C. elegans Gonad Dissection and Freeze Crack for Immunofluorescence and DAPI Staining
Summary
This is a commonly used method for C. elegans gonad dissection followed by freeze crack, which produces germline samples for immunofluorescence via antibody staining, or for simple DAPI staining to visualize DNA. This protocol has been successful for undergraduates in a research lab and in a course-based undergraduate research experience.
Abstract
The C. elegans germline makes an excellent model for studying meiosis, in part due to the ease of conducting cytological analyses on dissected animals. Whole mount preparations preserve the structure of meiotic nuclei, and importantly, each gonad arm contains all stages of meiosis, organized in a temporal-spatial progression that makes it easy to identify nuclei at different stages. Adult hermaphrodites have two gonad arms, each organized as a closed tube with proliferating germline stem cells at the distal closed end and cellularized oocytes at the proximal open end, which join in the center at the uterus. Dissection releases one or both gonad arms from the body cavity, allowing the entirety of meiosis to be visualized. Here, a common protocol for immunofluorescence against a protein of interest is presented, followed by DAPI staining to mark all chromosomes. Young adults are immobilized in levamisole and quickly dissected using two syringe needles. After germline extrusion, the sample is fixed before undergoing a freeze crack in liquid nitrogen, which helps permeabilize the cuticle and other tissues. The sample can then be dehydrated in ethanol, rehydrated, and incubated with primary and secondary antibodies. DAPI is added to the sample in the mounting medium, which allows reliable visualization of DNA and makes it easy to find animals to image under a fluorescent microscope. This technique is readily adopted by those familiar with handling C. elegans after a few hours spent practicing the dissection method itself. This protocol has been taught to high-schoolers and undergraduates working in a research lab and incorporated into a course-based undergraduate research experience at a liberal arts college.
Introduction
Meiosis is the specialized cell division used to create gametes (eggs and sperm/pollen) in all sexually-reproducing organisms1,2. Crossover recombination is the reciprocal exchange of DNA between homologous chromosomes; it is essential for meiosis, both providing an important source of genetic diversity and promoting genome stability through generations. Chromosomes that fail to form at least one crossover during meiosis will segregate randomly, which can result in chromosome nondisjunction, creating gametes with the incorrect number of chromosomes – a condition that is usually fatal for resulting progeny3. During meiosis, crossovers are induced by programmed double-strand DNA breaks4. A subset of these breaks will be repaired as crossovers that provide physical linkages of DNA, called chiasmata, that help orient homologous chromosomes in preparation for cell division5. Meiotic stages are highly conserved across all eukaryotes, and their chromosomal conformation allows them to be easily identified.
As a fundamental concept in biology, meiosis is a topic that students encounter multiple times in different biology courses. They are often introduced to the mechanics of meiotic chromosome segregation in high school, while college-level courses focus on the cell biology of segregation and the genetic impact of crossover recombination. However, meiosis is a notoriously tricky concept for many students1. A failure to understand the relationship between genes, DNA, chromosomes, and meiosis can generate student misconceptions and gaps in understanding that impede a full understanding of genetic inheritance6,7. One way to improve student understanding of abstract topics is to provide concrete, hands-on activities. For example, when teaching meiosis, instructors can choose from activities that emulate molecular analysis8, 3D models that allow students to manipulate molecules9, or role-play where students themselves act out the molecular choreography1. Incorporating research with unknown outcomes is a particularly effective way of improving student understanding. This practice is known as a course-based undergraduate research experience (CURE) and has the added benefit of strengthening student attitudes and agency, especially for those belonging to groups that remain underrepresented in STEM10,11. The nematode worm Caenorhabditis elegans is particularly amenable for classroom studies of behavior, fertility, and genetic crosses, and is an effective model to introduce students to biological research12.
C. elegans makes a powerful model organism for cell biology by combining molecular genetics with simple cytological analysis. It is also particularly well-suited for use in a biology classroom 13,14,15. They are easy and economical to maintain in a lab, producing hundreds of progeny every 3 days, both at standard room temperature or at 20 °C, the most common incubation temperature. Importantly, they can be frozen as glycerol stocks and kept in a -80 °C freezer, which means that any husbandry mistakes made by novice researchers can be easily corrected16. Furthermore, its well-annotated genome allows for forward and reverse genetic techniques17,18, allowing C. elegans to be used to address biological questions ranging from the molecular to evolutionary. Finally, C. elegans researchers have created a supportive community that is often willing to provide help and advice for budding scientists19. These advantages have led to C. elegans being incorporated into a number of CUREs at various types of institutions12,19,20,21,22,23.
In addition to its benefits for research and teaching, C. elegans has become a popular model for studies of meiosis and germline development24,25,26. The optical clarity of these animals simplifies cytological approaches27, and in adults, gonads represent nearly half of the animal, providing hundreds of meiotic cells to study. In gonads, meiotic germline nuclei are arranged like an assembly line (Figure 1); mitotic replication occurs at the distal tip of the gonad, with nuclei progressing through meiotic stages as they migrate toward the proximal end of the gonad, where fertilized embryos emerge from the vulva. Because the stereotyped spatial organization also represents a temporal progression through meiosis, different stages can be easily identified based on their chromosomal organization and location in the gonad. Finally, processes that disrupt meiosis and cause aneuploidy create phenotypes that are straightforward to characterize, even for novices: sterility, embryonic lethality, or a high incidence of males (Him phenotype)28.
This is a simple protocol for visualizing meiotic chromosomes in C. elegans. Mounting, dissection, fixing, and antibody staining are all performed on the same microscope slide, which simplifies the protocol and allows near-perfect sample recovery. This method works for simple DAPI staining to visualize chromosomes and can be used for immunofluorescence to visualize the localization of proteins in the gonad. Students dissect gonads using basic dissecting microscopes, generate whole-mount preparations for visualization of DNA or immunofluorescence, and image them on a compound fluorescent microscope. This protocol has been taught to high-school students and undergraduates working in a C. elegans research lab and incorporated into a CURE at a liberal arts college12. Although the CURE had a relatively small class size, this protocol would be amenable for classes at a range of institutions due to the relatively low cost of worm strains and reagents. Instructors would only be limited by the number of dissecting microscopes available for use. The previous implementation had students working in groups of three to share a single microscope and took place over three 90-minute sessions: the first to practice dissection, the second to implement dissection and DAPI staining, and the third to image slides on a widefield fluorescence microscope. Participation in undergraduate research provides many benefits for students11,29, both academic and personal. Embedding research in courses via CUREs allows students to participate in research during normal class time11,30,31, which makes exposure to these benefits more accessible and equitable.
Protocol
1. C. elegans husbandry
NOTE: See C. elegans maintenance protocol16 and Elgin et al.32 for more detail. C. elegans strains can be easily acquired from the Caenorhabditis Genetics Center (https://cgc.umn.edu) and are shipped through regular mail to any location in the US. Each strain costs $10, and each lab/user pays an annual fee of $30.
- Using sterile technique, prepare 6 cm nematode growth medium agar plates (NGM agar plates).
NOTE: Poured plates can be stored upside down in their original plastic sleeves or airtight containers at 4 °C for several months.- To make NGM agar plates, add 3 g of NaCl, 2.5 g of Bacto peptone, 20 g of NGM agar, and ddH2O to 1 L (~975 mL). Autoclave the media using a liquid cycle that holds at 121 °C for 20 min. Let cool to 55 °C, and using sterile technique, add 1 mL of cholesterol, 0.5 mL of 1 M CaCl2, 1 mL of 1 M MgSO4, and 25 mL of 1 M Potassium phosphate buffer (pH 6).
NOTE: To make 1 M Potassium phosphate buffer, mix 108.3 g of KH2PO4 (monobasic) and 35.6 g of K2HPO4 (dibasic); if needed, adjust pH to 6 using KOH. Add ddH2O up to 1 L (usually ~900 mL) and autoclave for 40 min at 121 °C. 1 L of NGM makes about 100 plates containing 10 mL each.
- To make NGM agar plates, add 3 g of NaCl, 2.5 g of Bacto peptone, 20 g of NGM agar, and ddH2O to 1 L (~975 mL). Autoclave the media using a liquid cycle that holds at 121 °C for 20 min. Let cool to 55 °C, and using sterile technique, add 1 mL of cholesterol, 0.5 mL of 1 M CaCl2, 1 mL of 1 M MgSO4, and 25 mL of 1 M Potassium phosphate buffer (pH 6).
- Using a serological or transfer pipet, spot the plates with three drops of E.coli OP50 bacterial cultures (~150 µL). Try to spot in the center of the plate and avoid placing spots too close to the plate edges; this will discourage the animals from crawling up the sides of the plates and desiccating.
- Once the bacterial lawn dries, store the spotted plates upside down at room temperature (RT) for up to 2 weeks. Spotting plates at least 1-2 days before using will allow the bacterial lawns to grow thicker and support a higher density of animals.
NOTE: To prepare OP50 bacterial cultures, OP50 can be ordered from the Caenorhabditis Genetics Center. OP50 bacteria is streaked from a glycerol stock onto an LB plate to ensure single colonies and kept at 4 °C for 4-6 weeks. OP50 bacterial cultures are grown in LB overnight at 37 °C from a single colony – no shaking is needed. To prepare LB media, add 10 g of Tryptone, 10 g of NaCl, 5 g of yeast extract, 950 mL of ddH2O, adjust the pH to 7 with 10 N NaOH and adjust the final volume to 1 L with ddH2O. Aliquot into 100-mL amounts and autoclave for 20 min at 121 °C. - To create a working stock for each strain, pick three L4-stage hermaphrodites onto a spotted NGM plate. Store the plates at 15-25 °C. The standard culturing condition is 20 °C. If access to temperature-controlled incubators is not available, maintain the cultures on the bench top at RT.
NOTE: L4-stage hermaphrodites can be easily staged both by size (smaller than adults) and the presence of a distinct half circle halfway through their bodies (the developing vulva). At 20 °C, animals will reach the L4 stage 34-46 h after hatching (which is about 40-52 h after embryos are laid). See Corsi et al.14 for more details about the timing of developmental stages.- Shift the cultures to various temperatures to control the rate of development. Animals will grow faster at higher temperatures and slower at lower temperatures. They will start to become sterile at temperatures higher than 25 °C.
NOTE: Some mutant genotypes are temperature-sensitive and will display different phenotypes depending on the culturing temperature.
- Shift the cultures to various temperatures to control the rate of development. Animals will grow faster at higher temperatures and slower at lower temperatures. They will start to become sterile at temperatures higher than 25 °C.
- Maintain the working stocks by picking three L4-stage hermaphrodites to a new spotted NGM plate every 4 days. Doing so will ensure a constant, well-fed population with a wide range of developmental stages.
2. Gonad dissection
- Collecting age-matched adults: 12-24 hours before dissection, pick L4-stage hermaphrodites to a new spotted NGM plate – these grow into young adults the following day, which is ideal for dissection. The number of L4-stage hermaphrodites will depend on the cytological analysis being performed; usually, 10-20 hermaphrodites are dissected per slide, with two to four slides generated per condition.
NOTE: Gonads are most efficiently extruded in well-fed animals. Be sure to pick L4 hermaphrodites from working stocks that are not starved (i.e., still have a visible bacterial lawn present on the plate). Starved animals tend to undergo incomplete gonad extrusions, making them difficult to visualize.- If assessing later stages of meiosis, like diakinesis, dissect older adults (48 h post-L4) because they accumulate more diakinesis-stage nuclei, simplifying analysis. However, researchers should note the possibility that some effects may be caused by advanced maternal age.
- Prepare for dissection.
- Prepare M9: Add 3 g of KH2PO4 (monobasic), 6 g of Na2HPO4 (dibasic), 5 g of NaCl, and add ddH2O to 100 mL. Autoclave the solution for 20 min at 121 °C, let it cool, and then add 1 mL of 1 M MgSO4 using sterile technique.
- Prepare dissecting buffer: Mix 1 µL of 10% Tween 20, 12 µL of 100 mM levamisole, 10 µL of 10x M9, and 77 µL of ddH2O.
NOTE: Tween 20 is included in the dissecting buffer to reduce surface tension and further permeabilize tissue membranes. - Prepare fix solution (2% PFA [100 µL]): Mix 12.5 µL of 16% paraformaldehyde (PFA), 10 µL of 10x M9, and 77.5 µL of ddH2O; be sure to use a fresh ampule of PFA (opened for no longer than 2 weeks).
CAUTION: PFA is a hazardous chemical, and fix solution should be prepared in the fume hood. - Set up the freezing method-liquid nitrogen is easier to manage, but if necessary, an aluminum block can be used atop dry ice.
- Liquid nitrogen method: Set a plastic beaker or a plastic Coplin jar in a polystyrene box. Fill the beaker with liquid nitrogen (overflow into the polystyrene container is fine). Set the tweezers nearby. Ideally, the beaker should be narrow enough to prevent microscope slides from falling completely horizontal-slides should lie at a tilt and be easy to grab with tweezers.
NOTE: It is important to use plastic containers when working with liquid nitrogen rather than glass to avoid shattering due to rapid cooling. - Dry ice method:
- Place a flat aluminum block on dry ice in a polystyrene container or rectangular ice bucket. It is easier to freeze the slides if the top of the block sits above the top of the container.
- Wearing gloves, press down gently on the aluminum block to ensure good contact with dry ice and hasten the cooling process (it may release a screech as the metal cools rapidly). Set a razor blade nearby.
- Leave the aluminum block on dry ice for at least 30 min prior to freeze-crack stop to allow full cool-down, which is necessary for a quick freeze.
NOTE: Ideally, solid blocks of dry ice should be used, which helps the surface remain level. If necessary, aluminum block can be placed on dry ice pellets, but care should be taken to ensure that the surface remains level. - The surface of the aluminum block will collect frost, which can prevent close contact between the slides and the block. Use the razor blade to scrape frost off the surface, clearing an area for each slide. Covering the polystyrene container with a lid will help to prevent excessive frost accumulation.
- Liquid nitrogen method: Set a plastic beaker or a plastic Coplin jar in a polystyrene box. Fill the beaker with liquid nitrogen (overflow into the polystyrene container is fine). Set the tweezers nearby. Ideally, the beaker should be narrow enough to prevent microscope slides from falling completely horizontal-slides should lie at a tilt and be easy to grab with tweezers.
- Fill a Coplin jar with ~40 mL of 95% ethanol.
- Fill three Coplin jars with ~40 mL of PBS-T.
NOTE: To make PBS-T, mix 100 mL of 10x PBS, 5 mL of Triton X-100, and 2 mL of 0.5 M EDTA (pH 8). Add 873 mL of ddH2O. Shake vigorously to dissolve Triton X-100. To make 10x PBS, mix 25.6 g of Na2HPO4·7H2O, 80 g of NaCl, 2 g of KCl, and 2 g of KH2PO4. Add ddH2O to bring the volume up to 1 L. Autoclave for 40 min at 121°C. The detergent Triton X-100 is included in the wash buffer PBS-T to reduce surface tension and enhance retention of whole-mounted animals on the slide.
- Dissect the animals on an 18 mm x 18 mm glass coverslip. This step is time-sensitive due to the evaporation of the dissecting buffer. It should take less than 5 min to finish dissections.
NOTE: Levamisole is used to immobilize animals to make them easier to dissect, but leaving them in levamisole for too long will result in poor gonad extrusion. It is easiest to reduce the number of animals dissected per slide in order to fit within the 5 min time frame.- Place a holding slide on the stage of a dissecting microscope-the holding slide is used to move the coverslip around more easily, and can be reused indefinitely (Figure 2A, left photo).
- Place the coverslip on top of the holding slide and pipet 4 µL of the dissecting solution into the center of the coverslip. Move the holding slide to one side of the stage to free up the view.
- Pick 10-20 hermaphrodites into the drop of dissecting solution. Choose enough animals to image ~10 per slide, but not so many that they cannot be dissected within 5 min.
- Dissect the animals using two syringe needles, one for each hand (Figure 2A).
- Cross the points of the needles to make an X-shape and use the bottom of the X to pin an adult down to the surface of the coverslip.
NOTE: It can take practice to discover the best way to hold each needle in terms of angle and rotation. Start by holding the needles with their bevels (slanted edge) facing down. See the discussion for suggestions on first learning to dissect in a larger volume (Figure 2D). - Position the X-shape immediately behind the pharynx, about 1/5 of the body length of a young adult, or just below the clear part of the head (Figure 2A, right diagram).
- Decapitate the animal with one scissoring motion (like slicing food with a knife and fork). A good extrusion will result in one arm of the gonad (or sometimes both arms) releasing fully from the body cavity, along with one or both halves of the gut (Figure 2B, left image). The gut will appear darker and of uniform width, while the gonad will appear clear with a distal tapered tip.
- Each animal should only be cut once; if the gonad does not extrude after making the cut, just move onto the next animal. The first cut significantly reduces the hydrostatic pressure in the body, which makes any further cuts unlikely to result in a better extrusion. Incomplete or poor extrusions are readily visible while imaging and can be ignored (Figure 2B, right image).
NOTE: Do not attempt to separate the gonad from the carcass-doing so will usually tear the tissue and limit the number of meiotic stages that can be analyzed.
- Each animal should only be cut once; if the gonad does not extrude after making the cut, just move onto the next animal. The first cut significantly reduces the hydrostatic pressure in the body, which makes any further cuts unlikely to result in a better extrusion. Incomplete or poor extrusions are readily visible while imaging and can be ignored (Figure 2B, right image).
- Repeat the dissection for the remaining animals on the cover slip.
- Cross the points of the needles to make an X-shape and use the bottom of the X to pin an adult down to the surface of the coverslip.
- Fix the sample with formaldehyde.
- Working gently, pipet 4 µL of the fix solution on the coverslip very close to the drop with animals. Ideally, the drops are close enough to merge; avoid pipetting directly into the dissection drop and displacing the dissected carcasses.
- Pin the coverslip to the holding slide using one finger. With the other hand, gently flick the coverslip a few times to mix the drops. The final fix concentration is 0.8% PFA.
- Holding a positively charged slide front-side down, use it to pick up the coverslip in the center of the slide. Gently touch the sample drop, and surface tension of the drop will pick up the coverslip.
NOTE: Positively charged slides have an electrostatic coating that helps tissues adhere to the surface to prevent sample loss.- If desired, position the coverslip diagonally, such that one corner hangs off the top or bottom edge of the slide by 1 mm. Leaving an overhang will make it easier to crack the coverslip off after freezing (Figure 2C).
- Set the slide on the bench and fix for exactly 5 min at RT. During this time, label the slide with a pencil.
NOTE: It is common to over-fix the samples, which can be detected by an exclusion of antibody from nuclei or even all tissues. Additionally, some antibodies are particularly sensitive to formaldehyde. In these cases, reduce the fixation time, or decrease the final concentration of formaldehyde used.
- Freeze-crack
- Immediately after the fix, freeze the slides.
- If using liquid nitrogen: Hold the labeled edge of the slide with tweezers and gently lower it into liquid nitrogen. Release the slide such that it leans against the side of the beaker, coverslip-side up. Slides are frozen within 10 s (once the liquid stops boiling).
- If using dry ice: scrape the frost off the surface of the aluminum block with a razor. Firmly hold the labeled edge of the slide and set it on the cleared surface, applying some pressure downward to ensure full contact with the block. The coverslip should be completely on the block surface. Freezing occurs within 10 s and can be visually confirmed as the sample under the coverslip turns to ice.
- For both methods, leave the slides for at least 5 min to fully freeze.
NOTE: Once frozen, the slides can be left in liquid nitrogen or on the block for 15-20 min, as long as they remain frozen. This allows multiple slides to be collected at this stage before proceeding to the cracking step.
- Working quickly, crack the coverslip off the sample. This step is time-sensitive. Remove the coverslip and immerse the slide in ethanol before the sample starts to thaw.
- Remove the frozen slide (using tweezers for liquid nitrogen).
- Firmly grip the slide's labeled short edge and brace one long edge against the bench.
- With the other hand, firmly grip a razor, and slide the razor's edge down the slide to flick the coverslip off and away from the sample. This step is slightly easier if the coverslip is positioned with a corner overhang (Figure 2C).
- Immediately after the fix, freeze the slides.
- Immediately place the slide in a Coplin jar containing 95% ethanol at RT. Leave the slides in ethanol for at least 5 min.
NOTE: Slides can be left in ethanol for a while-this allows multiple slides to be collected at this step before proceeding onto washes. Slides can also be stored at this step for several days. If storing, move the Coplin jar of slides in ethanol to -20 °C. - Wash the slides in PBS-T three times for 5 min each at RT. Use fresh PBS-T for each wash.
- If staining with antibodies, proceed to step 3 (antibody staining). If only staining with DAPI to visualize chromosomes, proceed to step 4 (mounting and imaging).
3. Antibody staining
- Primary antibody incubation
NOTE: When working out appropriate conditions for new antibodies, it is important to include the following negative controls to verify the specificity of the fluorescent signal: 1) A slide that lacks a primary antibody and has a secondary antibody only; 2) a slide with a primary antibody only that lacks a secondary antibody. Each of these negative controls should produce a complete lack of signal. If fluorescence is identified on the secondary antibody-only slide, the secondary antibody dilution should be increased or a different secondary should be used. If fluorescence is identified on the primary antibody-only slide, it is likely due to autofluorescence or other potential contaminants.- Dilute the primary antibody in antibody dilution buffer (50 mg of BSA + 50 µL of 10% sodium azide + 10 mL of PBS-T ) – prepare enough antibody dilution to use 20-30 µL per slide.
- Prepare a humid chamber: Line the bottom of a plastic container with an airtight lid with damp paper towels. Lay a single layer of glass Pasteur pipets on top of the paper towels. These pipets create a surface that allows the slides to stay level and keeps them elevated off the paper towels.
NOTE: For humid chambers, it is best to use plastic food storage containers with snap lids, which makes it easier to open and close the container without disrupting the slides within. Look for one that is long enough for the Pasteur pipets but also has a relatively flat bottom without indentations to allow the slides to rest fully horizontal. - Remove the slide from the final PBS-T wash. Work quickly from this step forward to keep the sample wet at all times and avoid evaporation. If the sample dries out, antibody staining will be negatively affected.
- Dry the entire surface of the slide using a wiping tissue folded into a compact rectangle. Wipe down the front and back of the slide, but leave plenty of space around the sample. Take special care when drying the surface near the sample-avoid getting too close to the sample, which leaves an area about the size of the coverslip undried. However, ensure that the perimeter of the sample is dry for the next step.
- Draw a circle around the sample using a hydrophobic PAP pen. Use care to enclose the entire sample area, but avoid disrupting animal carcasses visible on the surface of the slide. Wait for ~5 s to allow the barrier to dry completely.
NOTE: A hydrophobic barrier is necessary to contain the primary antibody solution because the surface of the slide has been coated in PBS-T, which contains a detergent. The PAP pen works best on a dry slide surface, so it is important to create a dry perimeter around the sample. Leaving the area closest to the sample undried protects the sample during this step - Working quickly, use the corner or edge of the folded wiping tissue to wick liquid away from the sample within the hydrophobic barrier. Take care to avoid disrupting animal carcasses.
- Immediately pipet 20 µL of the primary antibody solution into the ring created by the hydrophobic barrier. Take care to pipet gently and at an angle to prevent disrupting carcasses. Avoid pipetting directly upon any carcass.
- Tilt the slide gently to ensure that the entire surface within the hydrophobic barrier is covered by the solution.
NOTE: It is best if the hydrophobic barriers have an interior circumference of 1-1.5 cm, which is fully covered by 20 µL of the antibody solution. The circumference width will depend upon how the carcasses are distributed during the freeze-crack step. Wider barriers may require a higher volume of antibody solution. If desired, small squares of parafilm (cut to the size of the 18 mm x 18 mm coverslip) can be gently placed on top of the sample. The parafilm squares will ensure that the antibody solution covers the entire sample and will also help to prevent evaporation. - Repeat steps 3.1.3-3.1.8 for the remainder of the slides.
- Carefully transfer the slides to the humid chamber, ensuring that the solution remains contained within the hydrophobic barrier and that slides are resting completely level.
- Incubate the slides overnight at RT. Depending on the antibody, this step could be shortened to 6 h or performed overnight at 4 °C by keeping the humid chamber in a cold room or a refrigerator.
NOTE: Using a humid chamber and hydrophobic barriers is usually sufficient to prevent evaporation of the antibody solution, even for long overnight incubations. However, if evaporation proves to be a problem, small squares of parafilm can be placed gently upon each sample, which will help prevent evaporation. These can be removed in the first wash step: immerse each slide in a Coplin jar filled with PBS-T (containing no other slides), and allow the parafilm to float away from the sample. Remove the parafilm from the Coplin jar before using the same jar to remove the parafilm from the next slide.
- Wash the slides in Coplin jars containing ~40 mL of PBS-T three times for 5 min each at RT. Use fresh PBS-T for each wash.
- During these washes, dilute the secondary antibody in antibody dilution buffer. Prepare enough antibody dilution to use 20-30 µL per slide. It is common to use Alexa Fluor conjugated secondary antibodies diluted at 1:200.
- Secondary antibody incubation
- Remove the slide from the final PBS-T wash. Work quickly from this step forward to keep the sample wet at all times and avoid evaporation.
- Dry the slide using a folded wiping tissue. Avoid drying the area contained by the hydrophobic barrier.
- Working quickly, use the corner or edge of the folded wiping tissue to wick liquid away from the sample within the hydrophobic barrier. Take care to avoid disrupting the animal carcasses.
- Immediately pipet 20 µL of the secondary antibody solution into the ring created by the hydrophobic barrier. Take care to pipet gently and at an angle to prevent disrupting the carcasses.
- Avoid pipetting directly upon any carcass. Ensure that the entire surface within the hydrophobic barrier is covered by the solution. Wider barrier circumferences may require a higher volume of antibody solution. If desired, cover the sample with a small square of parafilm (see the note below step 3.1.8).
- Carefully transfer the slide to the humid chamber, ensuring that the solution remains contained within the hydrophobic barrier and that the slide is resting completely level. Replace the lid on the humid chamber to keep the slide in the dark.
- Repeat steps 3.3.1-3.3.6 for the remainder of the slides.
- Incubate in the dark for 4 h at RT.
NOTE: Depending on the antibody, this step can be shortened to 2 h or extended to 6 h. If the humid chamber is not dark, place it in a drawer or cover it with a cardboard box. Alternatively, the humid chamber can be wrapped in aluminum foil to keep the slides in the dark.
- Wash the slides in Coplin jars containing ~40 mL of PBS-T three times for 5 min each at RT. Use fresh PBS-T for each wash.
4. Mounting and imaging
- Prepare for mounting the sample by having mounting medium + DAPI (2 µg/mL), 18 mm x 18 mm glass coverslips, and nail polish within reach.
- Remove the slide from the final wash and dry using a folded wiping tissue. Avoid drying the area contained by the hydrophobic barrier.
- Using the corner of the folded wiping tissue, carefully wick away liquid from the sample inside the barrier. Remove most liquid at this step, but without letting the carcasses dry out completely.
- Working quickly, add 8 µL of the mounting medium to the sample. Pipet gently and at an angle to prevent disrupting the carcasses. Avoid pipetting directly upon the carcasses.
- Still working quickly, carefully lower a glass coverslip onto the sample.
- Air bubbles can disrupt imaging and lead to sample degradation. To minimize air bubbles, rest one edge of the coverslip on the slide next to the sample, so it is held at diagonal over the sample. It can help to rest the higher edge of the coverslip on a pencil or tweezers. Then slowly lower the higher edge of the coverslip down-this movement allows the mounting medium to create a liquid seal progressing from one edge to the opposite edge.
- Repeat steps 4.2-4.5 for the remainder of the slides.
- Seal the coverslips with nail polish. Take care to avoid pressing down upon the coverslip (which will crush the sample) or dislodging the coverslip horizontally (which will smear the sample).
- Add a small dot of nail polish to each corner of a coverslip (~1 mm wide). These will help hold the coverslip in place. Allow the dots to dry completely.
- When the corners are completely dry, seal the edges with nail polish. Ensure that the polish completely fills the gap between the coverslip and slide, but avoid covering the coverslip the sample too much. It is best to maintain a 1 mm overlap onto the coverslip. Only use as much nail polish as is needed. Avoid using too much or having excess polish, which can take a long time to dry and runs the risk of damaging the microscope objective.
NOTE: It is preferable to use colored nail polish instead of clear, which makes it easier to see the boundary of the seal and helps prevent imaging animals that lie under the nail polish boundary. - Allow the nail polish to dry completely before imaging. This step is important, as wet nail polish can severely damage microscope objectives.
NOTE: Slides should be kept in the dark as much as possible from here on out. Use a flat cardboard box to cover them as they dry on the bench.
- Store the slides in the dark at 4 °C for 1-2 weeks before imaging. If needed, store the slides at -20 °C for a longer duration.
- Image using standard fluorescence compound light microscopy.
- For each slide, first, examine the entire sample at 10x in the DAPI channel to identify well-extruded gonads and note their placement. For most applications, avoid imaging any gonads that are severed, incomplete, or partially covered by another part of the animal. For most animals, only one gonad arm will be fully extruded and visible.
NOTE: It can be helpful to take a lower-magnification image at 10x of the entire gonad in the DAPI channel to use for later orientation or identify the location of specific nuclei. - Depending on the application, images can be taken at 40x, 63x, or 100x. If the entire gonad is to be captured, create a montage with overlapping boundaries. While imaging, remember that the gonad is a hollow tube, with nuclei most dense at the top and bottom.
- For each slide, first, examine the entire sample at 10x in the DAPI channel to identify well-extruded gonads and note their placement. For most applications, avoid imaging any gonads that are severed, incomplete, or partially covered by another part of the animal. For most animals, only one gonad arm will be fully extruded and visible.
Representative Results
DAPI binds strongly to DNA, and its staining is robust even under a wide variety of conditions (Figure 3A,B). It should be present in all nuclei and therefore makes an effective positive control for the presence of any worm tissue on the slide and the ability to detect fluorescence on the microscope. Staining is effective when the antibody is present within meiotic nuclei (Figure 3A,B). For example, Figure 3A,B shows mid-pachytene nuclei stained with DAPI and an antibody targeting RAD-51, a marker for double-strand breaks. KLE-2 is a component of condensin, a highly conserved protein complex that structures chromosomes in preparation for mitosis and meiosis. kle-2/+ mutants have minor defects in chromosome structure, as shown by slightly disordered DAPI staining and an increase in double-strand break number reflected in the higher number of RAD-51 foci (Figure 3B). A common mistake for immunofluorescence is leaving the sample in the fix solution for too long. Over-fixing can lead to unsuccessful staining because it usually prevents antibodies from diffusing into nuclei. This mistake can be identified when the antibody is diffused in the cytoplasmic regions of the gonad, but appears to be excluded from nuclei.
In the germline (Figure 1), nuclei mitotically proliferate at the distal tip, enter meiosis during the transition zone, and progress through pachytene (which is often divided into three equal stages by a number of nuclei rows) before entering diplotene and finally diakinesis. Oocytes in diakinesis are numbered based on their proximity to the spermatheca, with the -1 oocyte being most proximal to the spermatheca, the -2 oocyte being the next distal, and so on. C. elegans have six chromosomes, which manifest as compact ovals in diakinesis nuclei. Each of these represents a homologous pair of two sister chromatids held together by a chiasma, also called a bivalent (Figure 3C). Mutants, like spo-11, that disrupt crossover formation will fail to form chiasmata; therefore, diakinesis nuclei will have 12 separate DAPI bodies (also called univalents), one for each sister chromatid (Figure 3D).
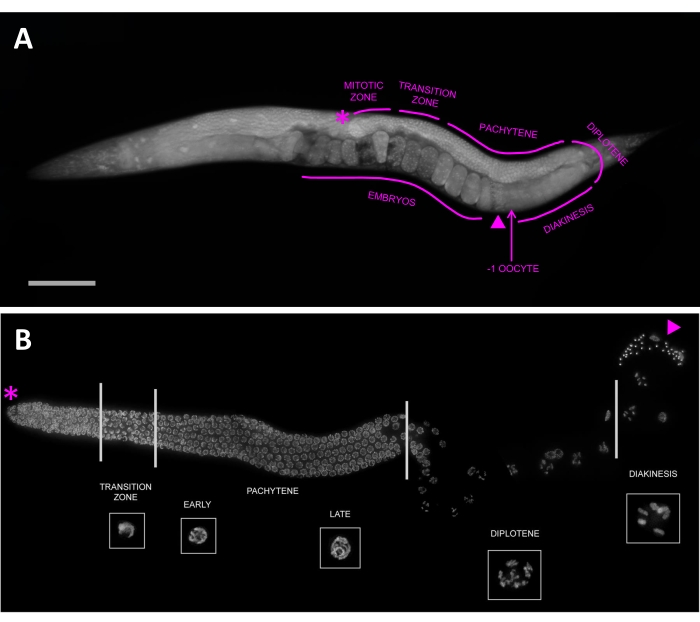
Figure 1: Germline nuclei are arrayed in a spatial-temporal manner that represents their progression through meiosis. (A) Whole-mount young adult hermaphrodite stained with DAPI. Gonad and meiotic stages are outlined, from the distal tip (asterisk) to the proximal region (the -1 oocyte, indicated with an arrow), which contains the spermatheca (triangle). Scale bar represents 100 µm. (B) Projected fluorescence image of a dissected hermaphrodite gonad stained with DAPI. Meiotic stages are denoted, with representative nuclei shown in insets (all insets are shown to scale with one another). Nuclei can be easily staged based on their location in the germline and characteristic chromosome morphology, progressing from the mitotic zone at the distal tip (asterisk), to the transition zone, through pachytene, diplotene, and diakinesis. The spermatheca is marked by a triangle. This figure has been adapted from Hillers et al. under license CC BY 3.028. Please click here to view a larger version of this figure.
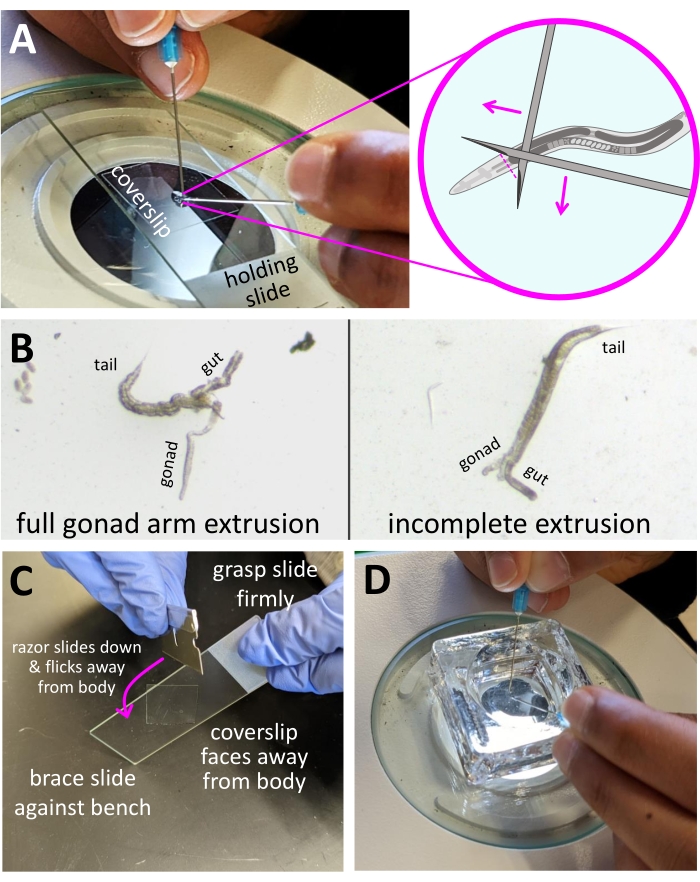
Figure 2: Demonstration of dissection setup. (A) Microscope stage during dissection. Animals are dissected in 4 µL of dissection solution on a coverslip placed on the holding slide, which is used to move the coverslip into view. The diagram shows ideal needle placement. Needles are held with a beveled edge facing down, crossed over the pharyngeal region. Pink arrows demonstrate a scissor-like motion for needles. The pink dashed line shows the ideal cut location. (B) Images of dissected animals, with an example of a full extrusion and an incomplete extrusion. Sections of extruded gonads and guts are labeled. (C) Image demonstrating the freeze-crack step. The slide should be held firmly in one hand, with the coverslip side facing away from the body. The opposite edge is braced against the bench. The razor should be held in the other hand. The pink arrow indicates the direction of movement for the razor to flick the coverslip off the slide, with a slight turn such that the coverslip flicks away from the body. Note that the coverslip has been placed such that one corner hangs over the long edge of the slide. (D) Image of the setup for dissection practice in a glass embryo staining dish. Animals are placed in 50-200 µL of dissection solution, which negates the problem of evaporation and extends the time for dissection. Please click here to view a larger version of this figure.
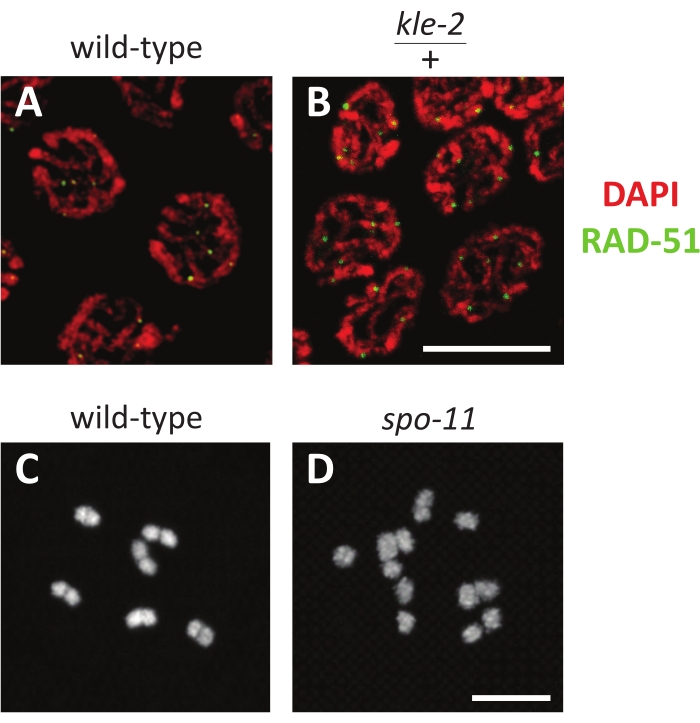
Figure 3: Representative fluorescence results of germline nuclei. (A,B) Z-stack projections of late pachytene nuclei stained with RAD-51 antibody (green) and DAPI (red) in (A) wild-type and (B) kle-2/+ heterozygotes. (C,D) Z-stack projections of a single diakinesis nucleus stained with DAPI in (C) wild-type (with six DAPI staining bodies) and (D) spo-11 mutants (with 12 DAPI staining bodies). Scale bars represent 5 µm. Please click here to view a larger version of this figure.
Discussion
Sexual reproduction requires the creation of haploid gametes, which are produced via the specialized cell division of meiosis. C. elegans has become a popular model for the cytological study of meiosis due to its optical transparency, convenient germline anatomy, and powerful genetics28. Simple organismal experiments assessing fertility and embryonic lethality can be combined with molecular genetics to address many questions in the lab or the classroom. For example, because crossovers are essential for proper chromosome segregation, processes that disrupt their formation or resolution will generate aneuploid gametes. In turn, aneuploidy leads to inviable progeny, which can easily be assessed by counting progeny, or through the slightly more complicated method of determining embryonic lethality. Cytologically, a lack of crossovers will affect the numbers of DAPI-staining bodies observed during diakinesis. The robustness of DAPI staining and the ease of scoring makes this an ideal experiment to teach cytological techniques. The temporal-spatial layout of nuclei in the hermaphrodite germline provides a snapshot of each stage of meiosis at a single moment in time. Nuclei take about 54 hours to proceed from the distal mitotic end of the germline to the proximal end (for examples, see Jaramillo-Lambert et al.33, Stamper et al.34 and Libuda et al.35). This well-established timing of meiotic progress allows for pulse-chase labeling or DNA damaging experiments.
Because DAPI staining nearly always works, it serves as a useful technical control for fluorescence microscopy (and can provide satisfaction for microscopists-in-training). Antibody staining can be more variable and is a good measure of reproducibility between replicates. C.elegans gonads can be difficult to fix consistently, as this step requires a balance between preserving chromosomal structure while also allowing enough antibody diffusion. Fixing with formaldehyde best preserves chromosomal morphology, and preparing formaldehyde fresh from paraformaldehyde ampules has provided the most reproducible results. It is also possible to use formaldehyde prepared from formalin (37% aqueous formaldehyde and methanol). Fixation strength and timing may need to be empirically determined for each antibody. Alternative methods involve skipping the formaldehyde fixation in step 2.4 and, following freeze-crack, immersion in 100% ethanol at -20 °C, or immersion in 100% methanol for 10 min followed by immersion in 100% acetone for 10 min at -20 °C. These fix conditions allow better antibody penetration but will negatively affect tissue morphology (chromosomes will look blown-out and puffier).
Timing is very important for the dissection and fix steps outlined in step 2. Because the volume of dissection solution is so small, evaporation can impact the final fix concentration, which will cause variable antibody staining. An experienced C. elegans handler can prepare a slide in less than 2 min from the start (step 2.3) to fix (step 2.4). The main technical hurdle is the ability to pick animals into the drop of dissection solution and quickly dissect them. It can be easier to learn how to dissect in larger volumes. New trainees first start by picking animals into 50-200 µL of dissection solution in a glass embryo staining dish (Figure 2D); the wider surface provides more room to maneuver when identifying an ideal needle position for good gonad extrusions, while the larger volume of liquid makes evaporation less of an issue. Once comfortable with the motions of dissecting, trainees start dissecting using only 50 µL in the dish, then switch to dissecting in 20 µL on a slide. Once dissecting on slides, trainees can quickly start reducing the amount of solution until they are quick enough at working in 4 µL to make evaporation negligible.
If the timing of dissection remains an issue, trainees can dissect in 8 µL of the dissection solution during step 2.3. Then, during step 2.4, they can add 8 µL of the fix solution, gently pipetting to mix thoroughly (watching closely to ensure that carcasses are not disrupted or pipetted away), and removing 8 µL from the mixed solution. This alternative approach will result in the same final volume of 8 µL and the same final fix concentration; this volume is important to ensure that carcasses contact both the coverslip and slide surface during the freeze-crack in step 2.5. If the timing of preparing each slide remains an issue even in larger dissection volumes, a suspension method for germline immunofluorescence protocol is described by Gervaise and Arur26.
The major limitation of using immunofluorescence to visualize proteins in situ is the availability of primary antibodies targeting a particular protein of interest. However, if a tagged version of the protein has been engineered, this protocol can be adapted to visualize the protein tag. Antibodies targeting common tags, like FLAG, HA, or GFP, are commonly available. Fluorescent tags like GFP can often be quenched by fixation steps, so it is recommended to use a primary antibody targeting GFP, rather than relying on the native fluorescence signal itself. Another limitation of this protocol is that it captures the germline within a single timeframe (although all stages of oogenesis will be represented within the germline). Therefore, this technique can miss dynamic changes that may occur as an oocyte progresses through oogenesis. However, the timing of gametogenesis has been well-studied in C. elegans; in a wild-type young adult, an oocyte takes about 60 h to progress from the distal tip of the germline (the mitotic zone) to diakinesis33. Therefore, a pulse chase or specific intervention like irradiation followed by a time course would allow for the observation of effects at different stages of meiosis.
In conclusion, this protocol describes C. elegans gonad dissection followed by DAPI and antibody staining for fluorescence microscopy. Dissection and fixing (step 2) takes 60-90 min, depending on the number of slides generated. Antibody staining (step 3) is mostly hands-off and can range from 7 h at minimum to 1.5 days, depending on antibody incubation times. Mounting (steps 4.1-4.7) takes about 15 min. This general approach to visualizing germline chromosomes and the gonad can be used for cytological studies of any protein if an antibody or fluorescent tag reagent exists. In its simplest form, DAPI staining of diakinesis nuclei can be used to screen for factors affecting meiotic recombination. When paired with organismal analyses of progeny number, the incidence of males (Him phenotype), and embryonic lethality, this cytological approach provides a single-cell counterpoint to population-based analyses.
Divulgazioni
The authors have nothing to disclose.
Acknowledgements
Work in the Lee lab was supported by the National Institute of General Medical Sciences under award number 1R15GM144861 and the National Institute for Child and Human Development under award number 1R15HD104115, both of the National Institutes of Health. DA was supported by the UML Kennedy College of Sciences Science Scholars program. NW was supported by a UML Honors College Fellowship and a UMLSAMP fellowship (funded by the National Science Foundation under grant number HRD-1712771). We thank A. Gartner for RAD-51 antibody. All C. elegans strains were provided by the Caenorhabditis Genetics Center, which is funded by the Office of Research Infrastructure Programs of the National Institutes of Health under award numberP40 OD010440.
Materials
| Alexa Fluor 488 Donkey Anti-Rabbit IgG Secondary Antibody | Molecular Probes | 711-545-152 | |
| Aluminum heating/cooling block | Millipore Sigma | Z743497 | |
| Bacto Peptone | Gibco | DF0118 17 0 | |
| Borosilicate Glass Pasteur Pipets, Disposable, 5.75 inches | Fisherbrand | 13 678 20B | Used to make worm picks |
| BSA, Bovine Serum Albumin | VWR | 97061 420 | |
| C. elegans wild type (ancestral) | Caenorhabditis Genetics Center | N2 (ancestral) | |
| C. elegans kle-2 (ok1151) mutants | Caenorhabditis Genetics Center | VC768 | The full genotype of this strain is: kle-2(ok1151) III/hT2 [bli-4(e937) let-?(q782) qIs48] (I;III). It has kle-2 balanced with a balancer chromosome marked with pharangeal GFP. Pick non-green animals to identify homozygous kle-2 mutants. |
| C. elegans spo-11 (me44) mutants | Caenorhabditis Genetics Center | TY4342 | The full genotype of this strains is: spo-11(me44) IV / nT1[qIs51] (IV:V). It has spo-11 balanced with a balancer chromosome marked with pharangeal GFP. Pick non-green animals to identify homozygous spo-11 mutants. |
| Calcium Chloride Anhydrous | VWR | 97062 590 | |
| Cholesterol | Sigma Aldrich | 501848300 | |
| DAPI | Life Technologies | D1306 | |
| EDTA, Disodium Dihydrate Salt | Apex Bioresearch Products | 20 147 | |
| Embryo Dish, glass, 30mm cavity | Electron Microscopy Services | 100492-980 | Used to practice dissecting; glass is preferred because dissected animals can stick to plastic |
| Escherichia coli | Caenorhabditis Genetics Center | OP50 | |
| Food storage container, plastic | various | n/a | Plastic containers with (1) relatively flat bottoms, to allow slides to rest level; (2) lids that are air-tight, but can still be easily removed without disturbing contents of container, and (3) are about 9 inches long by 6 inches wide are preferred |
| Glass Coplin Jar | DWK Life Sciences Wheaton | 08-813E | |
| Hydrophobic Barrier PAP Pen | ImmEdge | NC9545623 | |
| Kimwipes | Kimtech Science | 06 666A | |
| Levamisole Hydrochloride | Sigma Aldrich | L0380000 | |
| Magnesium Sulfate Anhydrous | Fisher Chemical | M65 500 | |
| Needles, Single-use, 25 G | BD PrecisionGlide | 14 821 13D | blue, 1 inch |
| NGM Agar, Granulated | Apex Bioresearch Products | 20 249NGM | |
| Parafilm | Bemis | 16 101 | |
| Paraformaldehyde (16% w/v) Aqueous Solution | Electron Microscopy Sciences | 50 980 487 | |
| PBS, Phosphate Buffered Saline (10x Solution) | Fisher BioReagents | BP399500 | |
| Petri Dishes, 60-mm | Tritech Research | NC9321999 | Non-vented, sharp edge |
| Platinum wire (90% platinum, 10% iridium) | Tritech Research | PT 9010 | Used to make worm picks |
| Potassium Chloride | Fisher | BP366-500 | |
| Potassium Phosphate Dibasic | VWR BDH Chemicals | BDH9266 500G | |
| Potassium Phosphate Monobasic | VWR BDH Chemicals | BDH9268 500G | |
| Premium Cover Glasses 18 mm x 18 mm | Fisherbrand | 12-548-AP | 0.13–0.17 mm thickness |
| Razor Blades, Single Edge | VWR | 55411 055 | |
| SlowFade Gold Antifade Mountant | Molecular Probes | S36937 | Alternatives: VectaShield Antifade Mounting Medium (Vector Laboratories, H-1000-10) or ProLong Diamond Antifade (Thermo Fisher Scientific, P36970). If using ProLong Diamond, no nail polish is required for sealing, but slides must cure for 24 hours before imaging. |
| Sodium Azide | Fisher BioReagents | BP922I 500 | |
| Sodium Chloride | Fisher Chemical | S271 500 | |
| Sodium Phosphate Dibasic Anhydrous | Sigma Aldrich | 7558 79 4 | |
| Sodium Phosphate Dibasic Heptahydrate | Sigma Aldrich | S9390 | |
| Styrofoam box | various | n/a | Approximate dimensions of interior: 12 inches long, 9 inches wide, 8 inches deep |
| Superfrost Plus Microscope Slides | Fisherbrand | 22 037 246 | |
| Triton X-100 | Fisher BioReagents | BP151 500 | |
| Tryptone | Apex Bioresearch Products | 20 251 | |
| Tween 20 | Fisher Chemical | BP337 500 | |
| Yeast Extract | Apex Bioresearch Products | 20 254 |
Riferimenti
- Newman, D. L., Wright, L. K. Meiosis: A play in three acts, starring DNA sequence. CourseSource. 4, (2017).
- Ohkura, H. Meiosis: An overview of key differences from mitosis. Cold Spring Harbor Perspectives in Biology. 7 (5), 015859 (2015).
- Hassold, T., Hunt, P. To err (meiotically) is human: the genesis of human aneuploidy. Nature Reviews Genetics. 2 (4), 280-291 (2001).
- Keeney, S., Giroux, C. N., Kleckner, N. Meiosis-specific DNA double-strand breaks are catalyzed by Spo11, a member of a widely conserved protein family. Cell. 88 (3), 375-384 (1997).
- Rabitsch, K. P., et al. Kinetochore recruitment of two nucleolar proteins is required for homolog segregation in meiosis I. Developmental Cell. 4 (4), 535-548 (2003).
- Brown, C. R. Some misconceptions in meiosis shown by students responding to an advanced level practical examination question in biology. Journal of Biological Education. 24 (3), 182-186 (1990).
- Kalas, P., O’Neill, A., Pollock, C., Birol, G. Development of a meiosis concept inventory. CBE-Life Sciences Education. 12 (4), 655-664 (2013).
- McDonnel, L. M., Klenz, J. Teaching genetic linkage and recombination through mapping with molecular markers. CourseSource. 2, (2015).
- Wright, L. K., Cortez, P., Franzen, M. A., Newman, D. L. Teaching meiosis with the DNA triangle framework: A classroom activity that changes how students think about chromosomes. Biochemistry and Molecular Biology Education. 50 (1), 44-54 (2021).
- Shortlidge, E. E., Bangera, G., Brownell, S. E. Each to their own CURE: Faculty who teach course-based undergraduate research experiences report why you too should teach a CURE. Journal of Microbiology & Biology Education. 18 (2), 18 (2017).
- Auchincloss, L. C., et al. Assessment of course-based undergraduate research experiences: A meeting report. CBE-Life Sciences Education. 13 (1), 29-40 (2014).
- Lee, T. W., Carpenter, B. S., Birol, O., Katz, D. J., Schmeichel, K. L. The pipeline CURE: An iterative approach to introduce all students to research throughout a biology curriculum. CourseSource. 6, (2019).
- Lu, F. -. M., Eliceiri, K. W., Stewart, J., White, J. G. WormClassroom.org: an inquiry-rich educational web portal for research resources of Caenorhabditis elegans. CBE Life Sciences Education. 6 (2), 98-108 (2007).
- Corsi, A. K., Wightman, B., Chalfie, M. A Transparent window into biology: A primer on Caenorhabditis elegans. Genetica. 200 (2), 387-407 (2015).
- JoVE Science Education Database. Biology I: yeast, Drosophila and C. elegans. An Introduction to Caenorhabditis elegans. JoVE Science Education Database. , (2022).
- JoVE. Science Education Database. Biology I: yeast, Drosophila and C. elegans. C. elegans Maintenance. JoVE Science Education Database. , (2022).
- Lee, R. Web resources for C. elegans studies. WormBook. , (2005).
- JoVE. Science Education Database. Biology I: yeast, Drosophila and C. elegans. RNAi in C. elegans. JoVE Science Education Database. , (2022).
- Meneely, P. M., Dahlberg, C. L., Rose, J. K. Working with worms: Caenorhabditis elegans as a model organism. Current Protocols Essential Laboratory Techniques. 19 (1), 35 (2019).
- Lemons, M. L. An inquiry-based approach to study the synapse: Student-driven experiments using C. elegans. Journal of Undergraduate Neuroscience Education. 15 (1), 44-55 (2016).
- Kowalski, J. R., Hoops, G. C., Johnson, R. J. Implementation of a collaborative series of classroom-based undergraduate research experiences spanning chemical biology, biochemistry, and neurobiology. CBE-Life Sciences Education. 15 (4), 55 (2016).
- Mordacq, J., Drane, D., Swarat, S., Lo, S. Research and teaching: Development of course-based undergraduate research experiences using a design-based approach. Journal of College Science Teaching. 46 (4), (2017).
- Palmisano, N. J., et al. A laboratory module that explores RNA interference and codon optimization through fluorescence microscopy using Caenorhabditis elegans. bioRxiv. , 344069 (2021).
- Hillers, K. J., Villeneuve, A. M. Analysis of meiotic recombination in Caenorhabditis elegans. Meiosis. 557, 77-97 (2009).
- Phillips, C. M., McDonald, K. L., Dernburg, A. F. Cytological analysis of meiosis in Caenorhabditis elegans. Meiosis. 558, 171-195 (2009).
- Gervaise, A. L., Arur, S. Spatial and temporal analysis of active ERK in the C. elegans germline. Journal of Visualized Experiments. (117), e54901 (2016).
- Shakes, D. C., Miller, D. M., Nonet, M. L. Immunofluorescence microscopy. Methods in Cell Biology. 107, 35-66 (2012).
- Hillers, K. J., Jantsch, V., Martinez-Perez, E., Yanowitz, J. L. Meiosis. Wormbook. , (2017).
- Hunter, A. -. B., Seymour, E., Laursen, S., Thiry, H., Melton, G. . Undergraduate Research in the Sciences: Engaging Students in Real Science. , (2010).
- Wei, C. A., Woodin, T. Undergraduate research experiences in biology: Alternatives to the apprenticeship model. CBE-Life Sciences Education. 10 (2), 123-131 (2011).
- Elgin, S. C. R., et al. Insights from a convocation: Integrating discovery-based research into the undergraduate curriculum. CBE-Life Sciences Education. 15 (2), (2016).
- Stiernagle, T. Maintenance of C. Elegant. WormBook. , (2006).
- Jaramillo-Lambert, A., Ellefson, M., Villeneuve, A. M., Engebrecht, J. Differential timing of S phases, X chromosome replication, and meiotic prophase in the C. elegans germ line. Biologia dello sviluppo. 308 (1), 206-221 (2007).
- Stamper, E. L., et al. Identification of DSB-1, a protein required for initiation of meiotic recombination in Caenorhabditis elegans, illuminates a crossover assurance checkpoint. PLoS Genetics. 9 (8), 1003679 (2013).
- Libuda, D. E., Uzawa, S., Meyer, B. J., Villeneuve, A. M. Meiotic chromosome structures constrain and respond to designation of crossover sites. Nature. 502 (7473), 703-706 (2013).

