Forming, Confining, and Observing Microtubule-Based Active Nematics
Summary
Presented here are methods for preparing active nematics from microtubules and kinesin motors, including protein preparation and construction and the use of wells for active nematic confinement.
Abstract
The formation of biopolymer-based active phases has become an important technique for researchers interested in exploring the emerging field of active liquid crystals and their possible roles in cell biology. These novel systems consist of self-driven sub-units that consume energy locally, producing an out-of-equilibrium dynamic fluid. To form the active liquid crystal phase described in this report, purified protein components including biopolymers and molecular motors are combined, and the active nematic phase spontaneously forms in the presence of adenosine triphosphate (ATP). To observe the nematic state, the material must be confined in a suitable geometry for microscopy at a high enough density. This article describes two different methods for the formation of an active nematic phase using microtubules and kinesin motors: assembly of a two-dimensional active layer at an oil and water interface and assembly under an oil layer using an elastomeric well. Techniques to insert the active material into small wells of different shapes are also described.
Introduction
Active fluids are composed of energy-driven particles or elements that draw fuel from their local environment. Under the right conditions, these motile active elements can act collectively to produce emergent fluid dynamics over long length-scales. There are a variety of examples of such out-of-equilibrium phase behavior in the literature and active phases can be found across the spectrum of living systems. Some notable examples are colonies of bacteria1, cell sheets2,3, and the flocking or swarming of organisms4,5. Active phases have also been studied extensively in condensed phases of cytoskeletal filaments, either as part of the cell6 or in synthetic systems designed to make use of biologically extracted components7,8,9. Liquid crystalline ordering and the formation of topological defects in both naturally occurring and synthetic systems assembled from biological extracts are of particular interest to the research community. In recent years, research groups have examined such systems, their fundamental physical properties, and their relevance to biology2,3,10,11.
This paper focuses on the formation of the active nematic state from a combination of microtubules and kinesin motor proteins. The traditional nematic liquid crystal is an equilibrium phase of matter in which the constituent molecules exhibit orientational ordering. For example, a fluid consisting of relatively rigid rod-like molecules may exhibit both the nematic phase and, at higher temperatures, an unoriented isotropic fluid phase12. The first experimental example of an active nematic phase was developed by Sanchez et al.13, adapting an earlier in vitro experiment14 in which clusters of motor proteins were used to produce a shearing motion between neighboring microtubule bundles. When this microtubule system was confined to a thin layer, spontaneous nematic ordering emerged. In recent years, the active nematic state has been studied intensively by several experimental15,16 and theoretical17,18 research groups, focusing on phenomena such as active turbulence — a state in which the fluid produces self-driven chaotic flows19 — and mobile topological defects. This paper describes methods to prepare and form the active nematic state from microtubules and kinesin motors in different experimental geometries. First, preparation methods for the different component solutions are described, followed by methods for forming the active nematic using two different flow chamber geometries. Typical imaging results are shown. Finally, methods for confining the active nematic in wells and channels are described.
Protocol
1. Preparing the active material
NOTE: The 2D active nematic is assembled in a three-step process. First, two separate solutions are prepared: a) polymerized, stabilized microtubules and b) MIX (a solution containing kinesin motors). These are combined and activity is initiated upon adding adenosine triphosphate (ATP). The material is then confined in a suitable geometry, such that its density is high enough for nematic order to emerge. Protocols are included for the preparation of all necessary components and how to assemble the active phase.
- Kinesin motor protein cluster preparation
- Express and purify recombinant K401-BIO motor proteins (K401 motors) from Escherichia coli following the protocol by Edgar C. Young20.
NOTE: K401-BIO motor proteins are dimeric and consist of two heads connected with a helical stalk. The motors were supplied by the Brandeis Biomaterials Facility and used as previously reported16. For the purposes of forming an active nematic, the kinesin molecules are biotinylated and then connected via a streptavidin linkage to form kinesin clusters of up to four motors13,15,21,22. It is helpful to express the kinesin with a green fluorescent protein (GFP) tag. - After purification and incubation of streptavidin and motors on ice for 40 min, flash-freeze the K401 motors in liquid nitrogen in 5 µL aliquots at a final concentration of 0.7 mg/mL, and store at -80 °C.
NOTE: The experiment can be paused here. Gently thaw the kinesin when needed and do not refreeze. - Prepare clusters of biotin-labeled kinesin (KSA) by mixing 24 vol% of 0.7 mg/mL K401 motors, 27 vol% of 0.325 mg/mL streptavidin, and 3 vol% of 5 mM dithiothreitol (DTT) (to prevent aggregation) at 4 °C in 46 vol% M2B buffer (80 mM PIPES [1,4-Piperazinediethanesulfonic acid, pH 6.8], 2 mM MgCl2, and 1 mM EGTA [ethylene glycol-bis(β-aminoethyl ether)-N,N,N′,N′-tetraacetic acid]). Let the KSA incubate on ice for 40 min.
- Express and purify recombinant K401-BIO motor proteins (K401 motors) from Escherichia coli following the protocol by Edgar C. Young20.
- Microtubule solution preparation
NOTE: Guanosine-5'-[(α,β)-methyleno]triphosphate, sodium salt (GMPCPP) is a slowly hydrolysable analog of Guanosine triphosphate (GTP), and microtubules formed in the presence of GMPCPP are three times stiffer than GTP microtubules23 and shorter. The use of short, stiff microtubules is favorable for the formation of the active nematic phase as these factors combine to promote liquid crystalline ordering.- Polymerize unlabeled cycled tubulin (99%, see Table of Materials) using 0.6 mM GMPCPP at a tubulin concentration of 6 mg/mL.
NOTE: High quality tubulin can be also purified from bovine or porcine brain following established protocols or obtained from another reliable source such as the Brandeis Biomaterials Facility where materials can be shipped frozen to prevent damage. For tubulin purification from bovine brain, please refer to the published protocol from Bate et al.24. For tubulin purification from porcine brain, refer to the published protocols from Castoldi et al.25 and Tayar et al.26. - In preparation for polymerization, prepare a heat bath at 37 °C and pre-cool the centrifuge to 4 °C. Combine the unlabeled tubulin solution in M2B buffer (step 1.1.3) in a 500 µL ultracentrifuge tube with 4 mol% rhodamine-labeled tubulin (see Table of Materials) to produce 4% labeled microtubules after polymerization.
- Verify tubulin concentration using a Bradford assay27. The total tubulin concentration in the centrifuge tube should be 6.5-6.9 mg/mL.
- Incubate the tubulin mixture on ice for 10 min and ultracentrifuge for 10 min at 352,700 x g at 4 °C. This step removes dysfunctional tubulin, which will be in the pellet.
- Using a pipette, carefully extract the supernatant containing functional tubulin into a microcentrifuge tube. Add GMPCPP to a final concentration of 0.6 mM to induce tubulin polymerization and DTT to a final concentration of 1 mM to prevent protein aggregation.
- Incubate the mixture in the heat bath at 37 °C for 30 min, then centrifuge again for 10 min at 14,000 x g at room temperature. Remove the supernatant, then dilute the pellet with M2B buffer to reach a final microtubule concentration of 6 mg/mL.
NOTE: This solution can be stored at room temperature for at least 4 h before use. - To check that the microtubules have polymerized successfully, dilute 1 µL of the microtubule solution 100:1 with M2B buffer and pipette on a microscope slide. Cover with a cover slip for imaging using a fluorescence microscope (see Table of Materials) with a 40x objective lens.
NOTE: Excitation and emission wavelengths are chosen according to the fluorescence labeling used on the microtubules. In this protocol, rhodamine labeling is used (see step 1.2.2), so imaging is carried out using an excitation band of 515-560 nm and a 590 nm long pass filter. Figure 1 shows a representative example. - After polymerization is complete, drop-freeze the microtubules as 2 µL aliquots in liquid nitrogen and store at -80 °C (if required).
- Polymerize unlabeled cycled tubulin (99%, see Table of Materials) using 0.6 mM GMPCPP at a tubulin concentration of 6 mg/mL.
- Preparation of MIX
NOTE: MIX is an aqueous solution that includes the kinesin-streptavidin clusters (KSA). Prepared MIX should be stored at -80 °C in 4 µL aliquots prior to the experiment. When MIX is combined with ATP and the microtubule solution described in step 1.2 at room temperature, activity is initiated. MIX is prepared as follows13,19.- Prepare a solution for preventing fluorescence fading (ANTIFADE) by mixing two antioxidant solutions. Combine AO1 (250 mM DTT, 65 mM catalase) and AO2 (750 µM catalase, 3 mM glucose oxidase) in a 1:1 volume ratio. Include 20 mM Trolox, another antioxidant used to reduce damage caused by fluorescence microscopy.
- Prepare MIX by making a solution of KSA (described in step 1.1.3) that includes 6 wt% 20 kDa polyethylene glycol (PEG) to induce bundling of the microtubules, 3 vol% ANTIFADE, and 5 vol% pyruvate kinase/lactic dehydrogenase (PKLDH) at 70 mg/mL for ATP regeneration.
2. Creating the active nematic
NOTE: Activity in the material is initiated by ATP addition. The active network is prepared fresh for each experiment by adding ATP at a concentration high enough to induce motor activity. To form a uniform, fully developed active nematic phase, the microtubules must be at a sufficiently high density. This can be achieved by confining the microtubules in between two immiscible fluids to form a two-dimensional (2D) active nematic layer. This method was originally developed at Brandeis University13 and remains a popular technique for producing a homogeneous, high quality, active nematic phase.
- Flow cell method for active nematic formation
- Prepare hydrophilic coverslips with an acrylamide coating.
- Clean the coverslips thoroughly with soapy water, ethanol, and 0.1 M NaOH with alternate rinses using nanopure water. Once rinsed, coat the coverslips with a silane solution composed of 100 mL of ethanol, 1 mL of acetic acid, and 500 µL of 3-(trimethoxysilyl) propylmethacrylate for 15 min, then rinse with nanopure water.
- Prepare an acrylamide solution from 95 mL of nanopure water and 5 mL of 40 wt% acrylamide, then degas the solution for 30 min in a vacuum oven.
- Add 35 μL of tetramethylethylenediamine (TEMED) for a 2.3 mM final concentration and then 0.07 g of ammonium persulfate. Pour the acrylamide solution over the coverslips while face up and incubate overnight at room temperature.
- Prepare hydrophobic microscope slides. Pipette 100 µL of a water repellent solution (see Table of Materials) onto a clean glass microscope slide, then place another clean glass slide on top. This ensures an even coating of the water repellent solution on the surface where it sits for 2 min. After 2 min, remove the second glass slide and rinse the first slide thoroughly with nano-pure water, then dry with nitrogen gas. Gently clean the region where the tape will be placed (around the pattern) with acetone to ensure the water repellent solution does not prevent adhesion to the glass.
- Prepare a mixture of engineered oil that includes 1.8% (v/v) 008-FluoroSurfactant (see Table of Materials).
- Assemble the glass slide and coverslip with the hydrophobic glass slide on the bottom of the flow cell and the hydrophilic cover slip as the upper surface in a sandwich geometry using 40 µm double-sided adhesive spacers. Place the spacers 1.5 mm apart on the hydrophobic microscope slide. Then place the acrylamide coated coverslip on top of the spacers with the treated side face down to adhere. Ensure the adhesion is complete with pressure from a blunt object.
NOTE: The goal is to assemble a flow cell with a flat oil/water interface inside (Figure 2A,B). This is where the active layer will form. Alternative spacer tapes and films can also be used to produce a similar cell thickness. - After the flow cell has been constructed, immediately pipette the oil mixture into the flow cell, filling the enclosed space.
- Using a pipette, in a separate vial gently mix 6 µL of active material with 3.73 µL of MIX, 1 µL of microtubule solution, 0.6 µL of ATP solution (the concentration can be varied to vary microtubule velocity), and 0.67 µL of M2B buffer.
- Pipette freshly mixed active material into one open end of the flow cell, volumes will vary but should exceed the volume of the flow cell (roughly 3-6 μL). Some oil will be displaced by the aqueous solution as it is injected into the channel; this can be wicked up at the opposite end of the flow channel using a small piece of tissue paper.
- After filling, seal both sides of the flow cell with an epoxy glue (see Table of Materials) that hardens when exposed to UV light for 20 s.
NOTE: At this point, the active material forms a 3D network that remains suspended in the water layer. - To confine the active layer between the two immiscible fluids in a quasi-2D layer, place the flow cell in a swinging bucket centrifuge (see Table of Materials) with the aqueous phase on top and the denser oil layer underneath. Centrifuge at 212 x g for 10 min. After this step is complete, the flow cell can be taken to an epi-fluorescence microscope for imaging with a 10x or 20x magnification objective. Figure 2C,D shows typical images before and after this step.
- Prepare hydrophilic coverslips with an acrylamide coating.
- Inverted method for active nematic formation
NOTE: An alternate method to that described in step 2.1 is to assemble the active nematic layer underneath a thick oil layer confined to a deep PDMS well28. This method produces similar results, and is somewhat easier to master; however, image quality using this method is usually not as good as the flow cell method.- Prepare the polydimethylsiloxane (PDMS) using an elastomer curing agent and an elastomer base (see Table of Materials). Mix the two components in a 1:10 ratio using a metal spatula. Tiny bubbles appear during mixing which are difficult to remove, and the mixture appears milky. To remove these bubbles, place the mixture under a vacuum to degas for 1 h, after which the uncured PDMS should appear transparent.
NOTE: The PDMS is now ready to form any shape using a mold, or it can cure in the container and then be cut or punched in the desired pattern. - Pour the PDMS into a suitable mold and leave overnight to cure at 60 °C.
NOTE: Untreated, the surface of cured PDMS is hydrophobic, but with surface treatment, it can be made hydrophilic. - To prepare a hydrophilic PDMS surface, coat the PDMS with an acrylamide polymer brush29. This step also prevents proteins from sticking to the surface.
- Start by cleaning the PDMS for 10 min with both ethanol and isopropanol, then rinse thoroughly with deionized water 3x and dry. Use a plasma cleaner for 5 min to clean the dry, cured PDMS. This step makes the surface more hydrophilic.
- Next, prepare a silane solution (98.5 wt% ethanol, 1 wt% acetic acid, and 0.5 wt% Trimethoxysilyl propyl methacrylate) and immerse the substrate in that solution for 15 min to prepare for the acrylamide coating. Rinse the substrate thoroughly with deionized water and immerse in acrylamide solution (2 wt% acrylamide/bis solution, 2.3 mM TEMED, and 3 mM ammonium persulfate).
NOTE: These substrates can be stored at room temperature covered in acrylamide solution in a glass Petri dish, and should be used within 2 weeks.
- When ready for use, rinse the surface with deionized water and dry with nitrogen for immediate use. Add the active mixture (described in step 2.1.6) to the wells and immediately add silicone oil on top to a thickness of approximately 2 mm.
NOTE: At this stage, the active mixture will be sandwiched between the oil and the hydrophilic coating on the PDMS, but it will still be somewhat three-dimensional. - To push the material further into a 2D layer, glue the PDMS device onto a glass slide, place it in a swinging bucket centrifuge and spin for 12 min at 212 x g. The device will need to be positioned such that the silicone oil is on the top of the aqueous layer. Representative results are shown in Figure 3.
- Prepare the polydimethylsiloxane (PDMS) using an elastomer curing agent and an elastomer base (see Table of Materials). Mix the two components in a 1:10 ratio using a metal spatula. Tiny bubbles appear during mixing which are difficult to remove, and the mixture appears milky. To remove these bubbles, place the mixture under a vacuum to degas for 1 h, after which the uncured PDMS should appear transparent.
3. Preparing active nematics in confined geometries
NOTE: Active nematics such as this quasi-two-dimensional system can be challenging to confine into small microfluidic geometries such as wells or channels. Here, a reliable method to confine the material into different shaped PDMS wells is described.
- First, design a master mold for the PDMS. This can be achieved by 3D printing pillars on a substrate. After 3D printing the resin master mold, clean with isopropanol and then cure the mold under a UV lamp for 45 min and in the oven at 120 °C for 2 h (Figure 4).
NOTE: Curing under UV and thermal post-curing improves the quality of replication in PDMS by removing monomers and photo inhibitor residues from the resin30. - Use the master mold to create wells from PDMS. Prepare the PDMS as described in step 2.2.1. Immerse the master mold in uncured PDMS and cure overnight in an oven at 60 °C.
- After PDMS curing is complete, carefully remove the master mold and cut the PDMS as desired to work with the wells (Figure 4). Treat the PDMS surface as described in step 2.2.3. Before the experiment, the surface can be attached to a glass slide with epoxy glue to make imaging easier.
- Pipette 1 µL of the active mixture described in step 2.1.6 onto the PDMS substrate and immediately add silicone oil with 100-1000 cSt viscosity28 on top of the active network droplet. The active network will move into the well; this process takes up to 60 min (Figure 4). As described in step 2.2.5, the 2D network can be enhanced by spinning down the PDMS well in the swinging bucket centrifuge for 12 min at 212 x g.
Representative Results
Figure 1 shows a representative image of single microtubules prepared from GMPCPP tubulin. The image depicts short microtubules of similar lengths (with some dispersity present). Sufficient dilution of the microtubule solution should produce an image of well separated microtubules for length verification. The individual microtubules may be challenging to image due to their small size. Use of a high sensitivity camera designed for fluorescence microscopy is best for this application. Figure 2 and Figure 3 show example fluorescence microscopy images of successful experiments performed using the flow cell method (section 2.1) and the inverted method (section 2.2), respectively. A well-formed active nematic layer is homogeneous in texture, with no significant void areas and mobile topological defects present. Note however that there may be some acceptable small voids in the defect cores. In addition to the examples shown in Figure 2 and Figure 3, three supplemental movies (Movie 1, Movie 2, and Movie 3) have been included to demonstrate how the active nematic should appear in a successful experiment. All the movies demonstrate the smooth continuous motion of the active nematic phase. No variations in microtubule concentration are apparent after the material has reached its steady state. As long as sufficient ATP is present in the system, the material will continue to move uniformly.
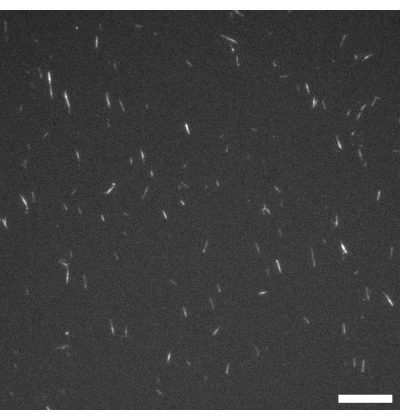
Figure 1: Fluorescence microscope image of GMPCPP microtubules. The GMPCPP microtubules were labeled at 4% with rhodamine tubulin and polymerized for 20 min at 37 °C. Imaging was performed at room temperature. Scale bar = 10 µm. Please click here to view a larger version of this figure.
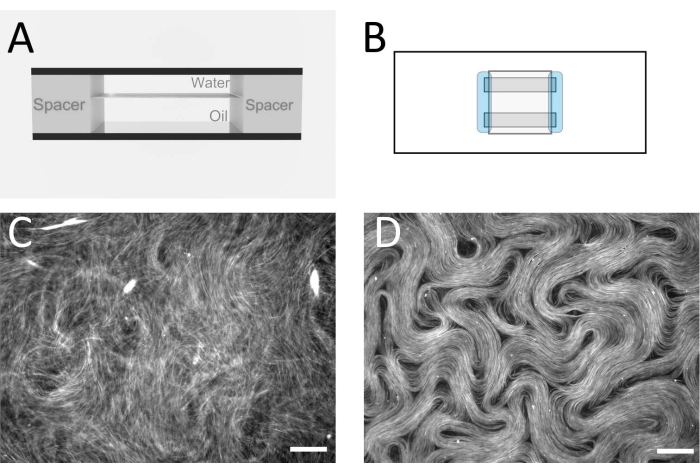
Figure 2: Microtubule nematics in a flow cell. (A) Cross sectional schematic of the flow cell, 1 mm x 18 mm geometry. (B) Top view schematic of the flow cell. (C) Fluorescence microscopy image demonstrating the typical appearance of the active solution before assembly at the oil/water interface. (D) Fluorescence microscopy image of the active nematic phase assembled at the oil/water interface inside the flow cell. Scale bar = 100 µm. Please click here to view a larger version of this figure.
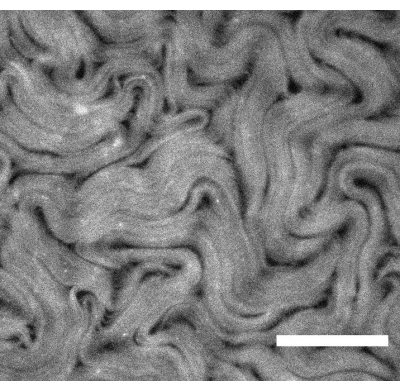
Figure 3: Fluorescence microscope image showing an active nematic prepared using the inverted method. Scale bar = 200 µm. Please click here to view a larger version of this figure.
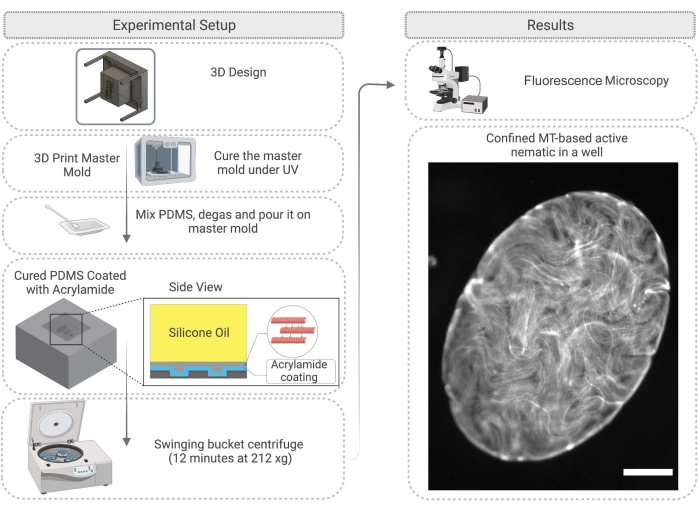
Figure 4: Flow diagram illustrating the method for active nematic confinement in a PDMS well, including mold fabrication and surface treatment. Scale bar on the right image (confined active material) = 200 µm. Please click here to view a larger version of this figure.
Movie 1: Representative result for active nematic prepared using the flow cell method. Please click here to download this Movie.
Movie 2: Representative result for active nematic prepared using the inverted method. Please click here to download this Movie.
Movie 3: Representative result for active nematic prepared using the inverted method confined to an elliptical well. Please click here to download this Movie.
Discussion
There are a few points throughout the protocols at which the experimenter can make some important checks. Before filling either of the devices with active material, fluorescence microscopy (see Figure 1) should be used to check that the microtubules are polymerized and ideally ~2-3 µm in length. If microtubules are not visible under the microscope, they may have depolymerized and the active nematic will not form. Because individual microtubules are very small, it may be challenging to observe them directly through the microscope. In this study, a high-quality fluorescence camera designed for challenging low-light applications was used with the associated software to verify filament growth. Significant fluorescent aggregates should not be present at this stage, as this may indicate depolymerization or the presence of denatured protein. It is also a good idea to make a simple microscope test slide by combining microtubules, MIX, and ATP in the same ratios as described in the protocols. Activity should begin on combining the components and the material should appear similar to that shown in Figure 2C with bundles present and noticeable filament movements visible throughout.
When using the flow cell method, the centrifuge time and orientation of the flow cell are important for the formation of a uniform active layer. This step may require some fine tuning depending on the centrifuge type used. Centrifuging the flow cell with the active plane oriented perpendicular to the plane of rotation gives the best results as material can be pushed onto the fluid interface uniformly. Double check that the flow cell is carefully sealed before centrifuging.
When using the inverted method to produce confined active nematics there are several steps to optimize. First, it is important to use a 3D printing method that produces high resolution structures. Uneven side walls can cause the microtubules to catch, which will disrupt the flows. The wells should not be too deep (150–200 µm deep wells with a 2 mm thick overlying oil layer were used in this study). Experimenters may need to adjust these parameters slightly by trial and error to get the best result.
The flow cell method and the inverted method have been used by different authors to look at a variety of effects that impact the active flows, including different oils12 and submersed structures13. The choice of method depends on the experimental objective. Using the flow cell method, optical imaging from above the active layer is clearer than for the inverted method due to the different overlying fluids. In the flow cell method, imaging is carried out through a glass cover slip and a thin layer of water, whereas the inverted method is designed to have the oil layer on top. This means that a long working distance objective is needed for the inverted method, and image quality is reduced. Image quality differences can be seen by comparing Figure 2D (flow cell method) and Figure 3 (inverted method), and Movie 1 and Movie 2, respectively. A lower magnification lens with a longer working distance was required for Figure 3 than that used for Figure 2. These imaging disadvantages for the inverted method can be avoided if a suitable inverted microscope is available, combined with objectives with an appropriate working distance for the microscope slide substrates. Thinner glass can be used as a substrate to allow the use of standard working distance objectives.
As an advantage, the inverted geometry allows for the use of a wider range of oil viscosities, does not necessarily require swinging bucket centrifugation (if this is not available), and preparation of the system is relatively easier once the mold is prepared. However, for confinement in wells using the inverted method, some centrifugation may be important to get the material into a well-defined 2D layer.
The flow cell method has recently been used very successfully in experiments where a continuous active layer is required. Our recent work has looked at the dynamics of topological defects in the active layer, where high quality imaging and texture analysis is important19. In addition, the flow cell method has been used to investigate the effects of oil-submersed microstructures on active flows16 and pillars to trap defects in the active flows31. This method works very well for the formation of a continuous active layer, and the image quality is excellent. However, the centrifugation step used to produce the final 2D active layer can be difficult to carry out, and the flow cells are prone to leaks and air bubbles. The inverted method is a very useful alternative with a high success rate, is easy to construct, and can be used for any substrate pattern or geometry provided a high-resolution 3D printed master mold can be created. This method is also useful for looking at the effects of geometrical confinement on active nematic dynamics because it makes filling wells relatively straightforward.
In this paper, two ways to form an active nematic from microtubules and kinesin motors are described, plus a technique to confine the materials in wells. The system presented represents the cleanest example of an active nematic phase currently in the literature and has been reproduced by several groups around the world. The significance of this material not only lies in the biological origins of its components, but also because it opens up an entirely new direction in active ordered fluids. By working with this system and elucidating its fundamental properties, scientists can move toward the design of fully synthetic active phases.
The experiments focused on the effects of confinement on active nematics have the potential to answer fundamental questions regarding the behavior of active flows and topological defect dynamics under topological confinement. The method presented here will aid in the performance of a variety of geometry-focused experiments and their analysis, including microfluidics and active mixing.
Divulgazioni
The authors have nothing to disclose.
Acknowledgements
The authors would like to acknowledge the National Science Foundation (NSF) award DMR-1808926 for generous funding. The project was also supported by the NSF through the Center of Research Excellence in Science and Technology: Center for Cellular and Biomolecular Machines at the University of California Merced (HRD-1547848) and the Brandeis Biomaterials Facility Materials Research Science and Engineering Center (DMR-2011846). We would like to thank Dr. Bin Liu at the University of California Merced for assistance in 3D printing the mold, and Dr. Jordi Ignes for scientific advice during the development of the inverted experimental method.
Materials
| 20 kD PEG (polyethylene glycol)) | Sigma Aldrich | 1419109 | Depletion agent CAS Number: 125061-88-3 |
| 3-(trimethoxysilyl)propyl methacrylate | Sigma Aldrich | M6514-50ML | CAS Number: 2530-85-0 |
| 3D printer & Resin | Phrozen | Phrozen sonic mini 8K 3D printer – Aqua Gray 8K resin | |
| 40% Acrylamide Solution | BIO-RAD | 1610140 | CAS Number: 7732-18-5, 79-06-1 |
| Acetic Acid | Fisher | CAS Number: 64-19-7 | |
| Acetone | Sigma Aldrich | CAS Number: 67-64-1 | |
| Adhesive sheets (NOTE: "Parafilm" is an alternative) | Grace Bio-Labs | 620001 | SecureSeal |
| Ammonium Persulfate | Sigma Aldrich | A3678 | CAS Number: 7727-54-0 |
| Aquapel (NOTE: "RainX" is an alternative) | Aquapel Glass Treatment | hydrophobic glass treatment | |
| ATP (Adenosine triphosphate) | Sigma Aldrich | A1852 | CAS Number: 34369-07-8 |
| Beakers | VWR | ||
| Catalase | Sigma Aldrich | C9322 | CAS Number: "9001-05-2" |
| Desiccator | Bel-art | ||
| Digital CMOS camera | Hamamatsu | ORCA – Flash4.0 LT+ | |
| DTT (Dithiothreitol) | Sigma Aldrich | D9779 | CAS Number: "3483-12-3" |
| EGTA (3,12-bis(carboxymethyl)-6,9-dioxa-3,12-diazatetradecane-1,14-dioic acid) | Sigma Aldrich | MFCD00004291 | CAS Number: 67-42-5 |
| Ethanol | Sigma Aldrich | CAS Number: 64-17-5 | |
| Fluorescence microscope | Leica | DM 2500P | |
| Glass Coverslips | VWR | 48368-040 | |
| Glass Slides | VWR | 16004-430 | |
| Glucose | Sigma Aldrich | G7021 | CAS Number: 50-99-7 |
| Glucose Oxidase | Sigma Aldrich | 345386 | CAS Number: 9001-37-0 |
| GMPCPP (guanylyl 5'-α,β-methylenediphosphonate) | Jena Bioscience | NU-405S | CAS Number: 14997-54-7 |
| HFE7500 Oil | 3M | ||
| Hot Plate | Fisher Scientific | Thermix hot plate model 100M | |
| Isopropyl Alcohol | VWR | ||
| KCl (potassium chloride) | Sigma Aldrich | P5405 | CAS Number: 7447-40-7 |
| Methanol | Sigma Aldrich | CAS Number: 67-56-1 | |
| MgCl2 (Magnesium Chloride) | Sigma Aldrich | 208337 | CAS Number: 7786-30-3 |
| Microcentrifuge tubes | Eppendorf – Thermo Fisher | 1.5 mL | |
| Nanopure water purifier | Sartorius | arium mini | |
| NaOH (Sodium hydroxide) | Sigma Aldrich | SX0603 | CAS Number: 1310-73-2 |
| Petri Dishes | VWR | ||
| PH Meter | Thermo Scientist | Orion 3 STAR | |
| Phosphoenol-pyruvate (PEP) | Sigma Aldrich | MFCD00044476 | CAS Number: 4265-07-0 |
| PIPES (1,4-Piperazinediethanesulfonic acid) | Sigma Aldrich | CAS Number: 5625-37-6 | |
| Pipettes (0.2 – 1000 µl) | VWR | ||
| Pluronic F-127 | Sigma Aldrich | 2594628 | |
| RAN Surfactant (NOTE: "FluoSurf" from Emulso is an alternative) | Ran Biotechnologies | 008-FluoroSurfactant-2wtH-50G | |
| Silicon Oil (100mpa s-1000 mpa s) | Sigma Aldrich | CAS Number: 63148-52-7 | |
| Streptavidin | Thermofisher | S888 | |
| Swinging Bucket Centrifuge | Thermo Scientist | Sorvall legend RT+ | |
| Sylgard 184 Elastomer base | World Precision Instruments | SYLG184 | |
| Sylgard 184 Elastomer Curing agent | World Precision Instruments | SYLG184 | |
| Table top centrifuge | Eppendorf | MiniSpin Plus | |
| TEMED (Tetramethylethylenediamine) | BIO-RAD | 1610800 | CAS Number: 110-18-9 |
| Trolox (6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid) | Sigma Aldrich | MFCD00006846 | CAS Number: 53188-07-1 |
| Tubulin | Cytoskeleton | T240-B | |
| Tubulin (Rhodamine labeled) | Cytoskeleton | TL590M-A | |
| Ultracentrifuge | Beckman | Optima Max-TL | |
| UV Light | RapidFix | ||
| UV-curable glue (NOTE: "Norland NO81" is an alternative) | RapidFix | ||
| Water Bath | Thelco | ||
| Whatman Filter paper | Sigma Aldrich | WHA1001325 |
Riferimenti
- Sokolov, A., Aranson, I. S., Kessler, J. O., Goldstein, R. E. Concentration dependence of the collective dynamics of swimming bacteria. Physics Review Letters. 98 (15), 158102 (2007).
- Saw, T. B., et al. Topological defects in epithelia govern cell death and extrusion. Nature. 544 (7649), 212-216 (2017).
- Kawaguchi, K., Kageyama, R., Sano, M. Topological defects control collective dynamics in neural progenitor cell cultures. Nature. 545 (97654), 327-331 (2017).
- Toner, J., Tu, Y. Long-range order in a two-dimensional dynamical XY model: how birds fly together. Physics Review Letters. 75 (23), 4326-4329 (1995).
- Katz, Y., Tunstrøm, K., Ioannou, C. C., Huepe, C., Couzin, I. D. Inferring the structure and dynamics of interactions in schooling fish. Proceedings of the National Academy of Sciences. 108 (46), 18720-18725 (2011).
- Needleman, D., Dogic, Z. Active matter at the interface between materials science and cell biology. Nature Reviews Materials. 2 (9), 17048 (2017).
- Weirich, K., Dasbiswas, K., Witten, T. Self-organizing motors divide active liquid droplets. Proceedings of the National Academy of Sciences. 116 (23), 11125-11130 (2019).
- Memarian, F. L., et al. Active nematic order and dynamic lane formation of microtubules driven by membrane-bound diffusing motors. Proceedings of the National Academy of Sciences. 118 (52), (2021).
- Bausch, A., Sciortino, A. R. Pattern formation and polarity sorting of driven actin filaments on lipid membranes. Proceedings of the National Academy of Sciences. 118 (6), (2021).
- Maroudas-Sacks, Y., et al. Topological defects in the nematic order of actin fibres as organization centres of Hydra morphogenesis. Nature Physics. 17 (2), 251-259 (2021).
- Liu, J., et al. Topological braiding and virtual particles on the cell membrane. Proceedings of the National Academy of Sciences. 118 (34), (2021).
- Hirst, L. S. . Fundamentals of Soft Matter Science 2nd ed. , (2019).
- Sanchez, T., Chen, D., DeCamp, S., Heymann, M., Dogic, Z. Spontaneous motion in hierarchically assembled active matter. Nature. 491 (7424), 431-434 (2012).
- Nedelec, F. J., Surrey, T., Maggs, A. C., Leibler, S. Self-organization of microtubules and motors. Nature. 389 (6648), 305-308 (1997).
- Guillamat, P., Ignés-Mullol, J., Sagués, F. Taming active turbulence with patterned soft interfaces. Nature Communications. 8, 564 (2017).
- Thijssen, K., et al. Submersed micropatterned structures control active nematic flow, topology, and concentration. Proceedings of the National Academy of Sciences. 118 (38), (2021).
- Shendruk, T. N., Doostmohammadi, A., Thijssen, K., Yeomans, J. M. Dancing disclinations in confined active nematics. Soft Matter. 13 (21), 3853-3862 (2017).
- Giomi, L. Geometry and topology of turbulence in active nematics. Physical Review X. 5 (3), 031003 (2015).
- Tan, A. J., et al. Topological chaos in active nematics. Nature Physics. 15 (10), 1033-1039 (2019).
- Young, E. C., Berliner, E., Mahtani, H. K., Perez-Ramirez, B., Gelles, J. Subunit interactions in dimeric kinesin heavy chain derivatives that lack the kinesin rod. The Journal of Biological Chemistry. 270 (8), 3926-3931 (1995).
- Gilbert, S. P., Johnson, K. A. Expression, purification, and characterization of the Drosophila kinesin motor domain produced in Escherichia coli. Biochimica. 32 (17), 4677-4684 (1993).
- Kuznetsov, S. A., Gelfand, V. I., Vernos, I. . Kinesin Protocol. 164, (2001).
- Hawkins, T. L., Sept, D., Mogessie, B., Straube, A., Ross, J. L. Mechanical properties of doubly stabilized microtubule filaments. Biophysics Journal. 104 (7), 1517-1528 (2013).
- Bate, T. E., Jarvis, E. J., Varney, M. E., Wu, K. Controlling flow speeds of microtubule-based 3D active fluids using temperature. Journal of Visualized Experiments. (153), e60484 (2019).
- Castoldi, M., Popov, A. V. Purification of brain tubulin through two cycles of polymerization-depolymerization in a high-molarity buffer. Protein Expression and Purification. 32 (1), 83-88 (2003).
- Tayar, A. M., Lemma, L. M., Dogic, Z. Assembling microtubule-based active matter.. Microtubules. , 151-183 (2022).
- Bradford, M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry. 72 (1-2), 248-254 (1976).
- Guillamat, P., Ignés-Mullol, J., Shankar, S., Marchetti, M. C., Sagués, F. Probing the shear viscosity of an active nematic film. Physical Review E. 94 (6), 060602 (2016).
- Rudy, A., et al. Lubricous hydrogel surface coatings on polydimethylsiloxane (PDMS). Tribology Letters. 65, 3 (2017).
- Venzac, B., et al. PDMS curing inhibition on 3D-printed molds: Why? Also, how to avoid it. Analytical Chemistry. 93 (19), 7180-7187 (2021).
- Khaladj, D. A., Hirst, L. S. Using curved fluid boundaries to confine active nematic flows. Frontiers of Physics. 10, 880941 (2022).

