Source and Route of Pyrrolizidine Alkaloid Contamination in Tea Samples
Summary
The present protocol describes the contamination of pyrrolizidine alkaloids (PAs) in tea samples from PA-producing weeds in tea gardens.
Abstract
Toxic pyrrolizidine alkaloids (PAs) are found in tea samples, which pose a threat to human health. However, the source and route of PA contamination in tea samples have remained unclear. In this work, an adsorbent method combined with UPLC-MS/MS was developed to determine 15 PAs in the weed Ageratum conyzoides L., A. conyzoides rhizospheric soil, fresh tea leaves, and dried tea samples. The average recoveries ranged from 78%-111%, with relative standard deviations of 0.33%-14.8%. Fifteen pairs of A. conyzoides and A. conyzoides rhizospheric soil samples and 60 fresh tea leaf samples were collected from the Jinzhai tea garden in Anhui Province, China, and analyzed for the 15 PAs. Not all 15 PAs were detected in fresh tea leaves, except for intermedine-N-oxide (ImNO) and senecionine (Sn). The content of ImNO (34.7 µg/kg) was greater than that of Sn (9.69 µg/kg). In addition, both ImNO and Sn were concentrated in the young leaves of the tea plant, while their content was lower in the old leaves. The results indicated that the PAs in tea were transferred through the path of PA-producing weeds-soil-fresh tea leaves in tea gardens.
Introduction
As secondary metabolites, pyrrolizidine alkaloids (PAs) protect plants against herbivores, insects, and pathogens1,2. Up to now, over 660 PAs and PA-N-oxides (PANOs) with different structures have been found in more than 6,000 plant species worldwide3,4. PA-producing plants are mainly found in the families Asteraceae, Boraginaceae, Fabaceae, and Apocynaceae5,6. PAs are easily oxidized to unstable dehydropyrrolizidine alkaloids, which have strong electrophilicity and can attack nucleophiles such as DNA and proteins, resulting in liver cell necrosis, venous occlusions, cirrhosis, ascites, and other symptoms7,8. The main target organ of PA toxicity is the liver. PAs can also cause lung, kidney, and other organ toxicity and mutagenic, carcinogenic, and developmental toxicity9,10.
Cases of human and animal poisoning have been reported in many countries from the ingestion of traditional herbs, supplements, or teas containing PAs or the indirect contamination of foods such as milk, honey, or meat (toxic from ingestion of pasturage containing PAs)11,12,13. The European Food Safety Authority (EFSA) findings indicate that substances such as (herbal) tea are an important source of human exposure to PAs/PANOs14. Tea samples do not produce PAs, whereas PA-producing plants are commonly found in tea gardens (e.g., Emilia sonchifolia, Senecio angulatus, and Ageratum conyzoides)15. It was previously suspected that the tea could be contaminated with PAs from their producing plants during picking and processing. However, PAs were also detected in some hand-picked tea leaves (i.e., no PA-producing plants), suggesting there must be other routes or sources of contamination16. A co-cultivation experiment of ragwort (Senecio jacobaea) with melissa (Melissa officinalis), peppermint (Mentha piperita), parsley (Petroselinum crispum), chamomile (Matricaria recutita), and nasturtium (Tropaeolum majus) plants was conducted, and the results showed that PAs were detected in all these plants17. It has been verified that PAs are indeed transferred and exchanged between living plants via soil18,19. Van Wyk et al.20 found that rooibos tea (Aspalathus linearis) was severely contaminated in weed-rich sites and contained PAs of the same type and proportion. However, no PAs were detected in rooibos tea in weed-free sites.
At present, ultra-high performance liquid chromatography tandem mass spectrometry (UPLC-MS/MS) with high selectivity and sensitivity has been widely used in the qualitative and quantitative analysis of PAs in agricultural products and food21,22. The sample treatment method is usually comprised of either solid phase extraction (SPE) or the QuEChERS (Quick Easy Cheap Effective Rugged Safe) clean-up of complex food matrices extracts, which can obtain the highest possible sensitivity12,19. However, robust analytical methods allowing the detection and quantification of PAs in complex matrices like soil, weeds, and fresh tea leaves are still missing.
This study analyzed 15 PAs in dried tea samples, fresh tea leaves, weeds, and weed rhizospheric soil samples with UPLC-MS/MS combined with an adsorbent purification method. Furthermore, 15 paired weed and weed rhizospheric soil samples and 60 fresh tea leaf samples were collected from five sampling sites in Jinzhai tea garden in Anhui Province, China, and were analyzed for 15 PAs. These results can provide a survey method and some information on the source and route of PAs (contamination) in tea samples to ensure the quality and safety of tea.
Protocol
For the present study, the following weed species were collected: Ludwigia prostrata Roxb., Murdannia triquetra (Wall. ex C. B. Clarke) Bruckn., Ageratum conyzoides L., Chenopodium ambrosioides, Trachelospermum jasminoide (L.) Lem., Ageratum conyzoides L., Emilia sonchifolia (L.) DC, Ageratum conyzoides L., and Crassocephalum crepidioides (Benth.) S. Moore. The fresh tea leaves were picked from the variety of Longjing 43# tea trees, and the dried tea samples were commercially available tea processed according to the green tea manufacturing process (see Table of Materials).
1. Sample collection
- Collect 40 real samples.
- Collect 10 weeds, 10 soils, and 10 fresh tea leaves randomly from multiple tea gardens.
NOTE: For the present study, the soil was sampled at a depth of 20 cm with a sample amount of 200 g. - Randomly collect 10 dried tea products (250 g) from a supermarket.
- Collect 10 weeds, 10 soils, and 10 fresh tea leaves randomly from multiple tea gardens.
- Collect samples of weeds, soil, and fresh tea leaves to study the contamination source of PAs in tea.
- Set five sampling points in the same tea garden, with three replicates at each point.
- Collect Ageratum conyzoides L. weed samples with the highest PA content commonly found in tea gardens.
NOTE: The sample amount was 250 g for the present study. - Collect the soil samples.
NOTE: The soil samples were A. conyzoides rhizospheric soil at a depth of 20 cm with a sample amount of 200 g. - Collect the fresh tea leaves from different parts of the tea plants, including one bud with two leaves, one bud with three leaves, one bud with four leaves, and mature leaves.
NOTE: The sample amount was 250 g.
2. Sample treatment
- Pretreat the samples following the steps below.
- Grind the dried tea and soil samples with a grinder, pass the pulverized samples through a 200-mesh sieve, and store them at −20 °C.
NOTE: The dried tea was a commercially available tea product (see Table of Materials), so it was directly crushed and sieved for storage. The soil samples (200 g) were placed in a ventilated place in the dark to air dry for about one week. - Homogenize the weed and fresh tea leaves with a homogenizer and store them at −20 °C.
- Grind the dried tea and soil samples with a grinder, pass the pulverized samples through a 200-mesh sieve, and store them at −20 °C.
- Perform sample treatment of the dried tea products, fresh tea leaves, and weeds.
- Weigh 1.00 g of each sample (dried tea products, fresh tea leaves, and weeds) and place it in a 50 mL centrifuge tubes.
- Add 10 mL of 0.1 mol/L sulfuric acid solution and vortex for 2 min for solid-phase extraction (using SPE cartridge, see Table of Materials) and 1 min for adsorbent purification. Perform ultrasonic extraction23 for 15 min, and then centrifuge for 10 min at a speed of 9,390 x g at room temperature.
NOTE: The power of the ultrasonic oscillator was 290 W, the oscillation frequency was 35 kHz, and the temperature was set to 30 °C. - Transfer the supernatant into a 50 mL centrifuge tube with a plastic-tip dropper.
- Follow the steps above to repeat the extraction once. Combine the two supernatants.
- Activate the SPE cartridges with 5 mL of methanol and 5 mL of deionized water. Add 10 mL of supernatant to the pre-activated cartridge and perform sample clean-up.
- After the sample solution level reached the the upper layer of cartridges, elute the analytes with with 5 mL of 1% formic acid solution and then 5 mL of methanol. Discard the eluate.
- Elute with 5 mL of methanol (containing 0.5% ammonia water), filter the eluate through a 0.22 µm membrane filter, and analyze by UPLC-MS/MS (see Table of Materials).
- Perform sample clean-up using adsorbents.
- Take 2 mL of the supernatant (step 2.2.4) into a 10 mL centrifuge tube filled with the adsorbents of GCB:PSA:C18 (10 mg:20 mg:15 mg, see Table of Materials), vortex for 1 min, and centrifuge at 9,390 x g for 8 min at room temperature.
- Pass 1 mL of the supernatant through a 0.22 µm membrane filter before analysis by UPLC-MS/MS.
- Perform treatment of the soil samples.
- Weigh a 1.00 g soil sample. Place it in a 50 mL centrifuge tube, and add 0.1 mL of 0.1 mol/L trisodium citrate solution (see Table of Materials) to adjust the soil pH value to 6.0.
- Allow to stand for 2 min, and then add 10 mL of 0.1 mol/L sulfuric acid methanol solution, vortex for 2 min and shake for 30 min, and then perform ultrasonic extraction for 30 min.
- Centrifuge at 9,390 x g for 10 min, and transfer the supernatant into a 50 mL centrifuge tube with a plastic-tip dropper.
- Follow the above steps to repeat the extraction and combine the supernatant twice.
NOTE: The purification method was the same as in step 2.2.5.1 and step 2.2.5.2.
3. Instrumental analysis
- Detect the 15 PAs in dried tea samples, fresh tea leaves, weed, and soil (samples from step 2) using a commercially available UPLC-MS/MS system (2.1 mm x 100 mm, 1.8 µm) (see Table of Materials).
- Set the column temperature to 40 °C, the flow rate to 0.250 mL/min, and the injection volume to 3 µL.
- Set the mobile phase A: methanol (containing 0.1% formic acid + 1 mmol/L ammonium formate), and mobile phase B: water (containing 0.1% formic acid + 1 mmol/L ammonium formate).
- Set a gradient elution procedure: 10% A from 0.0 min to 0.25 min, 10%-30% A from 0.25 min to 6.0 min, 30%-40% A from 6.0 min to 9.0 min, 40%-98% A from 9.0 min to 9.01 min that was held for 1.9 min, and 98%-100% A from 11.0 min to 11.1 min that was held for 2.9 min.
- Set the mass spectrometer parameters: ionization mode, electrospray positive ion source (ESI+); atomizer pressure, 7.0 bar; capillary voltage, 4.0 kV; taper hole back blowing flow, 150 L/h; solvent gas flow, 800 L/h; dissolvent temperature, 400 °C; impact gas flow, 0.25 mL/min.
Representative Results
The optimized adsorbent purification and analysis method of 15 PAs in dried tea samples, fresh tea leaves, weeds, and soil was established and compared with the commonly used purification method using the SPE cartridge. The results showed that the recoveries of the 15 PAs in dried tea samples, weed, and fresh tea leaves using the SPE cartridge were 72%-120%, while that using adsorbent purification was 78%-98% (Figure 1). The recoveries of the 15 PAs in soil using adsorbent purification were 79%-111% (Figure 1). Forty (40) real samples were randomly collected to detect the content of PAs to compare the two clean-up methods (Supplementary Tables 1–7). Heliotrine (He) was detected in all 10 dried tea samples using the adsorbent method with a content of 1.3-22 µg/kg, while it was only detected in three dried tea samples using the SPE cartridge with a content of 1.8-24.6 µg/kg (Supplementary Tables 3–4).
The adsorbent purification method (GCB:PSA:C18) was selected to detect PAs in weeds, weed rhizospheric soils, and fresh tea leaves in tea plantation systems. Five sampling sites were chosen in one tea garden in Jinzhai. In addition to jacobine (Jb), seneciphylline (Sp), seneciphylline N-oxid (SpNO), and senkirkine (Sk), a total of 11 PAs were detected in the weed A. conyzoides, of which the highest content of PAs was intermedine (Im) (2,006-2,970 µg/kg), heliotrine-N-oxide (HeNO) (2,446-2,731 µg/kg), and intermedine-N-oxide (ImNO) (13,535-17,345 µg/kg) (Table 1). In soil, only ImNO was detected at sampling site 5, with a content of 6.05 µg/kg (Supplementary Table 8). ImNO and Sn were detected in the fresh tea leaves from the five sampling sites (Figure 2). ImNO was detected in different parts of the tea plants, and its content ranged from 4.36-26.5 µg/kg, which was greater than that of Sn, except that Sn was not detected in mature leaves from sampling site 1 and sampling site 2. Sn was detected in different parts of the tea plants in the other sampling sites, and the content ranged from 1.0-3.14 µg/kg (Figure 2).
At sampling site 5, the transfer phenomenon of PAs among the weeds, weed rhizospheric soil, and fresh tea leaves was shown (Figure 3). Among the 11 PAs weeds, only ImNO was detected in the soil, with a content of 6.05 µg/kg, while ImNO and Sn were detected in different parts of the tea plants. The content of ImNO in one bud with two leaves was the highest, which was 12.6 µg/kg (Figure 3).
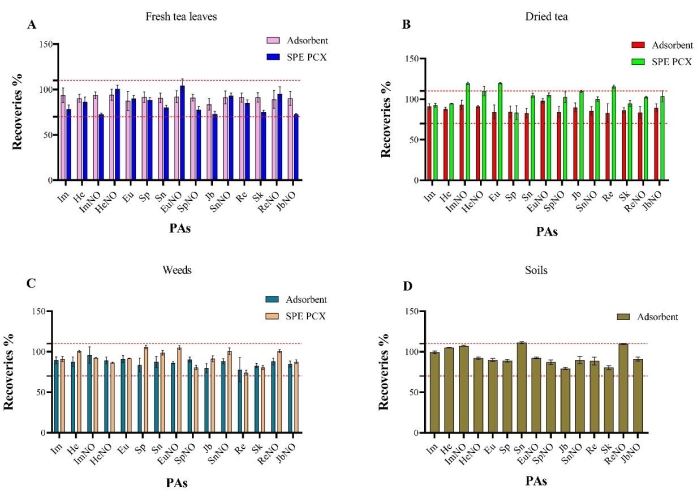
Figure 1: Recovery comparison. Comparison of the recoveries of 15 PAs (pyrrolizidine alkaloids) in extracts from (A) fresh tea leaves, (B) dried tea samples, (C) weed, and (D) soil samples upon clean-up with the adsorbent (spiked level = 0.02 mg/kg) and SPE cartridges (mixed cation exchange solid-phase extraction columns, spiked level = 0.01 mg/kg). The error bars show the standard deviation, and the significance test was performed by analysis of variance. Please click here to view a larger version of this figure.
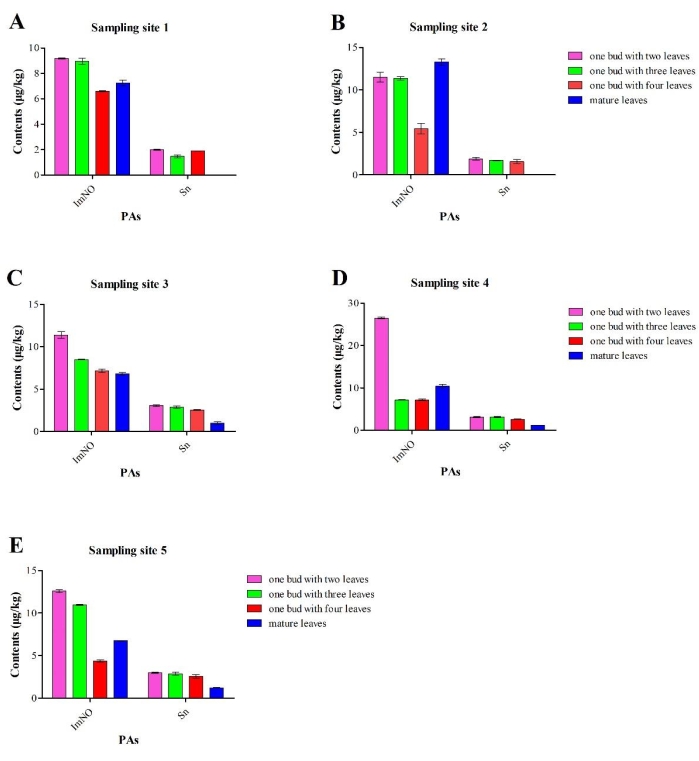
Figure 2: The content and types of PAs (pyrrolizidine alkaloids) in different parts of the tea plants collected from the five sampling sites. (A) Sampling site 1. (B) Sampling site 2. (C) Sampling site 3. (D) Sampling site 4. (E) Sampling site 5. The error bars show the standard deviation, and the significance test was performed by analysis of variance. Please click here to view a larger version of this figure.
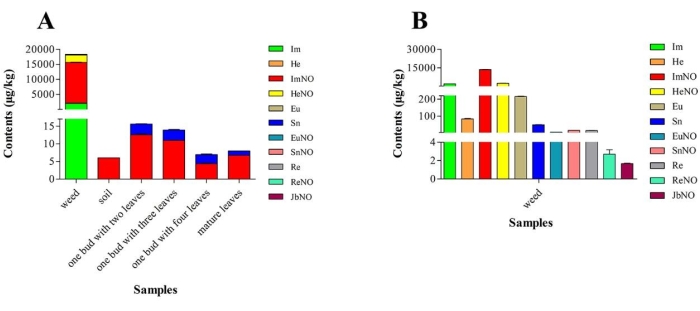
Figure 3: PAs contained in weeds and their transfer to soil and fresh tea leaves. (A) The content and type of PAs (pyrrolizidine alkaloids) detected in the weeds, soil, and fresh tea leaves. (B) The content and type of PAs detected in the weeds. The error bars show the standard deviation, and the significance test was performed by analysis of variance. Please click here to view a larger version of this figure.
| Samp-ling site | The mean content of single PAs (± relative standard deviations), μg/kg | The content of total PAs (μg/kg) | |||||||||||||||||||||||||||
| He | HeNO | Im | ImNO | Jb | JbNO | Re | ReNO | Sn | SnNO | Sp | Sp NO |
Eu | EuNO | Sk | |||||||||||||||
| 1 | 97.4 (2.43) | 2731.1 (2.04) | 2424.9 (1.84) | 13754 (0.56) | ND | 1.92 (1.54) | 21.2 (10.45) | 4.01 (5.72) | 58.4 (2.52) | 17.2 (9.03) | ND | ND | 224.0 (1.75) | 6.9 (2.02) | ND | 19341.03 | |||||||||||||
| 2 | 83.9 (1.21) | 2518.6 (0.81) | 2476.5 (1.15) | 13945 (0.30) | ND | 2.60 (2.52) | 28.8 (1.51) | 4.82 (3.66) | 63.7 (3.52) | 19.8 (10.2) | ND | ND | 248.6 (1.48) | 7.0 (1.58) | ND | 19399.32 | |||||||||||||
| 3 | 96.6 (1.67) | 2470.4 (1.08) | 2969.7 (1.02) | 16829 (0.36) | ND | 2.12 (1.08) | 20.9 (9.30) | 2.94 (1.08) | 51.0 (7.50) | 14.9 (8.25) | ND | ND | 252.1 (3.17) | 5.91 (0.35) | ND | 22715.57 | |||||||||||||
| 4 | 91.4 (1.98) | 2638.6 (2.75) | 2882.4 (1.98) | 17345 (0.76) | ND | 2.42 (10.59) | 15.4 (6.99) | 2.67 (10.59) | 51.6 (6.73) | 15.0 (0.92) | ND | ND | 281.3 (2.36) | 6.78 (2.15) | ND | 23332.57 | |||||||||||||
| 5 | 83.4 (3.79) | 2446.7 (6.0) | 2005.5 (3.79) | 13535 (1.96) | ND | 1.68 (4.94) | 15.2 (0.91) | 2.70 (4.94) | 49.4 (8.78) | 16.9 (10.7) | ND | ND | 215.2 (2.47) | 5.99 (3.76) | ND | 18377.67 | |||||||||||||
Table 1: The content of single and total PAs (pyrrolizidine alkaloids) of weeds in the five sampling sites. ND represents none detected.
Supplementary Table 1: The content of single and total PAs in fresh tea leaves purified by the adsorbent method. ND represents none detected. Please click here to download this Table.
Supplementary Table 2: The content of single and total PAs in fresh tea leaves purified by SPE. ND represents none detected. Please click here to download this Table.
Supplementary Table 3: The content of single and total PAs in dried tea purified by the adsorbent method. ND represents none detected. Please click here to download this Table.
Supplementary Table 4: The content of single and total PAs in dried tea purified by SPE. ND represents none detected. Please click here to download this Table.
Supplementary Table 5: The content of single and total PAs in weeds purified by the adsorbent method. ND represents none detected. Please click here to download this Table.
Supplementary Table 6: The content of single and total PAs in weeds purified by SPE. ND represents none detected. Please click here to download this Table.
Supplementary Table 7: The content of single and total PAs in soils purified by the adsorbent method. ND represents none detected. Please click here to download this Table.
Supplementary Table 8: The content of single and total PAs of soils in the five sampling sites. ND represents none detected. Please click here to download this Table.
Discussion
The present work was designed to develop an effective, sensitive method to explore the contamination routes and sources of PAs in tea samples as well as the distribution of PAs in different parts of the tea plants. However, in this study, only 15 PAs were successfully separated on the chromatographic column, which is a very small number in comparison to the large number of alkaloids in plant species3,4. This was not only related to the packing properties of the column itself but also to the complex matrix of the examined tea samples. Therefore, better separation and purification methods for detecting multi-PAs still need further exploration.
SPE cartridges and adsorbent methods have been applied to detect multi-PAs in a variety of sample matrices, but in the complex matrix of tea, the adsorbent method has not been reported24. Therefore, the adsorbent method with a ratio of GCB:PSA:C18 (10 mg:20 mg:15 mg) was developed in this work, and the recoveries of 15 PAs met the detection requirements for PAs in different sample matrices. In contrast, ImNO, Eu, and Re recoveries averaged 119%, 120%, and 115%, respectively, in dried tea upon clean-up with SPE cartridges, which showed a significant matrix effect. Moreover, compared with SPE cartridges, the adsorbent (GCB:PSA:C18) method had a shorter sample treatment time, lower cost, and better recoveries for PA analysis (Figure 1B). Establishing detection methods for 15 PAs in dried tea samples, fresh tea leaves, weeds, and soil provided an effective detection method for exploring the contamination source of PAs in tea samples. Moreover, according to the current knowledge, a multi-PA detection method in soil was established for the first time in this study.
The transfer route of PAs in the tea plantation system was studied. Our studies indicate that A. conyzoides was one of the weeds with the highest total PA content in the Jinzhai tea garden, and it grew beside the tea plants. Therefore, A. conyzoides, A. conyzoides rhizospheric soil, and the different parts of fresh tea leaves were collected from the five sampling sites in one tea garden in Jinzhai to analyze the 15 PAs. Figure 3 shows that, among the 11 PAs produced in A. conyzoides, only ImNO was detected in A. conyzoides rhizospheric soil, while ImNO and Sn were detected in fresh tea leaves. This indicates that not all the content of the PAs produced in A. conyzoides could be transported into the tea plants via the soil medium. Some content of the PAs transferred into the soil may be degraded by soil microorganisms.
ImNO and Sn were mainly distributed in one bud with two leaves and one bud with three leaves, while the content of PAs in mature leaves was relatively low. At sampling site 4, the content of ImNO in one bud with two leaves reached 26.5 µg/kg, while that in other parts of the tea plant ranged from 7.14-10.4 µg/kg. Sn was not detected in mature leaves at sampling site 1 and sampling site 2. This indicates that the enriched parts of PAs in tea plants were mainly concentrated in the young leaves, and the content was far below the maximum residue limit of PAs in tea samples set by the European Union (150 µg/kg for adults, 75 µg/kg for infants and young children)25. The results reveal that the PAs in tea samples may come from PA-producing weeds in tea gardens via the soil. Moreover, the results confirm the transfer and exchange of PAs among plants17.
Divulgazioni
The authors have nothing to disclose.
Acknowledgements
This work was supported by the National Natural Scientific Foundation of China (32102244), the National Agricultural Products Quality and Safety and Risk Assessment Project (GJFP2021001), the Natural Scientific Foundation of Anhui Province (19252002), and the USDA (HAW05020H).
Materials
| Acetonitrile (99.9%) | Tedia Company,Inc. | 21115197 | CAS No:75-05-8 |
| Ammonia (25%-28%) | Wuxi Zhanwang Chemical Reagent Co., Ltd. | 181210 | CAS No:1336-21-6 |
| Ammonium formate (97.0%) | Anpel Laboratory Technoiogies (shanghai) | G0860050 | CAS No:540-69-2 |
| Carbon-GCB | CNW | B7760030 | 120-400 MESH, 10g. per box |
| Centrifuge Z 36 HK | HERMLE | Z36HK | 30000 rpm (min:10 rpm), Dimensions (W x H x D): 71.5 cm× 42 cm × 51 cm |
| Commercially available tea product | Lvming, Qingshan, Luyuchun, Changling, Huixing, Wuyunjian, Heshengchun | loose tea | Green tea |
| Europine N-oxid (EuNO) (98.0%) | BioCrick | 323256 | CAS No:65582-53-8 |
| Europine (Eu) (98.0%) | BioCrick | 98222 | CAS No:570-19-4 |
| Formate (98.0%) | Aladdin | E2022005 | CAS No:64-18-6 |
| HC-C18 | CNW | D2110060 | 40-63 μm,100g.per box |
| Heliotrine (He) (98.0%) | BioCrick | 906426 | CAS No:303-33-3 |
| Heliotrine-N-oxide (HeNO) (98.0%) | BioCrick | 22581 | CAS No:6209-65-0 |
| High speed centrifuge TG16-WS | cence | 203158000 | Max:16000 r/min, 330 × 390 × 300 mm (L × W × H), Capacity: 6 × 50 mL |
| HSS T3 column | Waters | 186004976 | ACQUITY UPLC HSS T3 (2.1 × 100 mm 1.8 μm) |
| Intermedine (Im) (98.0%) | BioCrick | 114843 | CAS No:10285-06-0 |
| Intermedine-N-oxide (ImNO) (98.0%) | BioCrick | 340066 | CAS No:95462-14-9 |
| Jacobine (Jb) (98.0%) | BioCrick | 132282048 | CAS No:6870-67-3 |
| Jacobine-N-oxide (JbNO) (98.0%) | ChemFaces | CFN00461 | CAS No:38710-25-7 |
| Methyl Alcohol (99.9%) | Tedia Company,Inc. | 21115100 | CAS No:67-56-1 |
| PSA | Agela | P19-00833 | 40-60 μm, 60 Å 100g.per box |
| Retrorsine (Re) (98.0%) | BioCrick | 5281743 | CAS No:480-54-6 |
| Retrorsine-N-oxide (ReNO) (98.0%) | BioCrick | 5281734 | CAS No:15503-86-3 |
| Senecionine (Sc) (98.0%) | BioCrick | 5280906 | CAS No:130-01-8 |
| Senecionine-N-oxide (ScNO) (98.0%) | BioCrick | 5380876 | CAS No:13268-67-2 |
| Seneciphylline N-oxid (SpNO) (98.0%) | BioCrick | 6442619 | CAS No:38710-26-8 |
| Seneciphylline (Sp) (98.0%) | BioCrick | 5281750 | CAS No:480-81-9 |
| Senkirkine (Sk) (98.0%) | BioCrick | 5281752 | CAS No:2318-18-5 |
| SPE PCX | Agilent Technologies | 12108206 | Cation Mixed Mode, 6 mL |
| Sulfuric acid (97%) | Wuxi Zhanwang Chemical Reagent Co., Ltd. | 1003019 | CAS No:7664-93-9 |
| Trisodium citrate | Sinpharm Chemical Reagent Co., Ltd. | 20121009 | CAS No:6132-04-3 |
| Ultrasonic cleaner | Supmile | KQ-600B | Inner slot size: 500 × 300 × 150 mm; Capacity: 22.5 L |
| UPLC-xevoTQMS | Waters | ZPLYY-003 | Triple four-stage rod mass analyzer, Waters Alliance 2695/Waters ACQUITY UPLC Liquid Phase System |
| Water bath thermostat oscillator | Guoyu instrument | SHY-2AHS | Oscillation times: 60-300 times/min, Constant temperature range: room temperature to 100 °C |
Riferimenti
- Schramm, S., Kohler, N., Rozhon, W. Pyrrolizidine alkaloids: Biosynthesis, biological activities and occurrence in crop plants. Molecules. 24 (3), 498 (2019).
- EFSA Panel on Contaminants in the Food Chain (CONTAM). Scientific opinion on pyrrolizidine alkaloids in food and feed. EFSA Journal. 9 (11), 134 (2011).
- Ma, C., et al. Determination and regulation of hepatotoxic pyrrolizidine alkaloids in food: A critical review of recent research. Food and Chemical Toxicology. 119, 50-60 (2018).
- Keuth, O., Humpf, H. U., Fürst, P., Melton, L., Shahidi, F., Varelis, P. Pyrrolizidine Alkaloids: Analytical Challenges. Encyclopedia of Food Chemistry. 1, 348-355 (2019).
- Huang, D. Y., et al. Pyrrolizidine alkaloids and its source analysis in tea. Journal of Food Safety & Quality. 9 (2), 229-236 (2018).
- Liang, A. H., Ye, Z. G. General situation of the toxicity researches on Senecio. China Journal of Chinese Materia Medica. 31 (2), 93-97 (2006).
- Li, Y. H., et al. Proteomic study of pyrrolizidine alkaloid-induced hepatic sinusoidal obstruction syndrome in rats. Chemical Research in Toxicology. 28 (9), 1715-1727 (2015).
- Jia, Z. J., et al. Catalytic enantioselective synthesis of a pyrrolizidine-alkaloid-inspired compound collection with antiplasmodial activity. The Journal of Organic Chemistry. 83, 7033-7041 (2018).
- Yang, M., et al. First evidence of pyrrolizidine alkaloid N-oxide-induced hepatic sinusoidal obstruction syndrome in humans. Archives of Toxicology. 91 (12), 3913-3925 (2017).
- Chen, Z., Huo, J. R. Hepatic veno-occlusive disease associated with toxicity of pyrrolizidine alkaloids in herbal preparations. Netherlands Journal of Medicine. 68 (6), 252-260 (2010).
- Mattocks, A. R. . Chemistry and Toxicology of Pyrrolizidine Alkaloid. , (1986).
- Picron, J. F., Herman, M., Van Hoeck, E., Goscinny, S. Analytical strategies for the determination of pyrrolizidine alkaloids in plant based food and examination of the transfer rate during the infusion process. Food Chemistry. 266, 514-523 (2018).
- Kowalczyk, E., Kwiatek, K. Application of the sum parameter method for the determination of pyrrolizidine alkaloids in teas. Food Additives & Contaminants: Part A. 37 (4), 622-633 (2020).
- EFSA Panel on Contaminants in the Food Chain (CONTAM). Risks for human health related to the presence of pyrrolizidine alkaloids in honey, tea, herbal infusions and food supplements. EFSA Journal. 15 (7), 04908 (2017).
- Han, H., et al. Pyrrolizidine alkaloids in tea: A review of analytical methods, contamination levels and health risk. Food Science. 42 (17), 255-266 (2021).
- Nowak, M., et al. Interspecific transfer of pyrrolizidine alkaloids: An unconsidered source of contaminations of phytopharmaceuticals and plant derived commodities. Food Chemistry. 213, 163-168 (2016).
- Selmar, D., et al. Transfer of pyrrolizidine alkaloids between living plants: A disregarded source of contaminations. Environmental Pollution. 248, 456-461 (2019).
- Izcara, S., et al. Miniaturized and modified QuEChERS method with mesostructured silica as clean-up sorbent for pyrrolizidine alkaloids determination in aromatic herbs. Food Chemistry. 380, 132189 (2022).
- Izcara, S., Casado, N., Morante-Zarcero, S., Sierra, I. A miniaturized QuEChERS method combined with ultrahigh liquid chromatography coupled to tandem mass spectrometry for the analysis of pyrrolizidine alkaloids in oregano samples. Foods. 9 (9), 1319 (2020).
- Van Wyk, B. E., Stander, M. A., Long, H. S. Senecio angustifolius as the major source of pyrrolizidine alkaloid contamination of rooibos tea (Aspalathus linearis). South African Journal of Botany. 110, 124-131 (2017).
- Johnson, A. E., Molyneux, R. J., Merrill, G. B. Chemistry of toxic range plants. Variation in pyrrolizidine alkaloid content of Senecio, Amsinckia, and Crotalaria species. Journal of Agricultural and Food Chemistry. 33 (1), 50-55 (1985).
- Vrieling, K., de Vos, H., van Wijk, C. A. M. Genetic analysis of the concentrations of pyrrolizidine alkaloids in Senecio jacobaea. Phytochemistry. 32 (5), 1141-1144 (1993).
- Han, H. L., et al. Development, optimization, validation and application of ultra high performance liquid chromatography tandem mass spectrometry for the analysis of pyrrolizidine alkaloids and pyrrolizidine alkaloid N-oxides in teas and weeds. Food control. 132, 108518 (2022).
- Bodi, D., et al. Determination of pyrrolizidine alkaloids in tea, herbal drugs and honey. Food Additives & Contaminants: Part A. 31 (11), 1886-1895 (2014).
- European Union Commission. Commission Regulation (EU) 2020/2040 of 11 December 2020 amending Regulation (EC) No 1881/2006 as regards maximum levels of pyrrolizidine alkaloids in certain foodstuffs. Official Journal of the European Union. 14 (12), 1-4 (2020).

