Measuring Contralateral Silent Period Induced by Single-Pulse Transcranial Magnetic Stimulation to Investigate M1 Corticospinal Inhibition
Summary
Contralateral silent period (cSP) assessment is a promising biomarker to index cortical excitability and treatment response. We demonstrate a protocol to assess cSP intended for studying M1 corticospinal inhibition of upper and lower limbs.
Abstract
Contralateral silent period (cSP) is a period of suppression in the background electrical muscle activity captured by electromyography (EMG) after a motor evoked potential (MEP). To obtain this, an MEP is elicited by a suprathreshold transcranial magnetic stimulation (TMS) pulse delivered to the primary motor cortex (M1) of the target muscle selected, while the participant provides a standardized voluntary target muscle contraction. The cSP is a result of inhibitory mechanisms that occur after the MEP; it provides a broad temporal assessment of spinal inhibition in its initial ~50 ms, and cortical inhibition after. Researchers have tried to better understand the neurobiological mechanism behind the cSP to validate it as a potential diagnostic, surrogate, and predictive biomarker for different neuropsychiatric diseases. Therefore, this article describes a method to measure M1 cSP of lower and upper limbs, including a selection of target muscle, electrode placement, coil positioning, method of measuring voluntary contraction stimulation, intensity setup, and data analysis to obtain a representative result. It has the educational objective of giving a visual guideline in performing a feasible, reliable, and reproducible cSP protocol for lower and upper limbs and discussing practical challenges of this technique.
Introduction
The silent period (SP) is a period of electromyographic (EMG) silence that follows a motor-evoked potential (MEP) induced by transcranial magnetic stimulation (TMS) applied during sustained muscle contraction. The suprathreshold TMS pulse can either be applied to the contralateral or ipsilateral primary motor cortex (M1) of the target muscle from which the EMG activity is being recorded yielding two phenomena: contralateral silent period (cSP) and ipsilateral silent period (iSP).
Even though iSP and cSP share similar features, they may reflect slightly different components. The first is thought to reflect transcallosal inhibition and thus be entirely of cortical origin1,2. Conversely, cSP is investigated as a possible surrogate of corticospinal inhibition, most likely mediated by gamma-aminobutyric acid (GABA) B receptors within M13,4,5.
Supporting the role of cSP in GABA-mediated pathways, previous works have found an increase in cSP duration after oral administration of GABA-enhancing components5,6,7,8. Still, spinal processes are also involved in altering its duration. The earlier phase (<50 ms) of the cSP is associated with decreased H-reflex values3-a reflex that is a product of peripheral neurocircuitry and that quantifies the excitability of spinal neurons9. Spinal processing is thought to be mediated through the activation of Renshaw cells, motoneuron after-hyperpolarization, and postsynaptic inhibition by spinal interneurons10,11,12,13,14.
Despite spinal contribution, cSP results mainly from the activation of cortical inhibitory neurons, which are responsible for generating the later part of the cSP (50-200 ms)3,10,13,15,16. In that respect, the early part of cSP duration has been associated with spinal inhibition mechanisms, whereas long cSPs request larger cortical inhibitory mechanisms3,13,17,18.
Therefore, cSP is a promising biomarker candidate for corticospinal maladaptation due to neurological disorders, whereas more significant cSP durations potentially reflect an increase in corticospinal inhibition and vice versa5,11. Accordingly, previous works have found an association between cSP duration and pathologies such as dystonia, Parkinson's Disease, chronic pain, stroke, and other neurodegenerative and psychiatric conditions19,20,21,22. To illustrate, in a knee osteoarthritis cohort, a higher intracortical inhibition (as indexed by cSP) was associated with younger age, greater cartilage degeneration, and less cognitive performance in the Montreal cognitive assessment scale23. Moreover, cSP changes could also longitudinally index treatment response and motor recovery24,25,26,27,28,29,30.
As promising as the role of cSP in the neuropsychiatry field is, a challenging aspect of its assessment is that it can be too sensitive to protocol variations. For instance, the cSP duration (~100-300 ms)11 is distinguishable between upper and lower limbs. Salerno et al. found an average cSP duration of 121.2 ms (± 32.5) for the first dorsal interosseous muscle (FDI) and 75.5 ms (± 21) for the tibialis anterior muscle (TA), in a sample of fibromyalgia patients31. Thus, the literature conveys a myriad of divergences in the parameters used to elicit cSPs, which in turn jeopardizes comparability across studies and delays the translation to clinical practice. Within a similar population, protocols have been heterogeneous regarding the suprathreshold TMS pulse setting used to stimulate M1 and the target muscle, for example. On top of that, researchers have failed to properly report the parameters used in their protocols.
Therefore, the goal is to provide a visual guideline on how to apply a feasible, reliable, and easily reproducible cSP protocol for evaluating M1 corticospinal excitability of upper and lower limbs and to discuss the practical methodological challenges of that procedure. Also, to help illustrate the reasoning for the choice of parameters, we conducted a non-exhaustive literature review on Pubmed/MEDLINE to identify published papers on cSP in chronic pain and rehabilitation populations, using the search term: Rehabilitation (Mesh) or rehabilitation or chronic pain or stroke and terms such as transcranial magnetic stimulation and single pulse or cortical silent period. No inclusion criteria were defined for the extraction, and pooled results are displayed in Table 1 for illustrative purposes only.
Protocol
This protocol involves research on human subjects and is in alliance with institutional and ethical guidelines of local ethical committees and the Declaration of Helsinki. Informed consent was obtained from subjects for using their data in the study.
1. Pre-experimental procedures
- Screening of the subject. Screen the subject for intracranial implants, epilepsy, history of seizures, and pregnancy. Use questionnaire guidelines to ensure compliance with up-to-date safety precautions32.
- The delivery of electromagnetic pulses with TMS is contraindicated for individuals with intracranial implants of ferromagnetic material, such as shrapnel, aneurysm clips, or fragments from welding. Take precautions with individuals at increased likelihood of seizures.
- TMS assessment poses no fetal risk for pregnant women who are advised to have a conservative stance when dealing with this population. It is safe to apply TMS in pediatric populations, proceed cautiously in certain developmental stages (i.e., closure of the fontanelle, maturation of cortical excitability, and growth of the external auditory canal)33.
- Preparation of materials. For this procedure, other than the TMS and EMG devices, have at your disposal a swim cap, alcohol pads (with the preparation of 70% isopropyl alcohol), conductive gel, and a computer turned on with the EMG software setup and a dynamometer appropriate for the investigated muscle (see Table of materials).
NOTE: Swim caps have the advantage of being the cheapest and most accessible option that still allows for reliable and reproducible TMS assessments without causing the discomfort of marking the subjects' head.
2. Appropriate instructions to the patients
- Explain the basic steps of the procedure and how much time it will take.
- Instruct the participant to remain awake but not to perform cognitive activities that require extra attention and/or focus (e.g., mathematical calculations, meditation, etc.) and anticipate that they might experience hand/jaw twitching or plausible side effects. Such events might seem unexpected for an inexperienced subject and thus jeopardize the procedure.
NOTE: Single- and paired-pulse TMS have only been associated with mild, transient, adverse events, including headache, local pain, neck pain, toothache, and paresthesia. Seizures are rare, and no other serious adverse events have been associated33. For extra safety, it is recommended to offer earplugs, due to the possibility of harmful sounds, and bite-blocks for possible masseter contraction34.
3. Experimental procedures (Figure 1)
- Select the muscle for positioning the electrodes.
- Ask the subject to put their hand over the table, in a prone position. Select the FDI muscle, localized between the first and second metacarpal osseous. To identify the FDI, ask the subject to abduct their index finger against resistance, keeping the rest of the hand still and laying on the table, while you are palpating the area.
- Expose the selected area. Use a disposable razor to shave the area to improve electrode contact with the skin, if necessary, and clean the area with alcohol pads to remove skin oils and other factors that could increase impedance. Certify that there is free skin to ensure contact with the electrode.
NOTE: If evaluating lower limb activity, use the TA muscle for electrode placement. It is localized on the lateral side of the tibia and lies near the superficies of the skin. It can be identified by ankle dorsiflexion.
- Place the surface EMG electrodes
- With the area exposed and cleaned, apply the conductive gel to each electrode of the channel to ensure good impedance.
- Place the negative electrode on the belly of the FDI muscle (the center or the most prominent bulge of the muscle belly) and the positive on the distal interphalangeal joint, with an inter-electrode distance of at least 1.5 cm. Place the reference electrode (neutral) on the wrist, over the ulnar styloid process.
NOTE: The presence of motor endpoints, muscle tendons, or other active muscles can impact the stability of the recordings, so it is important to avoid these locations35. For the TA muscle, the electrodes should be placed at one-third on the line that connects the tip of the fibula and the tip of the medial malleolus. Provide a 20 mm distance between each electrode's pole and place the reference electrode in the ankle.
- Determine the required muscle contraction force
- Use a digital pinch dynamometer and a quadrangular pyramid support to minimize mechanical distortions and elevate the sensitivity for minimal contractions.
- Place the dynamometer between the first and second fingers with the help of the pyramidal support. Ensure that the third, fourth, and fifth fingers lay still on the table, while the 1st and 2nd generate the forces of the pinching movement.
- With the fixed position, ask the participant to press the dynamometer with the first finger and the side of the pyramid with the index finger, squeezing the dynamometer-pyramid system with their maximum force and creating a strong contraction of the FDI muscle.
- Using that value as reference, determine the 20% of maximum force. The participant must practice maintaining the target at 20% of sustained contraction. Allow for variations from 15%-25% of MVC.
NOTE: Alternatively, in case of unavailability of a dynamometer that can catch the isolated muscle activity being investigated, use EMG feedback to standardize force. The recording software will measure the maximum peak-to-peak amplitude that corresponds to the subject's maximum force, and using that value as reference, will determine the 20% MVC. Subjects can receive visual and/or auditory clues of when 20% is achieved.
- Identification of the initial location for hotspot searching
- Put a swim cap on the subject's head. All the reference points will be marked on it.
- Measure the sagittal circumference of the head from the nasion (the point between the forehead and the nose) to the inion (the most prominent point in the occipital region). Divide that value by two and mark that middle spot on the head.
- Mark the location of the patient's nasion, inion, the helix of both right and left external ears, and right and left supraorbital ridge. This is to certify that the cap has not slipped during the procedure, and/or that in future experiments it will be equally positioned on the patient's head.
- As described above, measure the tragus-to-tragus distance and add a mark halfway. Mark the intersection between them, a point identified as the vertex (Cz).
- From the vertex, move 5 cm laterally in parallel to the midsagittal line, on the contralateral side to the selected muscle. This mark approximately identifies the (M1), on the same coronal level as the hand motor cortex. Use this as the first spot to initiate the search for the hotspot.
- The hotspot is the area of the motor cortex where the lowest motor threshold is detectable. Set up a low intensity (e.g., 30% of maximum stimulator output [MSO]) and initiate the search by delivering multiple pulses to the first spot.
- Pursue with small intensity increments until identifying the lowest stimulus that detects an EMG-indexed response (i.e., MEP). For the delivery of the stimuli, angle the figure-of-eight coil at 45° in relation to the midsagittal line with the handle pointed toward the posterior of the patient.
- To ensure that the best spot was identified, move around the first spot and test the subsequent ~3 MEPs at 1 cm anterior, 1 cm lateral, 1 cm medial, and 1 cm posterior to it. Repeat this procedure as many times as needed for a consistent response; stick to the spot that elicits the largest MEP36.
- Once the hotspot is found, mark that spot in the patient's head (swim cap). Use this location during this experiment and the potential follow-up visits. Be cautious as not to cause discomfort to the subject due to extra pressure. Use both hands to support the coil on the subject's head.
- Determine resting motor threshold (RMT)
- Estimate the motor threshold as the minimum intensity required to promote an MEP of a minimal detectable amplitude (usually at least 50-100 µV).
- To determine the motor threshold, apply ten consecutive stimuli at the hotspot and select the lowest intensity that produced an MEP with a peak-to-peak amplitude of at least 50 µV on the target muscle, in 50% of the trials.
NOTE: This protocol can be done with the target muscle at rest (resting motor threshold [RMT]) or during active contraction (active motor threshold [AMT]). Both can further be used as references for the suprathreshold TMS pulses. The acquisition of the AMT is more prone to variability because it relies on the standardization of MVC, which can be an issue for longitudinal studies with multiple assessments.
- CSP protocol
- Deliver suprathreshold stimuli to elicit MEPs during tonic voluntary contraction of the target muscle.
- Deliver 10 stimuli with the stimulation intensity (SI) of 120% of the RMT with 10 s period in between them. During the application of the stimuli, ask the patient to maintain 20% of the maximum motor contraction of the target muscle, as practiced with the dynamometer.
- To ensure capturing the whole SP, certify that the EMG time window is long enough to capture up to 400 ms of EMG activity. Not infrequently – depending on the disease being studied – subjects might require higher SIs for a successful cSP to be obtained.
Representative Results
After following the step-by-step procedure, the delivery of a suprathreshold TMS pulse (120% of the RMT) will elicit an observable MEP in the EMG recording of the target muscle, and a subsequent period of background EMG activity suppression of approximately 150 ms to 300 ms (Figure 2). From that EMG pattern, it's possible to calculate the cSP metrics. The most reported outcomes are the duration (in the range of ms) of the relative and absolute SP. The relative SP is measured from the MEP start to the reappearance of EMG activity. One alternative is using the amplified motor stimulated output (MSO = 120% of RMT, as per the protocol) to establish the onset of relative SP. Since the true onset at a network level cannot be known, select the MEP onset as the initial start point to increase experimental reliability13. On the other hand, the absolute SP can be measured from the end of the MEP to the onset of the reemergence of voluntary EMG activity. For instance, using a recording of the resting EMG activity of the subject as reference for qualitative comparison. Those temporal parameters can be identified manually or using automated software37.
A fundamental methodological question for accurate cSP calculation is the definition of the reemergence of EMG background activity. Two approaches can be explored here: The first is using individual trial calculation. In this case, the calculation is based on trial-by-trial measure, using each recording to calculate the cSP duration. Then, a mean (or median) of the individual trials can be calculated and reported. The second approach is using rectified multiple trials. For this approach, all trials will be rectified, and then have to be averaged and overlapped with each other. Then, using the rectified and averaged traces, calculate the cSP duration using average temporal marks. The main advantage of this method is its precision and easier identification of the reappearance of voluntary EMG activity relative to the tonic baseline EMG level36. Using rectified average is advantageous because it is more comparable and reduces the between-subject variability.
It is important to mention that the cSP duration can be prolonged as a sigmoid function of stimulus intensity38, but it is barely affected by the degree of intentional contraction of the target muscle39. Moreover, MEP amplitudes increase with increases in stimulus intensities. Kojima et al. demonstrated that these increases in MEP amplitude (secondary to increases in intensity) is also accompanied by increases in cSP duration40. This behavior is expected since the MEP and cSP duration are thought to be influenced by common factors38. These common factors seem to be present throughout the cortical spinal tract and not in the motor unit; since the increases in stimulus intensity increases both, but increases in muscles contraction do not affect the cSP duration.
With this discussion, it is possible to conclude that stimulus intensity and muscle contraction must be carefully considered during the analysis and interpretation of findings. The cSP is characterized by a linear increase due to the SI, but then a plateau is reached at high intensities; this pattern is highly variable across subjects39, since they could have unique slopes and different plateau intensities. One alternative analysis could include evaluating the cSP during progressively increased intensities to perform an input-output (I/O) curve, and then the cSP can be obtained using the intensity at which the I/O curve reaches the plateau41,42. Finally, since cSP is affected by any activity or exposure that can produce cortical excitability and inhibition changes, it is recommended to assess and record general confounders in the analysis. For instance, using a reporting checklist for TMS experiments43.
cSP interpretation
The TMS test in the current study was used to show the implementation of a feasible and versatile biomarker of M1 inhibition. In general, the longer the duration of the SP, a higher corticospinal M1 inhibition is observed44. However, several factors need to be considered for its interpretation. First, the cSP is defined by both spinal and cortico-subcortical processes45. The spinal components account for approximately the first 50 ms46. The remaining duration is highly influenced by cortical mechanisms such as M1 interneurons inhibition and other inhibitory afferences within M1 (from subcortical regions and other cortices), mediated mainly by GABAergic B neurons after an important cortical activation eliciting MEPs6. It has been suggested that the role of this inhibition is to prevent unwanted movements and sustain motor control47. Second, behavioral and cognitive factors can impact the CSP duration, as well as motor and non-motor neuropsychiatric disorders45,48. Due to this dual nature of cSP, its values need to be interpreted within the experiment context (target population and the use of concomitant motor control tasks).
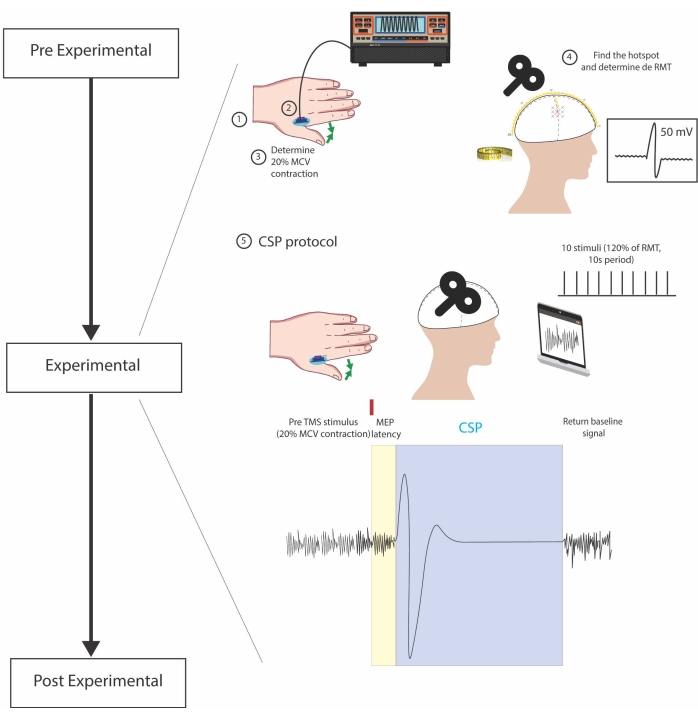
Figure 1: Experimental steps. 1. Electrode placement on the belly of the FDI muscle 2. Positioning of the dynamometer between fingers. 3. Voluntary contraction of the target muscle to test the standardization of 20% MVC 4. Head measurements and TMS pulses for identification of the hotspot and the RMT (lowest stimuli that elicits an MEP of at least 50 mV in five out of ten trials) 5. CSP protocol, consistent of 10 pulses with 120% RMT spaced out by 10s, during sustained muscle contraction. In the bottom center figure, the small red rectangle represents a single TMS pulse and divides pre-TMS stimulus (sustained muscle contraction and background EMG activity) and the cSP recording. CSP is considered from the start of the MEP until the reemergence of EMG baseline activity, represented inside the blue rectangle. In the yellow rectangle, the MEP latency is shown. Please click here to view a larger version of this figure.
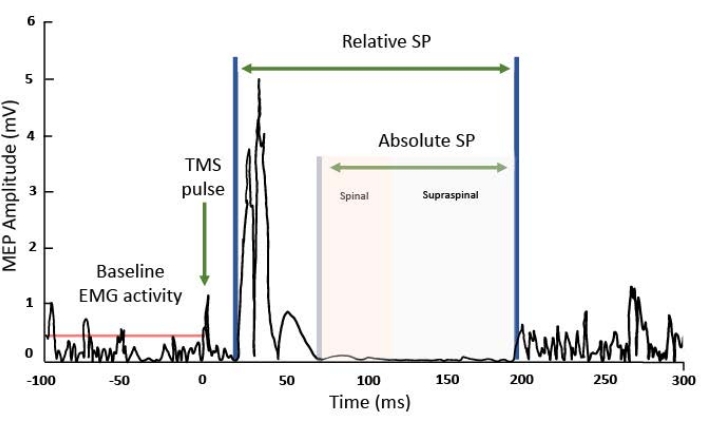
Figure 2: MEP in the EMG recording of the target muscle. On the X-axis, milliseconds (ms), and the Y axis, millivolts (mV) of the EMG signal. From left to right: the red line indicates the background electrical muscle activity before the MEP, subsequently, after the electric effect of the TMS pulse is observed it is followed by the motor-evoked potential. After the MEP, there is a suppression of the EMG signal known as the SP. It can be relative, counting the interval from the beginning of the MEP wave to the return of EMG background activity or absolute SP, counting the interval from the end of the MEP to the return of the background wave. Please click here to view a larger version of this figure.
Discussion
The default SI to elicit MEP and SPs can vary according to the population. Intensities as low as 80% RMT have been shown to elicit cSP in healthy individuals39, still studies on both healthy and diseased populations have used intensities as high as 150% RMT49,50,51. Although this source of heterogeneity can be inherent to the nature of the target population, it should not be neglected as different SIs have shown to independently (regardless of muscle contraction force) dictate the period of silent EMG activity following the MEP39,49,52. RMTs from 110% to 120% have successfully elicited SPs in a broad range of populations, while still being tolerable for participants53,54. However, 110% RMT might be borderline, since SIs lower than 110% have failed to elicit SPs or elicited SPs with duration shorter than 50 ms39, which might represent solely spinal rather than cortical or corticospinal components of M1 inhibition. Moreover, higher SIs are associated with diminished stimulation focality and increased patient discomfort-especially in diseased populations with higher RMTs55, in which high percentages of suprathreshold stimuli could correspond to close-to-maximum stimulator output. This can jeopardize participants' adherence to the used protocol56. Even though using 120% RMT seems to be the safest and most suitable SI setup overall, researchers should standardize the SI by checking previous successful experiments done in the population of interest. Standardization across similar populations is important to facilitate further pooled statistics.
The experiment is typically done using a single SI, but some studies have investigated cSP responses at more than one stimulus setup53,57,58,59,60. In the context of conditions with no clear pathophysiology or previous literature, or in which the understanding of the SP behavior is the objective of the study, it is recommended to plot cSPs against subsequent increasing stimulus intensities (i.e., 10% increment in a sigmoid stimulus-response curve)42. In that case, the researcher should consider adding to the protocol resting breaks to avoid muscle fatigue. Even though still contradictory, there is a considerable amount of evidence that cSPs are not influenced by the level of muscle contraction39,61,62; however, it is impacted by muscle fatigue63,64,65,66. A value of 20% of maximum voluntary contraction (MVC) has consistently shown to successfully elicit SPs with being less likely to induce fatigue60,67,68,69.
Another significant parameter that may contribute to the heterogeneity of cSP results in the literature is the selected muscle to evaluate cSP after the TMS stimuli. Studies have found that different muscles may recruit distinctive motor neuron networks, which in turn will have diverging cSP effects. This is true not only for upper versus lower limb musculature, but also for proximal and distal muscles of the same limb. For instance, in two separate studies, Van Kuijk and colleagues have conveyed a more significant sensitivity to TMS parameters, such as cSP, in distal upper-extremity muscles as compared to proximal muscles70,71. And although this difference has not always been statistically significant71, it is still noteworthy and may contribute to heterogeneous results. Moreover, a significant difference in cSP responses in upper and lower limb muscles has also been denoted in studies about fatigue, with upper limbs conveying 30% longer SPs than lower limbs72. Thus, to reduce heterogeneity in cSP results, it is important to standardize the muscle in which the cSP assessment will be evaluated as some are more sensitive to TMS stimuli than others. Therefore, different muscles can drastically change the procedure specificities and interpretation. To illustrate, cSP is also used to evaluate cortical excitability in deeper muscles, such as the laryngeal motor units. Applying the cSP protocol to these structures comes with unique challenges. An example is that of the laryngeal motor cortex; the stimulation site of this protocol is near the EMG electrode, which can increase the number of artifacts requiring adjustments to the EMG amplifier73. Also, needle electrodes that penetrate the skin are needed to measure the EMG activity of these muscles, making the placement, and if needed, reallocation of the electrodes difficult, as well as changing the interpretation of results. Therefore, a limitation of this methodological paper is that its scope is restricted to illustrating a protocol for upper and lower limbs, and that does encompass, for example, the field that explores cSP as a marker of corticobulbar inhibition or psychiatry conditions.
In that matter, literature search supports that the FDI is the most commonly used target muscle for studying upper limb M1 corticospinal inhibition. Reasons include but are not limited to its superficial and large cortical representation in the motor cortex, the lowest motor threshold for stimulation, and the simplicity to perform its isolated and sustained contraction as well as the positioning of the electrodes73,74. For the lower limbs, the use of the TA muscle is most frequent-likely due to its larger cortical representation in comparison to other leg muscles75. Also, the easiness to be isolated from the activity of large muscle groups that compose the lower limbs' musculature plays a role. Despite the importance of lower limb (LL) rehabilitation in the field, fewer studies use the LL MEP given its particular challenges. The brain anatomical region of the LL is more medial and deeper in the inter-hemispheric fissure compared to the upper limbs. However, the use of Neuronavigation has improved the accuracy of the stimuli36, while the use of a double cone coil has successfully targeted LL regions, including the TA muscle, showing lower LL MT than other coil types76,77,78,79, and currently being the standard recommendation to target LL36,44. However, the usage of modern navigation technologies should be contemplated parallel to the feasibility of the protocol. Jung et al. (2010) revealed no significant difference in MEP variability and reproducibility between non-navigated TMS and TMS navigation, which reach a comparable performance level80. Using non-navigated TMS can be more cost-effective in specific circumstances (i.e., limited resources), and therefore was the preferred approach for this protocol that aims to demonstrate a feasible, easy, and reproducible cSP assessment.
Given the promising and versatile use of cSP as a corticospinal inhibition biomarker in different neurological disorders, it is essential to provide researchers with a feasible, reproducible, and still reliable cSP protocol for upper and lower limbs. We highlight that only a few muscles could be represented in the experiment, leading to the absence of investigation of cSP for corticobulbar inhibition. Moreover, the results of the non-exhaustive search provided in Table 1 are not an attempt to summarize existing data but rather to illustrate part of the rationale behind the choice of parameters and insights, therefore, conducted with no scientific rigor. Hopefully, this methodological paper will help researchers advance the investigation of cSP as a biomarker for M1 corticospinal inhibition.
Table 1: Different parameters used on cSP protocols. We extracted data of cSP experiments from 117 different articles. Results are reported if the paradigm was used in ≥2 experiments, otherwise they were gathered within others. †Includes articles that either did not report the method of standardization or that reported not applying standardization. Abbreviations: MVC = maximum voluntary contraction. Please click here to download this Table.
Divulgazioni
The authors have nothing to disclose.
Acknowledgements
No acknowledgments.
Materials
| Alcohol pads | Medline | Preparation with 70% isopropyl alcohol | |
| Conductive gel | Weaver and Company | Used on the electrode | |
| Echo Pinch | JTECH medical | 0902A302 | Digital dynamometer. |
| Mega-EMG | Soterix Medical | NS006201 | Digital multiple channel EMG with built in software. |
| MEGA-TMS coil | Soterix Medical | NS063201 | 8 shaped TMS coil |
| Mega-TMS stimulator | Soterix Medical | 6990061 | Single Pulse TMS |
| Neuro-MEP.NET | Soterix Medical | EMG software used to analyse the muscles eletrical activity. | |
| Swim cap | Kiefer |
Riferimenti
- Li, J. Y., Lai, P. H., Chen, R. Transcallosal inhibition in patients with callosal infarction. Journal of Neurophysiology. 109 (3), 659-665 (2013).
- Wassermann, E. M., Fuhr, P., Cohen, L. G., Hallett, M. Effects of transcranial magnetic stimulation on ipsilateral muscles. Neurology. 41 (11), 1795-1799 (1991).
- Fuhr, P., Agostino, R., Hallett, M. Spinal motor neuron excitability during the silent period after cortical stimulation. Electroencephalography and Clinical Neurophysiology. 81 (4), 257-262 (1991).
- Meyer, B. U., Röricht, S., Gräfin von Einsiedel, H., Kruggel, F., Weindl, A. Inhibitory and excitatory interhemispheric transfers between motor cortical areas in normal humans and patients with abnormalities of the corpus callosum. Brain. 118, 429-440 (1995).
- Hupfeld, K. E., Swanson, C. W., Fling, B. W., Seidler, R. D. TMS-induced silent periods: A review of methods and call for consistency). Journal of Neuroscience Methods. 346, 108950 (2020).
- Siebner, H. R., Dressnandt, J., Auer, C., Conrad, B. Continuous intrathecal baclofen infusions induced a marked increase of the transcranially evoked silent period in a patient with generalized dystonia. Muscle Nerve. 21 (9), 1209-1212 (1998).
- Vallence, A. M., Smalley, E., Drummond, P. D., Hammond, G. R. Long-interval intracortical inhibition is asymmetric in young but not older adults. Journal of Neurophysiology. 118 (3), 1581-1590 (2017).
- Manconi, F. M., Syed, N. A., Floeter, M. K. Mechanisms underlying spinal motor neuron excitability during the cutaneous silent period in humans. Muscle Nerve. 21 (10), 1256-1264 (1998).
- Romanò, C., Schieppati, M. Reflex excitability of human soleus motoneurones during voluntary shortening or lengthening contractions. The Journal of Physiology. 390, 271-284 (1987).
- Cantello, R., Gianelli, M., Civardi, C., Mutani, R. Magnetic brain stimulation: the silent period after the motor evoked potential. Neurology. 42 (10), 1951-1959 (1992).
- Rossini, P. M., et al. Non-invasive electrical and magnetic stimulation of the brain, spinal cord, roots and peripheral nerves: Basic principles and procedures for routine clinical and research application. An updated report from an I.F.C.N. Committee. Clinical Neurophysiology. 126 (6), 1071-1107 (2015).
- Classen, J., Benecke, R. Inhibitory phenomena in individual motor units induced by transcranial magnetic stimulation. Electroencephalography and Clinical Neurophysiology. 97 (5), 264-274 (1995).
- Inghilleri, M., Berardelli, A., Cruccu, G., Manfredi, M. Silent period evoked by transcranial stimulation of the human cortex and cervicomedullary junction. The Journal of Physiology. 466, 521-534 (1993).
- Roick, H., von Giesen, H. J., Benecke, R. On the origin of the postexcitatory inhibition seen after transcranial magnetic brain stimulation in awake human subjects. Experimental Brain Research. 94 (3), 489-498 (1993).
- Chen, R., Lozano, A. M., Ashby, P. Mechanism of the silent period following transcranial magnetic stimulation. Evidence from epidural recordings. Experimental Brain Research. 128 (4), 539-542 (1999).
- Schnitzler, A., Benecke, R. The silent period after transcranial magnetic stimulation is of exclusive cortical origin: evidence from isolated cortical ischemic lesions in man. Neuroscience Letters. 180 (1), 41-45 (1994).
- Cantello, R., Tarletti, R., Civardi, C. Transcranial magnetic stimulation and Parkinson’s disease. Brain Research. Brain Research Reviews. 38 (3), 309-327 (2002).
- Ziemann, U., Netz, J., Szelényi, A., Hömberg, V. Spinal and supraspinal mechanisms contribute to the silent period in the contracting soleus muscle after transcranial magnetic stimulation of human motor cortex. Neuroscience Letters. 156 (1-2), 167-171 (1993).
- Paci, M., Di Cosmo, G., Perrucci, M. G., Ferri, F., Costantini, M. Cortical silent period reflects individual differences in action stopping performance. Scientific Reports. 11 (1), 15158 (2021).
- Poston, B., Kukke, S. N., Paine, R. W., Francis, S., Hallett, M. Cortical silent period duration and its implications for surround inhibition of a hand muscle. The European Journal of Neuroscience. 36 (7), 2964-2971 (2012).
- Vidor, L. P., et al. Association of anxiety with intracortical inhibition and descending pain modulation in chronic myofascial pain syndrome. BMC Neuroscience. 15, 42 (2014).
- Bradnam, L., et al. Afferent inhibition and cortical silent periods in shoulder primary motor cortex and effect of a suprascapular nerve block in people experiencing chronic shoulder pain. Clinical Neurophysiology. 127 (1), 769-778 (2016).
- Simis, M., et al. Increased motor cortex inhibition as a marker of compensation to chronic pain in knee osteoarthritis. Scientific Reports. 11 (1), 24011 (2021).
- List, J., et al. Cortical reorganization due to impaired cerebral autoregulation in individuals with occlusive processes of the internal carotid artery. Brain Stimulation. 7 (3), 381-387 (2014).
- Gray, W. A., Palmer, J. A., Wolf, S. L., Borich, M. R. Abnormal EEG responses to TMS during the cortical silent period are associated with hand function in chronic stroke. Neurorehabilitation and Neural Repair. 31 (7), 666-676 (2017).
- Braune, H. J., Fritz, C. Transcranial magnetic stimulation-evoked inhibition of voluntary muscle activity (silent period) is impaired in patients with ischemic hemispheric lesion. Stroke. 26 (4), 550-553 (1995).
- Goodwill, A. M., Teo, W. -. P., Morgan, P., Daly, R. M., Kidgell, D. J. Bihemispheric-tDCS and upper limb rehabilitation improves retention of motor function in chronic stroke: A pilot study. Frontiers in Human Neuroscience. 10, 258 (2016).
- Cincotta, M., et al. Suprathreshold 0.3 Hz repetitive TMS prolongs the cortical silent period: potential implications for therapeutic trials in epilepsy. Clinical Neurophysiology. 114 (10), 1827-1833 (2003).
- Langguth, B., et al. Transcranial magnetic stimulation for the treatment of tinnitus: effects on cortical excitability. BMC Neuroscience. 8, 45 (2007).
- Priori, A., et al. Rhythm-specific pharmacological modulation of subthalamic activity in Parkinson’s disease. Experimental Neurology. 189 (2), 369-379 (2004).
- Salerno, A., et al. Motor cortical dysfunction disclosed by single and double magnetic stimulation in patients with fibromyalgia. Clinical Neurophysiology. 111 (6), 994-1001 (2000).
- Rossi, S., Hallett, M., Rossini, P. M., Pascual-Leone, A. Screening questionnaire before TMS: an update. Clinical Neurophysiology. 122 (8), 1686 (2011).
- Rossi, S., Hallett, M., Rossini, P. M., Pascual-Leone, A. Safety of, T.M.S.C.G. Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clinical Neurophysiology. 120 (12), 2008-2039 (2009).
- Rossi, S., et al. Safety and recommendations for TMS use in healthy subjects and patient populations, with updates on training, ethical and regulatory issues: Expert Guidelines. Clinical Neurophysiology. 132 (1), 269-306 (2021).
- Hermens, H. J., Freriks, B., Disselhorst-Klug, C., Rau, G. Development of recommendations for SEMG sensors and sensor placement procedures. Journal of Electromyography and Kinesiology. 10 (5), 361-374 (2000).
- Groppa, S., et al. A practical guide to diagnostic transcranial magnetic stimulation: report of an IFCN committee. Clinical Neurophysiology. 123 (5), 858-882 (2012).
- Daskalakis, Z. J., et al. An automated method to determine the transcranial magnetic stimulation-induced contralateral silent period. Clinical Neurophysiology. 114 (5), 938-944 (2003).
- Orth, M., Rothwell, J. C. The cortical silent period: intrinsic variability and relation to the waveform of the transcranial magnetic stimulation pulse. Clinical Neurophysiology. 115 (5), 1076-1082 (2004).
- Säisänen, L., et al. Factors influencing cortical silent period: optimized stimulus location, intensity and muscle contraction. Journal of Neuroscience Methods. 169 (1), 231-238 (2008).
- Kojima, S., et al. Modulation of the cortical silent period elicited by single- and paired-pulse transcranial magnetic stimulation. BMC Neuroscience. 14 (1), 43 (2013).
- Poston, B., Kukke, S. N., Paine, R. W., Francis, S., Hallett, M. Cortical silent period duration and its implications for surround inhibition of a hand muscle. The European Journal of Neuroscience. 36 (7), 2964-2971 (2012).
- Kimiskidis, V. K., et al. Silent period to transcranial magnetic stimulation: construction and properties of stimulus-response curves in healthy volunteers. Experimental Brain Research. 163 (1), 21-31 (2005).
- Chipchase, L., et al. A checklist for assessing the methodological quality of studies using transcranial magnetic stimulation to study the motor system: an international consensus study. Clinical Neurophysiology. 123 (9), 1698-1704 (2012).
- Rossini, P. M., et al. Non-invasive electrical and magnetic stimulation of the brain, spinal cord, roots and peripheral nerves: Basic principles and procedures for routine clinical and research application. An updated report from an I.F.C.N. Committee. Clinical Neurophysiology. 126 (6), 1071-1107 (2015).
- Zeugin, D., Ionta, S. Anatomo-Functional origins of the cortical silent period: Spotlight on the basal ganglia. Brain Sciences. 11 (6), 705 (2021).
- Person, R. S., Kozhina, G. V. Investigation of the silent period by a poststimulus histogram method. Neurophysiology. 10 (2), 123-129 (1978).
- Stinear, C. M., Coxon, J. P., Byblow, W. D. Primary motor cortex and movement prevention: where Stop meets Go. Neuroscience and Biobehavioral Reviews. 33 (5), 662-673 (2009).
- Mathis, J., de Quervain, D., Hess, C. W. Dependence of the transcranially induced silent period on the ‘instruction set’ and the individual reaction time. Electroencephalography and Clinical Neurophysiology. 109 (5), 426-435 (1998).
- Chandra, S. R., Issac, T. G., Nagaraju, B. C., Philip, M. A study of cortical excitability, central motor conduction, and cortical inhibition using single pulse transcranial magnetic stimulation in patients with early frontotemporal and Alzheimer’s Dementia. Indian Journal of Psychological Medicine. 38 (1), 25-30 (2016).
- Bocci, T., et al. Spinal direct current stimulation modulates short intracortical inhibition. Neuromodulation. 18 (8), 686-693 (2015).
- Zunhammer, M., et al. Modulation of human motor cortex excitability by valproate. Psychopharmacology (Berl). 215 (2), 277-280 (2011).
- Ho, K. H., Nithi, K., Mills, K. R. Covariation between human intrinsic hand muscles of the silent periods and compound muscle action potentials evoked by magnetic brain stimulation: evidence for common inhibitory connections. Experimental Brain Research. 122 (4), 433-440 (1998).
- Acler, M., Fiaschi, A., Manganotti, P. Long-term levodopa administration in chronic stroke patients. A clinical and neurophysiologic single-blind placebo-controlled cross-over pilot study. Restorative Neurology and Neuroscience. 27 (4), 277-283 (2009).
- Volz, M. S., et al. Dissociation of motor task-induced cortical excitability and pain perception changes in healthy volunteers. PLoS One. 7 (3), 34273 (2012).
- Veldema, J., Nowak, D. A., Gharabaghi, A. Resting motor threshold in the course of hand motor recovery after stroke: a systematic review. Journal of Neuroengineering and Rehabilitation. 18 (1), 158 (2021).
- Rossi, S., et al. Safety and recommendations for TMS use in healthy subjects and patient populations, with updates on training, ethical and regulatory issues: Expert Guidelines. Clinical Neurophysiology. 132 (1), 269-306 (2021).
- Ortu, E., et al. Primary motor cortex hyperexcitability in Fabry’s disease. Clinical Neurophysiology. 124 (7), 1381-1389 (2013).
- Goodwill, A. M., Teo, W. P., Morgan, P., Daly, R. M., Kidgell, D. J. Bihemispheric-tDCS and upper limb rehabilitation improves retention of motor function in chronic stroke: A pilot study. Frontiers in Human Neuroscience. 10, 258 (2016).
- Mayorga, T., et al. Motor-Evoked potentials of the abductor hallucis muscle and their relationship with foot arch functional anatomy. Journal of American Podiatric Medical Association. 107 (5), 467-470 (2017).
- Matsugi, A., et al. Cerebellar transcranial magnetic stimulation reduces the silent period on hand muscle electromyography during force control. Brain Science. 10 (2), 63 (2020).
- van Kuijk, A. A., Pasman, J. W., Geurts, A. C., Hendricks, H. T. How salient is the silent period? The role of the silent period in the prognosis of upper extremity motor recovery after severe stroke. Journal of Clinical Neurophysiology. 22 (1), 10-24 (2005).
- Wu, L., Goto, Y., Taniwaki, T., Kinukawa, N., Tobimatsu, S. Different patterns of excitation and inhibition of the small hand and forearm muscles from magnetic brain stimulation in humans. Clinical Neurophysiology. 113 (8), 1286-1294 (2002).
- Hunter, S. K., Todd, G., Butler, J. E., Gandevia, S. C., Taylor, J. L. Recovery from supraspinal fatigue is slowed in old adults after fatiguing maximal isometric contractions. Journal of Applied Physiology. 105 (4), 1199-1209 (2008).
- Yoon, T., Schlinder-Delap, B., Keller, M. L., Hunter, S. K. Supraspinal fatigue impedes recovery from a low-intensity sustained contraction in old adults. Journal of Applied Physiology. 112 (5), 849-858 (2012).
- Kennedy, D. S., McNeil, C. J., Gandevia, S. C., Taylor, J. L. Effects of fatigue on corticospinal excitability of the human knee extensors. Experimental Physiology. 101 (12), 1552-1564 (2016).
- Goodall, S., Howatson, G., Thomas, K. Modulation of specific inhibitory networks in fatigued locomotor muscles of healthy males. Experimental Brain Research. 236 (2), 463-473 (2018).
- Neva, J. L., et al. Multiple measures of corticospinal excitability are associated with clinical features of multiple sclerosis. Behavioural Brain Research. 297, 187-195 (2016).
- Caumo, W., et al. Motor cortex excitability and BDNF levels in chronic musculoskeletal pain according to structural pathology. Frontiers in Human Neuroscience. 10, 357 (2016).
- Chen, M., Deng, H., Schmidt, R. L., Kimberley, T. J. Low-Frequency repetitive transcranial magnetic stimulation targeted to premotor cortex followed by primary motor cortex modulates excitability differently than premotor cortex or primary motor cortex stimulation alone. Neuromodulation. 18 (8), 678-685 (2015).
- van Kuijk, A. A., et al. Definition dependent properties of the cortical silent period in upper-extremity muscles, a methodological study. Journal of Neuroengineering and Rehabilitation. 11, 1 (2014).
- van Kuijk, A. A., et al. Stimulus-response characteristics of motor evoked potentials and silent periods in proximal and distal upper-extremity muscles. Journal of Electromyography and Kinesiology. 19 (4), 574-583 (2009).
- Vernillo, G., Temesi, J., Martin, M., Millet, G. Y. Mechanisms of fatigue and recovery in upper versus lower limbs in men. Medicine and Science in Sports and Exercise. 50 (2), 334-343 (2018).
- Chen, M., et al. Evaluation of the cortical silent period of the laryngeal motor cortex in healthy individuals. Frontiers in Neuroscience. 11, 88 (2017).
- Masakado, Y., Akaboshi, K., Nagata, M., Kimura, A., Chino, N. Motor unit firing behavior in slow and fast contractions of the first dorsal interosseous muscle of healthy men. Electroencephalography and Clinical Neurophysiology. 97 (6), 290-295 (1995).
- Petersen, N. T., Pyndt, H. S., Nielsen, J. B. Investigating human motor control by transcranial magnetic stimulation. Experimental Brain Research. 152 (1), 1-16 (2003).
- Dharmadasa, T., et al. The effect of coil type and limb dominance in the assessment of lower-limb motor cortex excitability using TMS. Neuroscience Letters. 699, 84-90 (2019).
- Kesar, T. M., Stinear, J. W., Wolf, S. L. The use of transcranial magnetic stimulation to evaluate cortical excitability of lower limb musculature: Challenges and opportunities. Restorative Neurology and Neuroscience. 36 (3), 333-348 (2018).
- Proessl, F., et al. Characterizing off-target corticospinal responses to double-cone transcranial magnetic stimulation. Experimental Brain Research. 239 (4), 1099-1110 (2021).
- Dharmadasa, T., et al. The effect of coil type and limb dominance in the assessment of lower-limb motor cortex excitability using TMS. Neuroscience Letters. 699, 84-90 (2019).
- Jung, N. H., et al. Navigated transcranial magnetic stimulation does not decrease the variability of motor-evoked potentials. Brain Stimulation. 3 (2), 87-94 (2010).

