Isolation and Culture of Bone Marrow-Derived Macrophages from Mice
Summary
The present protocol describes the isolation and culture of bone marrow-derived macrophages from mice.
Abstract
Macrophages have important effector functions in homeostasis and inflammation. These cells are present in every tissue in the body and have the important ability to change their profile according to the stimuli present in the microenvironment. Cytokines can profoundly affect macrophage physiology, especially IFN-γ and interleukin 4, generating M1 and M2 types respectively. Because of the versatility of these cells, the production of a population of bone marrow-derived macrophages can be a basic step in many experimental models of cell biology. The aim of this protocol is to help researchers in the isolation and culture of macrophages derived from bone marrow progenitors. Bone marrow progenitors from pathogen-free C57BL/6 mice are transformed into macrophages upon exposure to macrophage colony-stimulating factor (M-CSF) that, in this protocol, is obtained from the supernatant of the murine fibroblast lineage L-929. After incubation, mature macrophages are available for use from the 7th to the 10th day. A single animal can be the source of approximately 2 x 107 macrophages. Therefore, it is an ideal protocol for obtaining large amounts of primary macrophages using basic methods of cell culture.
Introduction
Monocytes and macrophages are mononuclear phagocytes that can be derived from progenitors in the bone marrow. Recent studies have reported that macrophages also originate from yolk sac-derived erythro-myeloid progenitors1. Regardless of their derivation, these leukocytes have important effector functions in homeostasis and inflammation2,3. Monocytes are cells from peripheral blood that can further differentiate into macrophages in the tissue2,4, whereas macrophages are heterogeneous cells that exhibit phenotypes and functions regulated by the local exposure of growth factors and cytokines5. Since macrophages show such functional diversity, they have been studied in many disease models. Thus, the in vitro culture of macrophages has become an important tool for understanding their physiology and their role in different diseases. The bone marrow is an important source of progenitor cells, including macrophage progenitors, which can be isolated and multiplied, exponentially increasing the number of macrophages obtained. In addition, bone marrow-derived macrophages are especially important to avoid the effects generated by the tissue microenvironment, since macrophages change their phenotype in response to different stimuli in tissues6,7. Bone marrow progenitors transform into macrophages upon exposure to macrophage colony-stimulating factor (M-CSF)8. Bone marrow-derived macrophages cannot be distinguished from monocyte-derived macrophages by biochemical markers in tissue. These cells represent a highly homogeneous population of primary cells, that in many other respects are comparable to peritoneal macrophages6,9.
Because of their heterogeneous set of cellular functions, macrophages have long been thoroughly studied by investigators. These cells can be used in different experimental models, including infectious and inflammatory diseases, as they feature in these processes10,11. They can also be useful to investigate macrophage polarization in response to various microenvironmental stimuli12,13. Thus, a simple and reliable protocol is provided here for the purpose of obtaining high numbers of primary macrophages from mouse bone marrow.
Protocol
This protocol was carried out in accordance with The National Council for the Control of Animal Experimentation (Concea) and with the approval of the Ethics and Use of Animals Committee (CEUA). C57BL/6 mice were purchased from Biotério Central of Federal University of Minas Gerais (UFMG), Belo Horizonte, Brazil. Personal protective equipment (PPE) such as laboratory coats, gloves, and eye protection should be used at all steps described in this protocol.
1. Preparation of fibroblast L-929 lineage supernatant as a source of M-CSF
- Defrost L-929 fibroblast lineage cells (American Type Culture Collection Certified Cell Line-ATCC CCL1) and start the culture process immediately to preserve the viability.
- In a laminar flow biosafety cabinet, using a 1,000 µL pipette, transfer the L-929 fibroblast lineage cells from the vial to a 50 mL sterile centrifuge tube.
- Use a serological pipette to add 50 mL of sterile phosphate-buffered saline (PBS), free from calcium and magnesium.
- Centrifuge at 300 x g, 4 °C for 10 min. Discard the supernatant.
- Resuspend the pellet in 20 mL of Dulbecco′s modified Eagle′s medium-F12 supplemented with 10% fetal bovine serum (FBS) and 1 mL of penicillin/streptomycin (P/S) (DMEM/F12-10) using a serological pipette.
- Transfer the resuspended cells to a T75 cell culture flask.
- Incubate in a 37 °C, 5% CO2 incubator until full confluence.
NOTE: While manipulating the flask, be careful to avoid wetting the cover with solution, as this can be a source of contamination. To obtain a convenient amount of supernatant, it is necessary to replicate the cells after they cover the full surface of the cell culture flask. L-929 cells are inhibited by contact. Warm trypsin/ethylenediaminetetraacetic acid (EDTA) solution, DMEM/F12-10, and sterile PBS to 37 °C to start the cell culture expansion, as described in the following steps. - Once full confluence is reached, discard the supernatant from the cell culture flask, inside the laminar flow biosafety cabinet.
- Wash the cell culture flask with 20 mL of sterile PBS using a serological pipette and discard the solution.
- Add 10 mL of trypsin/EDTA (0.05% solution) to the cell culture flask containing the adhered cells.
- Transfer the cell culture flask from laminar flow to a 37°C, 5% CO2 incubator and incubate for 3 min.
- Shake the cell culture flask to dissociate the cells from the surface of the culture flask and use a microscope to ensure that all the cells have been dissociated.
- Inactivate the trypsin/EDTA (0.05% solution) with 10 mL of DMEM/F12-10 medium.
- Transfer the solution using a serological pipette to a 50 mL sterile centrifuge tube.
- Centrifuge at 300 x g at 4 °C for 10 min. Discard the supernatant.
- Resuspend the pellet in 10 mL of DMEM/F12-10 medium.
- Add 1 mL of the cell suspension to a T175 cell culture flask (1:10 culture).
- Repeat the procedure to obtain ten T175 cell culture flasks.
- Add 50 mL of DMEM/F12-10 to each cell culture flask.
- Incubate in a 37 °C, 5% CO2 incubator until full confluence.
NOTE: These steps guarantee 500 mL of L-929 cell supernatant. On the day 5, after the cells have covered the T175 cell culture flask surface, the supernatant will be enriched for M-CSF and must be collected14. - Remove the cell culture flask from the incubator on day 5 after contact inhibition.
- Transfer the medium to a 50 mL sterile centrifuge tube.
- Centrifuge at 3,200 x g, at 4 °C for 10 min.
- Collect the supernatant enriched with M-CSF and transfer the solution to a 50 mL sterile centrifuge tube. Store in a freezer at -20 °C.
NOTE: Centrifugation is extremely important to remove cells and debris from the supernatants. - L-929 supernatant is a convenient and inexpensive source of macrophage colony-stimulating factor (M-CSF) and typically contains 5 to 10 ng/mL of M-CSF8. These supernatants also contain trace amounts of other cytokines and growth factors, but they do not exert a profound influence on macrophage physiology15. However, purified M-CSF can be purchased and used as an alternative to L-929 supernatants at concentrations of 1 to 2 ng/mL8.
2. Removal of the femur and tibia
- Proceed with 8-week-old, C57BL-6 wild-type male mouse euthanasia by cervical dislocation after carbon dioxide asphyxiation, in accordance with normative resolution number 18 of the National Council for Animal Experimentation (Concea) and with approval of the Ethics and Use of Animals Committee (CEUA).
- Soak the mouse with 70% ethanol solution and use sterile scissors to make a 1 cm incision along the abdomen.
- Remove the skin until the muscle of the hind legs is fully exposed.
- Remove the hind legs at hip height, being careful not to break the femur and tibia.
- Put the hind legs in a conical centrifuge tube with 70% ethanol solution.
NOTE: Always use pathogen-free mice. Be sure to collect both legs to achieve a greater number of cells. Step 2 is performed in a non-sterile environment (benchtop). Hence, it is important to remove the femur and tibia carefully so they remain intact to avoid bacterial contamination. The remaining steps of this procedure are carried out in a laminar flow biosafety cabinet. The time of exposure of the legs to 70% ethanol solution should be limited to 10 min. If the time is longer, bone marrow progenitors can be affected.
3. Obtain bone marrow progenitors from the lumen of the tibia and femur
- Remove the legs from the 70% ethanol solution and transfer them to sterile PBS.
- Using forceps and disinfectant wipes to remove all muscle and fascia. The bone should be clean after this step.
- Use a sterile surgical scalpel blade to cut the bone epiphysis on both ends to expose the bone marrow.
- Fill a 20 mL syringe with 10 mL of sterile PBS supplemented with 2% penicillin/streptomycin solution and connect a 26 G needle.
- Hold the bone using anatomical dissection forceps with one hand, and use the other to insert the needle into the bone cavity. Be careful to not crush the bone with the forceps.
- Wash out the inside of the bone with the PBS and collect the bone marrow in a sterile conical centrifuge tube. After this step, the bone cavity should appear white.
- Centrifuge the collected cells for 10 min at 300 x g at 4 °C.
- Discard the supernatant and resuspend the cell pellet in 1 mL of DMEM/F12-10.
- Homogenize and add another 9 mL of DMEM/F12-10 medium to bring the cells up to a total of 10 mL.
4. Macrophage culture
- Add 1 mL of cell progenitors to each of the 100 mm x 20 mm round plastic Petri dishes (total of 10 dishes), spreading the cells across the plates with a pipette to obtain a uniform distribution. Do not use tissue-culture treated dishes.
- Add 9 mL of DMEM/F12-10 supplemented with 20% of the L-929 cell supernatant to each dish.
- Incubate in a 37 °C, 5% CO2 incubator.
- On the 3rd day, add 10 mL of DMEM/F12-10 medium supplemented with 20% of the supernatant of L-929 cells to each dish. In this way, the culture dishes now have a total of 20 mL of culture medium, sufficient for the growth of the cells until the end of their maturation.
NOTES: Tissue culture-treated dishes must not be used because macrophages, as natural adherent cells marked by strong interactions with surfaces, will not easily dissociate from the dish later. Usually, cell progenitors from one mouse (two femurs) can be seeded in 10 culture dishes. Cell progenitors will transform to macrophages, which can be used from incubation day 7 to 10. The maturation confirmation is identified by changes on the morphology of macrophages. Mature macrophages are adherent and show heterogeneous morphology, explained by the emission of pseudopodia in different directions. Examples of these mature cells are shown in the representative results.
5. Harvesting macrophages
- Discard the supernatants from all the culture dishes.
- Wash each culture dish with 10 mL of warm (37 °C) sterile Mg2+ and Ca2+ free PBS.
- Discard the solution from each dish.
- Pre-warm non-enzymatic cell dissociation solution to 37 °C and add 3 mL to each dish. Incubate for 10 min in a 37 °C, 5% CO2 incubator. Use an inverted microscope to visualize if the macrophages are dissociating from the culture dishes.
NOTE: The use of non-tissue culture treated plastic plates should allow the easy dissociation of cells and avoid the need to scrape the cells off the plate. - Wash the dishes using a serological pipette with the non-enzymatic cell dissociation solution over and over, performing circular movements to confirm all the macrophages are dissociated from the dish.
- Collect the solution of all the culture dishes together to a conical 50 mL centrifuge tube.
- Add 10 mL of sterile pre-warmed PBS to the empty culture dishes and proceed as in step 5.4. This procedure is a repetition of step 5.4 and intends to guarantee that all the macrophages are collected.
- Centrifuge at 300 x g, 4 °C for 10 min.
- Discard the supernatant and resuspend the pellet with 1 mL of DMEM/F12-10. Homogenize slowly and carefully up and down using the pipette to avoid damaging the cells.
- Prepare an aliquot of 10 µL of macrophage solution diluted with 10 µL of Trypan Blue to count the cells using the hemocytometer.
NOTE: At day 7, each dish will produce approximately 2 x 106 macrophages. This means that one mouse can produce approximately 2 x 107 primary macrophages when harvested in ten plates. Since M-CSF only drives the maturation of macrophages, the procedure guarantees a population of cells that is essentially just macrophages and free from other contaminating leukocytes16.
Representative Results
Macrophages are large and adherent cells with special physiological characteristics. They show a diversity of morphologic presentations in culture because of the ability to adhere to glass and plastic, and their typical spread-out morphology is related to the emission of cytoplasmic extensions (Figure 1). Once bone marrow progenitors are exposed to M-CSF from L-929 cell supernatant and start the transformation to mature macrophages, they become adherent to the Petri plate.
Figure 2 shows the kinetics of the transformation of bone marrow progenitors to mature macrophages. On day 3, a few immature macrophages appear, most showing a typical round-shape morphology with few membrane projections (Figure 3). Although they transform along the entire process, 7 days is usually necessary to achieve an adequate number and maturity to guarantee maximum efficiency of the present protocol.
In addition, macrophages are phagocytes (macro = large; phagos = eat) capable of phagocytizing large amounts of antigenic or non-antigenic particles. Phenotypically, they are characterized by the expression of surface molecules F4/80 and CD11b. The analysis by flow cytometry shows that the macrophages obtained through the present protocol represent a homogeneous population in terms of size and granularity (Figure 4). Furthermore, 100% of the population expressed F4/80 and CD11b, forming a single, well-defined population of cells. Besides, analysis of phagocytosis of Leishmania major parasites showed an enormous capacity for phagocytosis, proving that they are mature and well-differentiated macrophages (Figure 5).
The microscopic data shown in this work was obtained using fluorescence and phase contrast microscopy in the Image Acquisition and Processing Center (CAPI-ICB/UFMG).
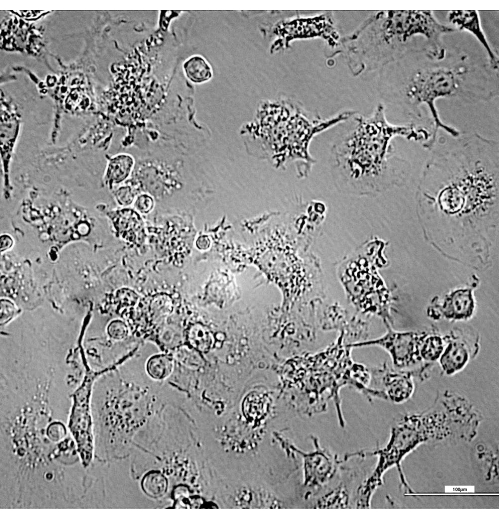
Figure 1: Culture of macrophages derived from bone marrow progenitors, showing different morphologies while adhered to the plate, at day 5. Macrophages emit long cytoplasmic extensions that allow them to move and phagocytize. Brightness and contrast were adjusted after the acquisition of images using a differential interference contrast (DIC) 20x filter. Bar = 100 µm. Please click here to view a larger version of this figure.
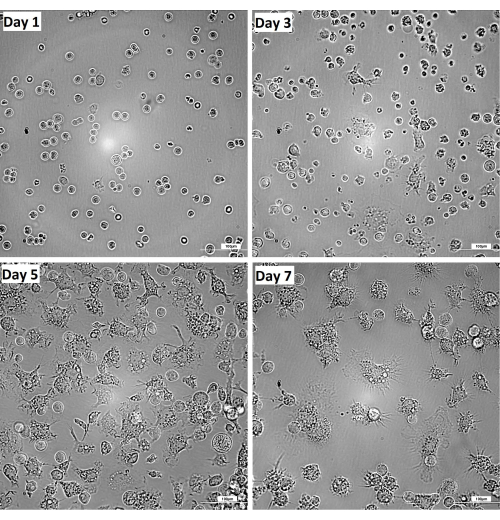
Figure 2: Kinetics of transformation from bone marrow progenitors to mature macrophages at day 1, 3, 5, and 7. Brightness and contrast were adjusted after the acquisition of images using a DIC 20x filter. Bar = 100 µm. Please click here to view a larger version of this figure.
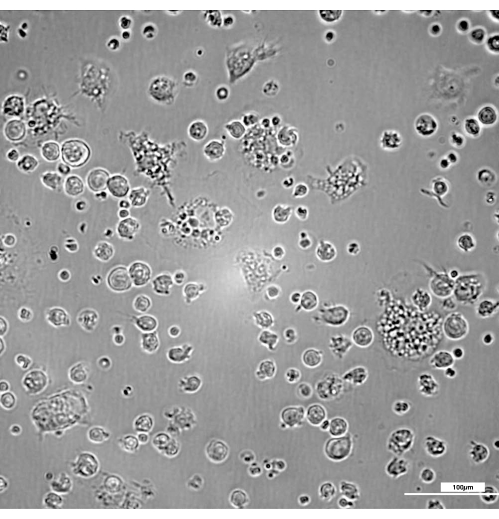
Figure 3: Presence of lightly adherent immature macrophages at day 3 with a typical round-shape morphology with few membrane projections. Brightness and contrast were adjusted after the acquisition of images using a DIC 20x filter. Bar = 100 µm. Please click here to view a larger version of this figure.
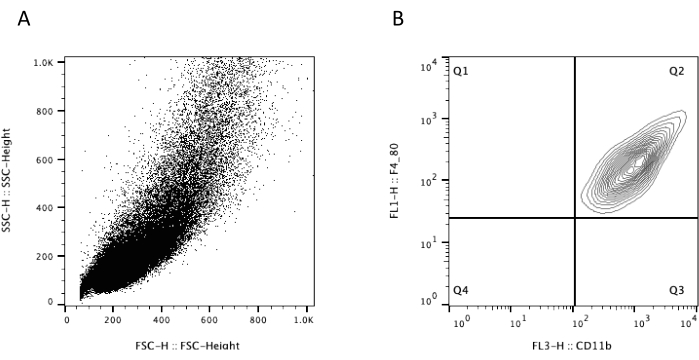
Figure 4: Flow cytometry analysis of bone marrow-derived macrophages. Macrophages were detached from the dish plates (cell stripper) and stained with anti-F4/80 FITC and anti-CD11b PE-Cy7. (A) The aspect of the cells based on size and granularity (FSC x SSC). (B) The expression of F4/80 and CD11b cell markers. Please click here to view a larger version of this figure.
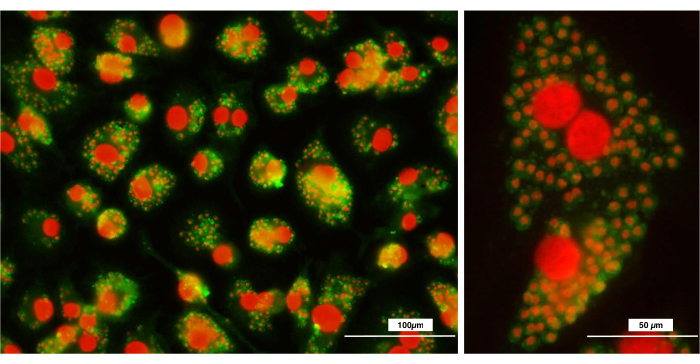
Figure 5: Fluorescence microscopy analysis after phagocytosis of Leishmania major parasites. Macrophages were detached from the plates and seeded on round glass coverslips, to which Leishmania major parasites were added. The figure shows the phagocytosed parasites inside the macrophages. Leishmania major parasites were stained with FITC anti-Leishmania antibodies and cell nuclei (macrophages and Leishmania major) were counterstained with propidium iodide. (A) 20x; (B) 40x. Bar = 100 µm. Please click here to view a larger version of this figure.
Discussion
Producing a population of bone marrow-derived macrophages is a basic step in many cell biology experimental models, especially when it is important to achieve a homogeneous population of primary cells. As mentioned, cell progenitors can only transform into macrophages in the presence of M-CSF. L-929 cell supernatants can be used as the main source of M-CSF. Other than the cost, there is no problem using recombinant M-CSF itself8,17. There is some evidence that recombinant M-CSF macrophages exhibit better uniformity in culture; however, using r-M-CSF or L-929 supernatant does not make any significant difference in the gene expression of main macrophage markers and cytokine production15.
Bone marrow progenitor cells are very susceptible. To avoid contamination, the usage of a clean laminar flow biosafety hood is essential. In the first steps of this protocol, removing muscle from the bone's surface produces residues that can be a source of contamination. It is recommended to clean the hood before cutting bone epiphysis and exposing the bone marrow. Once progenitors are out of the bone cavity, it is important to remember to handle these cells carefully to maintain sterility and optimum viability.
C57BL/6 mice are used in this protocol, but BALB/C mice are equally suitable. Mice aged 6-10 weeks are recommended for harvesting a good population of bone marrow derived-macrophages, with the estimation of 2 x 107 cells per mouse. Younger mice typically provide similar numbers of cells, but the smaller femurs may present some challenges to the isolation procedure. We typically do not observe much variation between macrophage yield from different wild-type strains of mice, in our previous studies, but we acknowledge that some strains of knockout mice may present some variability in the yield6,8.
The present protocol describes a reliable way of acquiring a primary macrophage colony using L-929 supernatant, a technique which has been well-established and used by researchers over many years18,19. In respect to other existing methods, this procedure excels by the number of cells and homogeneity of the population, especially when compared to peritoneal macrophages. The number of peritoneal resident macrophages is often insufficient, as millions of cells are usually required for each assay, and macrophages are only a small fraction found in peritoneal wash8,20. Peritoneal wash also provides other cell types, such as lymphocytes and dendritic cells, making it impossible to avoid interaction and obtain a culture of unstimulated macrophages. Since the peritoneum has a much more complex microenvironment than in vitro, variability can be a disadvantage for some applications6,21.
Divulgazioni
The authors have nothing to disclose.
Acknowledgements
This work was supported by grants from Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG), through Rede de Pesquisa em Doenças Infecciosas Humanas e Animais do Estado de Minas Gerais (RED-00313-16) and Rede Mineira de Engenharia de Tecidos e Terapia Celular – REMETTEC (RED-00570-16), and the Brazilian National Council for Scientific and Technological Development (CNPq).
Materials
| 20-cc syringe | DESCARPACK | SI100S4G | Sterile syringe |
| 26-G needles | BD | 497AQDKT7 | Sterile needles |
| 50-ml conical centrifuge tube | SARSTEDT | 62547254 | Plastic conical tubes suitable for centrifugation |
| 70% ethanol solution | EMFAL | 490 | Ethanol solution for esterilization |
| Anatomical dissection forceps | 3B SCIENTIFIC | W1670 | Maintain in sterile beaker containing 70% ethanol solution |
| C57Bl/6 wild type mouse | Purchased from Biotério Central at Federal University of Minas Gerais | Not applicable | Mice must be specific-pathogen-free, age between 6 and 10 weeks. Mice need be accommodated at least one week earlier for recovering from the stress of transportation |
| Cell culture flask T175 | GREINER | C7481-50EA | T-175 flask, canted neck, surface area 75 cm2, with filter cap, DNase free, RNase free |
| Cell culture flask T75 | GREINER | C7231-120EA | T-75 flask, canted neck, surface area 75 cm2, with filter cap, DNase free, RNase free |
| Disinfecting or baby Wipes₁ | CLOROX | Not applicable | It helps cleaning the bone |
| Distilled Water | GIBCO | 15230 | Sterile distilled water |
| DMEM/F12-10 | Not applicable | Not applicable | Add 10 mL of Fetal Bovine Serum (FBS) and 1 mL of Penicillin/ Streptomycin (P/S) to DMEM/F12 (q.s.p. 100 mL) |
| DMEM/F12-10 + supernatant of L-929 cells | Not applicable | Not applicable | Add 20% of supernatant of L929 cells culture on DMEM/F-12-10 |
| Dulbecco′s Modified Eagle′s Medium – F12 (DMEM/F12) | GIBCO | 12500096 | DMEM: F-12 Medium contains 2.5 mM L-glutamine, 15 mM HEPES, 0.5 mM sodium pyruvate, and 1200 mg/L sodium bicarbonate.Resuspend powder to 1 liter of distilled water and add 3,7 g of sodium bicarbonate. Adjust pH to 7,2 and filter with 0,22 µM. Storage at 2 – 8 °C freezer |
| Fetal Bovine Serum, certified, heat inactivated, United States (FBS) | GIBCO | 10082147 | Enrichment for DMEM-F12 |
| Hemocytometer | SIGMA-ALDRICH | Z359629 | Used to count macrophages at microscopy |
| L-929 cells | SIGMA-ALDRICH | ATCC # CCL-1 | L-929 is a lineage of mouse fibroblast cells used as a source of macrophage colony stimulating factor (M-CSF) |
| Nikon TI eclipse | NIKON | Not applicable | Nikon TI Eclipse is a fluorescence and phase contrast microscope |
| Non-enzymatic cell dissociation solution | CELLSTRIPER (CORNING) | 25-056-CI | Non-enzimatic cell dissociation solution remove macrophages from the plate without damaging them |
| Non-treated round culture dishes 100 × 20–mm | CORNING | CLS430591 | Do not use tissue culture treated petri dishes or any tissue culture treated plate |
| P3199 Penicillin G Potassium Salt | USBIOLOGICAL | 113-98-4 | Antibiotics |
| Penicillin and streptomycin (P/S) solution | Use 0,26 grams of penicillin and 0,40 grams of streptomycin. Mix with 40 mL of sterile PBS. Inside a horizontal laminar flow cabinet, use a 0,22 µM filter and store aliquots of 1.0 mL in 1.5 ml microfuge tubes in the freezer (-20°C) | ||
| Phosphate Buffered Saline free from Calcium and Magnesium (PBS) | MEDIATECH | 21-040-CM | Sterile |
| S7975 Streptomycin Sulfate, 650-850U/mg | USBIOLOGICAL | 3810-74-0 | Antibiotics |
| Serological pipettes of 10mL or 25 mL | SARSTEDT | 861254001 | Serological pipettes is used volumes higher than 1 mL |
| Sodium Bicarbonate | SIGMA-ALDRICH | 144-55-8 | pH correction for DMEM-F12 |
| Software Nis Elements Viewer | NIKON | Not applicable | NIS-Elements Viewer is a free standalone program to view image files and datasets |
| Sterile PBS and 2% of P/S solution | LABORCRIN | 590338 | Add 1 mL of P/S in 40 mL of sterile PBS |
| Straight iris scissors | KATENA | Not applicable | Maintain in sterile beaker containing 70% ethanol solution |
| Supernatant of L-929 cells | Not applicable | Not applicable | L-929 is a lineage of mouse fibroblast cells used as a source of macrophage colony stimulating factor (M-CSF). L-929 supernatant was obtain from the protocol |
| Surgical Scalpel Blade No.24 Stainless Steel | SWANN-MORTON | 311 | Used for exposing epiphysis from bones |
| Trypan Blue Solution, 0.4% | GIBCO | 15250061 | Trypan Blue Solution, 0.4%, is routinely used as a cell stain to assess cell viability using the dye exclusion test |
| Trypsin/EDTA solution 0,05% | GIBCO | 25300-062 | Used to dissociate cells from culture bottle |
| Water for Injection (WFI) for Cell Culture | GIBCO | A12873 | Sterile and endotoxin-free water |
Riferimenti
- Gomez Perdiguero, E., et al. Tissue-resident macrophages originate from yolk-sac-derived erythro-myeloid progenitors. Nature. 518 (7540), 547-551 (2015).
- van Furth, R., Cohn, Z. A. The origin and kinetics of mononuclear phagocytes. The Journal of Experimental Medicine. 128 (3), 415-435 (1968).
- Wynn, T. A., Chawla, A., Pollard, J. W. Macrophage biology in development, homeostasis and disease. Nature. 496 (7446), 445-455 (2013).
- Medzhitov, R. Origin and physiological roles of inflammation. Nature. 454 (7203), 428-435 (2008).
- Shapouri-Moghaddam, A., et al. Macrophage plasticity, polarization, and function in health and disease. Journal of Cellular Physiology. 233 (9), 6425-6440 (2018).
- Davies, J. Q., Gordon, S. Isolation and culture of murine macrophages. Methods in Molecular Biology. 290, 91-103 (2005).
- Jin, X., Kruth, H. S. Culture of macrophage colony-stimulating factor differentiated human monocyte-derived macrophages. Journal of Visualized Experiments. (112), e54244 (2016).
- Gonçalves, R., Mosser, D. M. The isolation and characterization of murine macrophages. Current Protocols in Immunology. 111 (1), 1-16 (2015).
- Varol, C., Mildner, A., Jung, S. Macrophages: development and tissue specialization. Annual Review of Immunology. 33, 643-675 (2015).
- Andrés, V., Pello, O. M., Silvestre-Roig, C. Macrophage proliferation and apoptosis in atherosclerosis. Current Opinion in Lipidology. 23 (5), 429-438 (2012).
- Pineda-Torra, I., Gage, M., de Juan, A., Pello, O. M. Isolation, culture, and polarization of murine bone marrow-derived and peritoneal macrophages. Methods in Molecular Biology. 1339, 101-109 (2015).
- Edwards, J. P., Zhang, X., Frauwirth, K. A., Mosser, D. M. Biochemical and functional characterization of three activated macrophage populations. Journal of Leukocyte Biology. 80 (6), 1298-1307 (2006).
- Hamczyk, M. R., Villa-Bellosta, R., Andrés, V. In vitro macrophage phagocytosis assay. Methods in Molecular Biology. 1339, 235-246 (2015).
- Hosoe, S., et al. Induction of tumoricidal macrophages from bone marrow cells of normal mice or mice bearing a colony-stimulating-factor-producing tumor. Cancer Immunology, Immunotherapy. 28 (2), 116-122 (1989).
- Rice, H. M., et al. rM-CSF efficiently replaces L929 in generating mouse and rat bone marrow-derived macrophages for in vitro functional studies of immunity to intracellular bacteria. Journal of Immunological Methods. 477, 112693 (2020).
- Boltz-Nitulescu, G., et al. Differentiation of rat bone marrow cells into macrophages under the influence of mouse L929 cell supernatant. Journal of Leukocyte Biology. 41 (1), 83-91 (1987).
- Weischenfeldt, J., Porse, B. Bone marrow-derived macrophages (BMM): isolation and applications. Cold Spring Harbor Protocols. 2008 (12), (2008).
- Austin, P. E., Mcculloch, E. A., Till, J. E. Characterization of the factor in L-cell conditioned medium capable of stimulating colony formation by mouse marrow cells in culture. Journal of Cellular Physiology. 77 (2), 121-134 (1971).
- Stanley, E. R. Macrophage colony-stimulating factor (CSF-1). Encyclopedia of Immunology. 116 (1983), 1650-1654 (1998).
- Bou Ghosn, E. E., et al. Two physically, functionally, and developmentally distinct peritoneal macrophage subsets. Proceedings of the National Academy of Sciences. 107 (6), 2568-2573 (2010).
- Ray, A., Dittel, B. N. Isolation of mouse peritoneal cavity cells. Journal of Visualized Experiments. (35), e1488 (2010).

