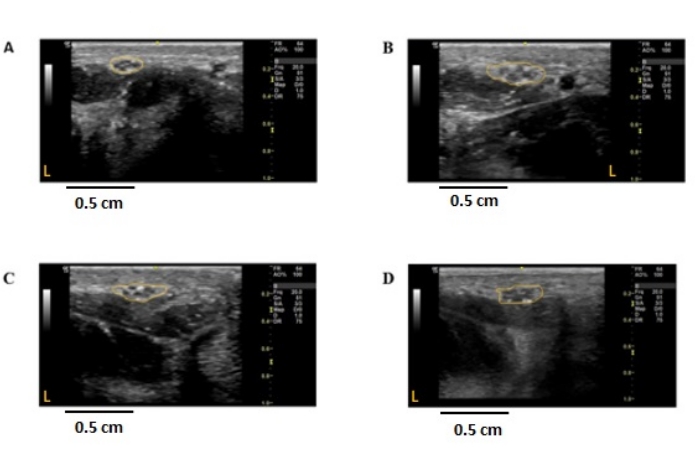
Figure 3: Ultrasound images of the non-dominant median common palmar nerve to digit 2 in individuals with peripheral nerve diseases. Cross-sectional, 2-dimensional images were obtained at the radial palmar flexor crease of the non-dominant hand. The nerve is outlined in yellow, demarcating the outer epineural border. The cross-sectional area was measured using ImageJ. (A) Control; CSA = 2.29 mm2. (B) CMT1A individual; CSA = 6.02 mm2. (C) CMT2A individual; CSA = 3.67 mm2. (D) HNPP individual; CSA = 4.09 mm2. Orange L represents the left side of the image concerning the nerve imaged. CSA: cross-sectional area, CMT1A/2A: Charcot-Marie-Tooth disease 1A/2A, HNPP: hereditary neuropathy with pressure palsies. Please click here to view a larger version of this figure.
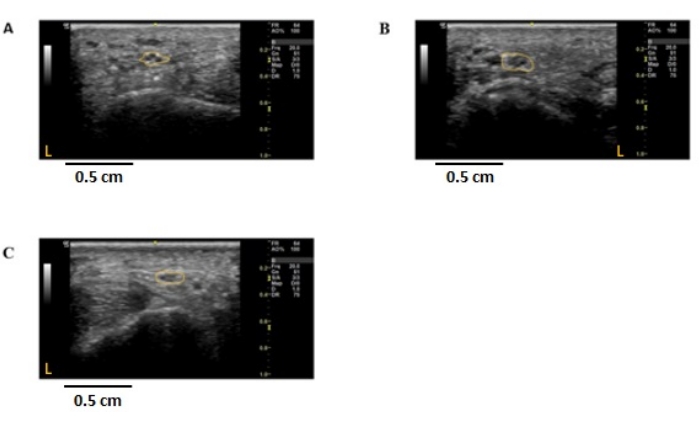
Figure 4: Ultrasound images of the non-dominant lateral digital branch to digit 2 in individuals with peripheral nerve diseases. Cross-sectional, 2-dimensional images were obtained from the radial side of digit 2 in the non-dominant hand, approximately 1 cm distal to the metacarpophalangeal joint of the non-dominant hand. The nerve is outlined in yellow, demarcating the outer epineural border. The cross-sectional area was measured using ImageJ. (A) Control, CSA = 1.48 mm2. (B) CMT1A individual; CSA = 2.49 mm2. (C) CMT2A individual; CSA = 1.46 mm2. Orange L represents the left side of the image concerning the nerve imaged. CSA: cross-sectional area, CMT1A/2A: Charcot-Marie-Tooth disease 1A/2A, HNPP: hereditary neuropathy with pressure palsies. Please click here to view a larger version of this figure.
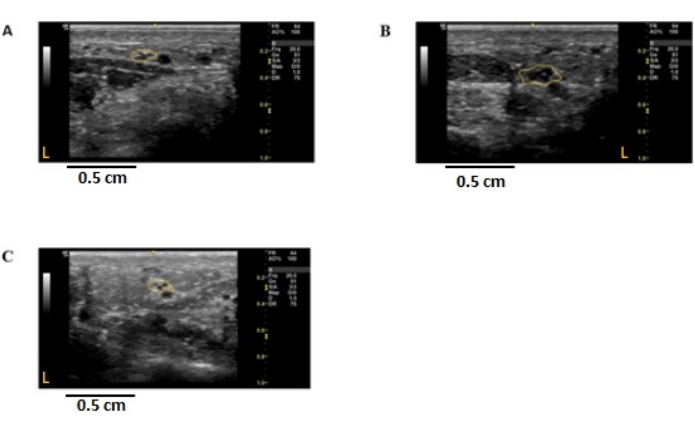
Figure 5: Ultrasound images of the ulnar common palmar branch to digit 5 in individuals with peripheral nerve disease. Cross-sectional, 2-dimensional images were obtained at the ulnar palmar crease of the non-dominant hand. The nerve is outlined in yellow, demarcating the epineural border. The cross-sectional area (CSA) was measured using ImageJ. (A) Control; CSA = 1.29 mm2. (B) CMT1A individual; CSA = 3.33 mm2. (C) CMT2A individual; CSA = 1.16 mm2. Orange L represents the left side of the image concerning the nerve imaged. CSA: cross-sectional area, CMT1A/2A: Charcot-Marie-Tooth disease 1A/2A. Please click here to view a larger version of this figure.
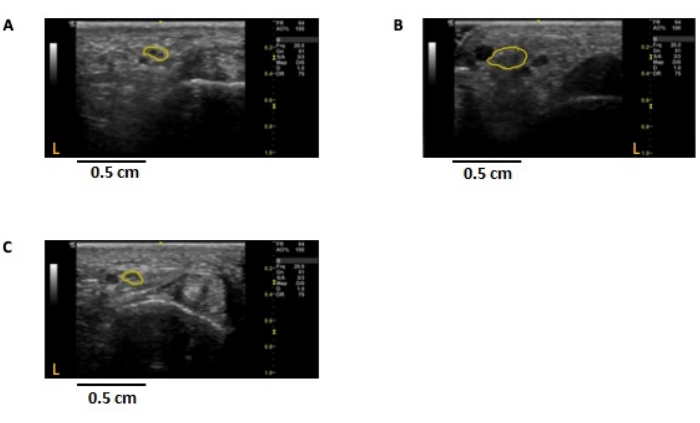
Figure 6: Ultrasound images of the medial digital branch to digit 5 in individuals with peripheral nerve disease. Cross-sectional, 2-dimensional images were obtained at the ulnar palmar crease of the non-dominant hand. The nerve is outlined in yellow, demarcating the epineural border. The cross-sectional area (CSA) was measured using ImageJ. (A) Control; CSA = 1.29 mm2. (B) CMT1A individual; CSA = 3.33 mm2. (C) CMT2A individual; CSA= 1.16 mm2. CSA: cross-sectional area, CMT1A/2A: Charcot-Marie-Tooth disease 1A/2A. Please click here to view a larger version of this figure.
| Demographic | Range | |
| N | 20 | |
| % Female | 50 | |
| Age (range) | 41 | (18-77) |
| Mean Height (cm) | 165.3 | (154.9-190.5) |
| Weight (kg) | 73.9 | (52.1 -99.7) |
| Mean CSA (mm2) (range) | Standard Deviation | Side to Side difference (mm2) | Intraclass Correlation Coefficent | Upper Limit (mm2) | |
| Median Common Palmar Branch to Digit 2 | 2.10 (1.57-2.38) | 0.39 | 0.16 (0.07-0.29) | 0.9 | 3.16 |
| Lateral Digital Branch to Digit 2 at MCP | 1.30 (0.89-1.58) | 0.34 | 0.10 (0.05-0.27) | 0.85 | 2.26 |
| Ulnar Common Branch to Digit 5 | 1.31 (0.75-1.56) | 0.38 | 0.10 (0.02-0.21) | 0.89 | 2.28 |
| Medial Digital Branch to Digit 5 at MCP | 0.92 (0.54-1.22) | 0.33 | 0.07 (0.01-0.17) | 0.83 | 1.88 |
Table 1: Demographics of control data and normative cross-sectional area for the median lateral common palmar nerve to digit 2, the lateral digital nerve to digit 2 at the MCP, the ulnar lateral common palmar nerve to digit 5, and the lateral digital nerve to digit 5 at the MCP. MCP: metacarpophalangeal joint, CSA: cross-sectional area. The upper limit is calculated as (maximum of the range) + (2 × [standard deviation]).
Discussion
The present protocol describes high-frequency ultrasound of the hand's digital and palmar nerve branches. This study was designed to test the hypothesis that cross-sectional area enlargement in distal nerve branches correlates with axonal loss. Extensive multicenter natural history studies of individuals with different subsets of axonal diseases will be needed to resolve this hypothesis. In addition to its potential research benefits, this protocol can also be applied clinically to localize peripheral nerve complaints.
The normative data here represent a small subset of healthy individuals who demonstrated no neurologic complaints and did not have any known disease of the peripheral nerves. With the enrollment limitations, these individuals represent a phenotypically homogenous group, which was ideal for testing the reproducibility of the current protocol. Due to the small diameters of these nerve branches, which approach the limits of point-of-care ultrasound, the variation in repeated measurements is critically important.
To test the reproducibility, each of the 20 individuals was examined by three separate ultrasonographers, one with 4 years of experience and the other two who had no prior ultrasound experience before a 2 week training course on this protocol. Each ultrasonographer was blinded to the results of the others. The intraclass correlation coefficients are shown in Table 1. These findings show slightly higher intraclass correlation coefficients reported in more proximal nerve locations. More importantly, these data demonstrate that point-of-care ultrasound can reproducibly measure nerve branches of 1-2 mm2.
To illustrate that this protocol could detect pathological processes, examples were taken from individuals with inherited peripheral polyneuropathies. These Mendelian inherited disorders26 are often symptomatically and pathologically length-dependent, referring to the distribution of symptoms and nerve pathology in the branches distal from the spinal cord27. In these diseases, the symptoms and nerve pathology ascend in a given nerve or limb over time. In light of the length-dependent pattern in these individuals, we hypothesized that, if the axonal loss correlates with cross-sectional area enlargement, it may be greater at the distal nerve branches.
The clinical cases presented cross-sectional enlargement in the digital and palmar branches in both demyelinating (CMT1A, HNPP) and axonal (CMT2A) polyneuropathies. The enlargement was more profound in the CMT1A individual, which is in line with reports7,28 of more proximal measurements of the median and ulnar nerve. Mild cross-sectional enlargement was seen only in the median common palmar branch to digit 2 in an individual with CMT2A, an axonal polyneuropathy, but not in the more distal, lateral digital branch to digit 2, which does not support the hypothesis. One explanation could be that individuals affected by inherited axonal polyneuropathies often have lower body mass indexes than the general population29. Due to this factor, further stratified normalized data is needed to improve the capabilities of this protocol both clinically and as a research tool.
As highlighted in the case of HNPP, this protocol can also be clinically valuable for further localization of peripheral nerve complaints. HNPP is an inherited polyneuropathy in which nerves are more susceptible to demyelination from constant or repetitive pressure, leading to focal demyelination26. Ultrasound studies in HNPP have shown cross-sectional area enlargement in locations susceptible to pressure or impingement, such as the carpal or cubital tunnels8,30. Palmar or digital branches are not commonly known to be invaded like the more proximal parts of the median or ulnar nerve. However, because of their extremely superficial location, it has been suggested that these branches may be more susceptible to repeated or prolonged pressure related to overuse of the digit31. These findings suggest that this protocol could also be valuable for localizing focal nerve damage in the palm and digits in HNPP or other traumatic nerve injuries.
Due to the protracted disease course in inherited polyneuropathies, complicated foot deformities are often encountered32. Due to this, the evaluation of digital branches in the feet would be severely limited and highly variable. This protocol focuses on the upper extremities to reduce these effects, as hand deformities are less common in peripheral diseases. Some individuals may not be symptomatic in the upper extremities early in their disease course because of the length-dependent nature of polyneuropathies. Therefore, the correlation of symptoms, electrophysiological pathology, and ultrasound findings in the upper extremities is imperative to expand this protocol further.
This protocol has limitations in its clinical application and as a research measure. First, anastomosis between the median and ulnar nerves in the hand is predicted to be relatively common; however, these are often too small or difficult to detect by nerve conduction studies33. Although no gross anastomosis was detected in this subset of individuals, variable contributions to the palmar branches may affect the cross-sectional area and correlations of the cross-sectional area with electrodiagnostic results. Additionally, this method relies on identifying palmar and wrist creases, which may have aberrant forms in small subsets of the population34,35.
In conclusion, this protocol represents a highly reproducible method to measure the hand's palmar and digital nerve branches. Furthermore, utilizing this protocol in a small subset of controls determined that these small nerve branches could be measured with high accuracy using point-of-care ultrasound. Lastly, this protocol expands the utility of ultrasound in localizing peripheral nerve disease by increasing the population of nerves for evaluation.
Divulgazioni
The authors have nothing to disclose.
Acknowledgements
This work was supported by the Wayne State University School of Medicine Departments of Neurology and Physical Medicine and Rehabilitation.
Materials
| 10-22mHz Transducer | General Electric Health Care | H48062AB | Small foot print transducer |
| ImageJ | NIH | N/A | https://imagej.nih.gov/ij/ |
| Logiq eR8 Ultrasound Beam Former | General Electric Health Care | H48522AS | This is the beamformer and image processor which includes Power Doppler Imaging |
| Ultrasound Gel | Parker Labratories | 44873 | Standard ultrasonoic gel, non sterile |
Riferimenti
- Gonzalez, N. L., Hobson-Webb, L. D. Neuromuscular ultrasound in clinical practice: A review. Clinical Neurophysiology Practice. 4, 148-163 (2019).
- Watson, J. C., Dyck, P. J. B. Peripheral neuropathy: A practical approach to diagnosis and symptom management. Mayo Clinic Proceedings. 90 (7), 940-951 (2015).
- Elnady, B., et al. Diagnostic potential of ultrasound in carpal tunnel syndrome with different etiologies: correlation of sonographic median nerve measures with electrodiagnostic severity. BMC Musculoskeletal Disorders. 20 (1), 634 (2019).
- Walker, F. O., et al. Indications for neuromuscular ultrasound: Expert opinion and review of the literature. Clinical Neurophysiology. 129 (12), 2658-2679 (2018).
- Hommel, A. L., Cartwright, M. S., Walker, F. O. The use of ultrasound in neuromuscular diagnoses. Neurology: Clinical Practice. 7 (3), 266-273 (2017).
- Merola, A., Rosso, M., Romagnolo, A., Peci, E., Cocito, D. Peripheral nerve ultrasonography in chronic inflammatory demyelinating polyradiculoneuropathy and multifocal motor neuropathy: Correlations with clinical and neurophysiological data. Neurology Research International. 2016, 9478593 (2016).
- Zanette, G., et al. Nerve ultrasound findings differentiate Charcot-Marie-Tooth disease (CMT) 1A from other demyelinating CMTs. Clinical Neurophysiology. 129 (11), 2259-2267 (2018).
- Schreiber, S., et al. Sonography of the median nerve in CMT1A, CMT2A, CMTX, and HNPP. Muscle & Nerve. 47 (3), 385-395 (2013).
- Prior, R., Van Helleputte, L., Benoy, V., Van Den Bosch, L. Defective axonal transport: A common pathological mechanism in inherited and acquired peripheral neuropathies. Neurobiology of Disease. 105, 300-320 (2017).
- Cashman, C. R., Höke, A. Mechanisms of distal axonal degeneration in peripheral neuropathies. Neuroscience Letters. 596, 33-50 (2015).
- Cartwright, M. S., Baute, V., Caress, J. B., Walker, F. O. Ultrahigh-frequency ultrasound of fascicles in the median nerve at the wrist. Muscle & Nerve. 56 (4), 819-822 (2017).
- Mitchell, C. H., Fayad, L. M., Ahlawat, S. Magnetic resonance imaging of the digital nerves of the hand: Anatomy and spectrum of pathology. Current Problems in Diagnostic Radiology. 47 (1), 42-50 (2018).
- Ortiz, R., et al. Nerve diameter in the hand: A cadaveric study. Plastic and Reconstructive Surgery Global Open. 7 (3), 2155 (2019).
- Fisse, A. L., Pitarokoili, K., Gold, R. Nerve ultrasound protocol to detect dysimmune neuropathies. Journal of Visualized Experiments. (176), e62900 (2021).
- Schneider, C. A., Rasband, W. S., Eliceiri, K. W. NIH Image to ImageJ: 25 years of image analysis. Nature Methods. 9 (7), 671-675 (2012).
- Sternberg, S. R. Biomedical image processing. Computer. 16 (1), 22-34 (1983).
- Connolly, D. J., Berman, L., McNally, E. G. The use of beam angulation to overcome anisotropy when viewing human tendon with high frequency linear array ultrasound. The British Journal of Radiology. 74 (878), 183-185 (2001).
- Suk, J. I., Walker, F. O., Cartwright, M. S. Ultrasonography of peripheral nerves. Current Neurology and Neuroscience Reports. 13 (2), 328 (2013).
- Depukat, P., et al. Anatomy of Guyon’s canal – A systematic review. Folia Medica Cracoviensia. 54 (2), 81-86 (2014).
- Grimm, A. -. S., et al. Normative observational nerve ultrasound values in school-age children and adolescents and their application to hereditary neuropathies. Frontiers in Neurology. 11, 303 (2020).
- Shen, J., Cartwright, M. S. Neuromuscular ultrasound in the assessment of polyneuropathies and motor neuron disease. Journal of Clinical Neurophysiology. 33 (2), 86-93 (2016).
- Cartwright, M. S., et al. Diagnostic nerve ultrasound in Charcot-Marie-Tooth disease type 1B. Muscle & Nerve. 40 (1), 98-102 (2009).
- Noto, Y., et al. Nerve ultrasound depicts peripheral nerve enlargement in patients with genetically distinct Charcot-Marie-Tooth disease. Journal of Neurology, Neurosurgery, and Psychiatry. 86 (4), 378-384 (2015).
- Carroll, A. S., Simon, N. G. Current and future applications of ultrasound imaging in peripheral nerve disorders. World Journal of Radiology. 12 (6), 101-129 (2020).
- Attarian, S., Fatehi, F., Rajabally, Y. A., Pareyson, D. Hereditary neuropathy with liability to pressure palsies. Journal of Neurology. 267 (8), 2198-2206 (2020).
- Li, J. Inherited neuropathies. Seminars in neurology. 32 (3), 204-214 (2012).
- Kramarz, C., Rossor, A. M. Neurological update: Hereditary neuropathies. Journal of Neurology. , (2022).
- Niu, J., Cui, L., Liu, M. Multiple sites ultrasonography of peripheral nerves in differentiating Charcot-Marie-Tooth type 1A from chronic inflammatory demyelinating polyradiculoneuropathy. Frontiers in Neurology. 8, 181 (2017).
- Donlevy, G. A., et al. Association between body mass index and disability in children with Charcot-Marie-Tooth disease. Neurology. 97 (17), 1727-1736 (2021).
- Bayrak, A. O., et al. Ultrasonographic findings in hereditary neuropathy with liability to pressure palsies. Neurological Research. 37 (2), 106-111 (2015).
- Cacciavillani, M., Padua, L., Gasparotti, R., Briani, C. HNPP: Not only entrapment sites. Ultrasound digital nerve abnormalities in a guitar player. Neurological Sciences. 37 (6), 999-1000 (2016).
- Joo, S. Y., et al. Foot deformity in Charcot Marie Tooth disease according to disease severity. Annals of Rehabilitation Medicine. 35 (4), 499-506 (2011).
- Smith, J. L., Siddiqui, S. A., Ebraheim, N. A. Comprehensive summary of anastomoses between the median and ulnar nerves in the forearm and hand. Journal of Hand and Microsurgery. 11 (1), 1-5 (2019).
- Afework, M. Prevalence of the different types of palmar creases among medical and dental students in Addis Ababa, Ethiopia. Ethiopian Journal of Health Sciences. 29 (3), 391-400 (2019).
- Sunilkumar, M. N. The enigma of the simian crease: Case series with the literature review. International Journal of Contemporary Pediatrics. 1 (3), 175-177 (2017).

.
