Sonodynamic Therapy for the Treatment of Glioblastoma Multiforme in a Mouse Model Using a Portable Benchtop Focused Ultrasound System
Summary
Here, we describe a protocol that details how to perform sonodynamic therapy in an in vivo mouse glioblastoma model using magnetic resonance-guided focused ultrasound.
Abstract
Sonodynamic therapy (SDT) is an application of focused ultrasound (FUS) that enables a sonosensitizing agent to prime tumors for increased sensitivity during sonication. Unfortunately, current clinical treatments for glioblastoma (GBM) are lacking, leading to low long-term survival rates among patients. SDT is a promising method for treating GBM in an effective, noninvasive, and tumor-specific manner. Sonosensitizers preferentially enter tumor cells compared to the surrounding brain parenchyma. The application of FUS in the presence of a sonosensitizing agent generates reactive oxidative species resulting in apoptosis. Although this therapy has been shown previously to be effective in preclinical studies, there is a lack of established standardized parameters. Standardized methods are necessary to optimize this therapeutic strategy for preclinical and clinical use. In this paper, we detail the protocol to perform SDT in a preclinical GBM rodent model using magnetic resonance-guided FUS (MRgFUS). MRgFUS is an important feature of this protocol, as it allows for specific targeting of a brain tumor without the need for invasive surgeries (e.g., craniotomy). The benchtop device used here can focus on a specific location in three dimensions by clicking on a target on an MRI image, making target selection a straightforward process. This protocol will provide researchers with a standardized preclinical method for MRgFUS SDT, with the added flexibility to change and optimize parameters for translational research.
Introduction
Glioblastoma (GBM) is a form of highly aggressive brain cancer that has an incidence of 3.21 per 100,000 people and is the most common malignant brain tumor1. The current standard of care includes surgical resection, radiation, and chemotherapy2. Due to the tumor's invasive and infiltrative nature, complete tumor resection is rare. Residual tissue at the tumor margins results in a high rate of tumor recurrence and a low survival rate of less than 6% after 5 years1.
Due to this prognosis, researchers are exploring new therapeutic options to combat this deadly disease. Sonodynamic therapy (SDT) is a noninvasive treatment that combines low-intensity focused ultrasound (FUS) and sonosensitizing agents to produce a cytotoxic effect in targeted cells3. As an example, porphyrin-based sonosensitizers such as 5-aminolevulinic acid (5-ALA) are preferentially taken up by tumor cells and increase reactive oxidative species (ROS) production to damaging levels when exposed to focused ultrasound. Overexpressed levels of ROS in cells can damage cellular structures and trigger apoptosis. Since 5-ALA is preferentially taken up by tumor cells, healthy tissue within the treatment region is unharmed3,4. Preliminary in vitro studies have revealed that many cancer cells are lysed by SDT treatment, though the rate of cell death is dependent on the cell line. Preliminary in vivo studies yield similar results, confirming that SDT can trigger apoptosis5.
This protocol aims to describe effective techniques and parameters for the SDT treatment of rodent models with intracranially implanted GBM cells using a benchtop FUS research platform. Researchers can use this protocol to perform and optimize SDT for translational FUS research.
Protocol
All animal studies were approved and conducted in accordance with the Johns Hopkins University Institutional Animal Care and Use Committee (IACUC). Athymic nude female mice (age: 10 weeks) were obtained from commercial sources (see Table of Materials). All Biosafety Level 2 (BSL-2) regulations were followed, including the usage of masks, gloves, and gowns.
1. Tumor implantations and bioluminescence imaging
- During the initial phase of the study, perform intracranial tumor implantation, following a previously published report6.
NOTE: In this study, 100,000 M59 human GBM xenograft cells in 4 µL of cell suspension were used for implantation at a 3.0 mm depth into the skull. - Prior to treatment, quantify the tumor size in each mouse noninvasively using an in vivo luminescence imaging system (see Table of Materials) following a previously published report7.
NOTE: This was done on the date of treatment, 7 days following initial tumor implantations. - Using imaging quantification, divide the mice into comparable subgroups for treatment.
NOTE: In this study, two subgroups were included: (1) untreated tumor-bearing mice (n = 4) and (2) tumor-bearing mice undergoing SDT (n = 4). No statistically significant difference in pre-treatment tumor size was observed between the two groups (p > 0.05).
2. Treatment day setup
- Remove 5-ALA hydrochloride (see Table of Materials) from the freezer and weigh out 200 mg/kg mouse weight of 5-ALA (e.g., for a 25 g mouse, weigh out 5 mg.). Do so under ambient light only.
NOTE: Sterile all the equipment before use. - Dissolve the total amount of 5-ALA in phosphate-buffered saline (PBS), such that each animal is administered a 60 mg/mL solution of 5-ALA with the correct weight dosage (e.g., for a 25 g mouse, dissolve 5 mg of 5-ALA into 83.33 µL of PBS).
- For each animal in the SDT testing group, inject the proper amount of solution of 5-ALA into the animal intraperitoneally (calculated in steps 2.1 and 2.2).
- Leave the animals in their cages for 3 h to metabolize the 5-ALA.
3. Preparation of requisites for experiments
- Fill a reservoir with deionized (DI) water.
NOTE: The amount of water needed is based on the number of mice used in the study. - Attach the Flow in and Flow out tubes to the ultrasonic degasser (see Table of Materials) and plug in the degasser to the power (1 = ON, 0 = OFF).
- Place the other end of the Flow in and Flow out tubes into the DI water reservoir.
- Turn on the degasser and run for 45 min.
- Fill the newly degassed water to the top of an airtight, sealed container to be used during the study.
- Pour ultrasound gel (see Table of Materials) into a conical tube, and then place into a centrifuge and spin at 160 x g for 5 min to remove any air from the gel.
NOTE: Enough gel is needed to form a dollop of gel on the head of each animal.
4. MRgFUS system setup
- Connect the MRgFUS system (see Table of Materials) using the wiring diagram as seen in Figure 1 (bottom panel).
- Connect all necessary components (desktop computer, monitor, MRgFUS platform, oscilloscope, amplifier) to power.
- Connect all cables to the correct locations.
- Connect the desired transducer to the MRgFUS platform via the BNC and coaxial cables, and then connect the corresponding impedance matching box to the correct wires.
NOTE: A 515 kHz transducer was used for the present study.
- Turn on all devices.
- On the desktop computer operating system, open the AUREUS application, which is already integrated into the FUS system software.
- Select Connect All Hardware to connect the hardware to the system and make sure that the components are communicating with the software.
- Select the transducer used by selecting the drop-down menu and choosing the desired transducer.
5. Initialization
- Unscrew the bottom screw from the phantom and fill the cavity with deionized and degassed water until it overflows, and then replace the screw again.
- Insert the phantom in its corresponding MRI bed location (see Figure 2), and then place the MRI bed into the MRI cradle in its corresponding slot.
- Place the MRI cradle in its corresponding location in the MRI scanner (see Table of Materials). Adjust the cradle so that the phantom MRI bed can slide into the MRI bore without any obstructions to obtain a high-quality image of the phantom. Mark this location so that it is easily replicable in the future.
NOTE: Once this location is found, the location will not be changed for the remainder of the treatment. Therefore, make sure that it is an adequate location for animal MRI placement, or the entire registration will need to be repeated. - Take an MRI scan of the phantom using the settings in Table 1.
- Remove the cradle from the magnet bore but keep it on the scanner. Remove the MRI bed containing the phantom from the cradle, and then place the bed with the phantom onto the MRgFUS system by sliding the peg on the bottom into its correct slot.
- Slot the registration tip onto the magnetic slots on the transducer arm so that it points downward toward the phantom (see Figure 2).
- On the software, choose Select a New Home Position, and then select Jog Mode On to begin guided focused finding. After jog mode is toggled, use the left, right, up, down, page up, and page down keys to manually move the transducer arm around in the left, right, forward, backward, up, and down directions, respectively.
- Adjust the pointer manually in all three dimensions until the tip of the pointer touches the middle of the cross pattern that lies on the top of the phantom (see Figure 2).
- Download the phantom MRI images to the computer and place them in a folder in the chosen directory, and then load the MRI images into the software by selecting Load Phantom Images. The axial phantom slices will then populate the screen on the right side. Scroll through these slices via the mouse scroll bar. Adjust the brightness by clicking and holding on the image and then moving the mouse up or down.
NOTE: If any of the files in the folder are not uncompressed DICOM files, then the software will not be able to read and import them, so remove any other files from that folder. - Scroll to any image of the phantom where there is a clear circle with three dark holes in each. Click on the middle of the phantom, and a red circle will pop up. Click and drag on the circle until it is the same diameter and lines up with the circumference of the phantom (see Figure 2).
- The coordinates of this position are referred to as 'home position'; save this by clicking Set L/R, A/P, and S/I.
- Click Jog Mode Off, remove the registration tip from the transducer arm, and then select Exit Focus Finding. Confirm the home position to complete the initialization sequence.
6. Animal preparation for SDT
- Anesthetize the mouse using an isoflurane-O2 gas mixture in an induction chamber. Set the gas flow rate to 1.0 mL/min and the vaporizer to 2.0% for anesthesia induction, typically requiring 3-5 min in the chamber (see Table of Materials).
- Assess the animal for adequate sedation by pinching the toe. Apply an ophthalmic ointment to the eyes to avoid dryness of the corneas.
NOTE: Monitor the anesthesia depth throughout the procedure. - Inject the mouse with 40 µL of gadolinium contrast agent through the tail vein (see Table of Materials).
- Remove any hair obstructing the scalp above the skull using a hair removal cream and an electronic shaver.
- Secure the animal to the MRI bed using the following steps (see Figure 3).
- Connect the inlet tube of the MRI bed (Figure 3A) to a source of isoflurane for anesthesia, and set the gas flow rate to 1.0 mL/min and the vaporizer to 2.0% for anesthesia induction. Connect the outlet tube to a charcoal filter canister for anesthesia absorption.
- Place the nose cone piece into its slot, as shown in Figure 3B. Slide the bite bar through the bite bar holes in both the nose cone and at the end of the bed, as shown, with the bite guard end hovering over the open well in the MRI bed.
- Place the animal on the MRI bed with its ears lined up with the stereotactic ear bar holes and latch its teeth through the bite guard to keep it in place. Slide the nose cone forward so that it is over top of the animal's snout to provide a steady stream of anesthesia.
- Slide the ear bars through the holes on both sides of the MRI bed and raise the animal's head until both ear bars can fit into the mouse's ear canals.
NOTE: Do not push too far, as this can damage the animal's ear drums. - Ensure that the animal is in a comfortable position. Then, using a flathead screwdriver, screw in the MRI-compatible screws to lock in both ear bars, the nose cone, and the bite bar. This will lock the animal into place and prevent any head movement until the animal is removed from the bed.
NOTE: Make sure that there is no disturbance to the animal's positioning on the MRI bed following this step. If the animal moves, then the entire setup (step 6.5) will need to be repeated, regardless of how far along the protocol is. - While the animal is waiting, place the MRI bed on a warm heat pad to maintain the body temperature.
NOTE: Monitor and maintain the body temperature throughout the procedure.
- Isoflurane is continuously supplied to the animal via tubes attached to the nose cones throughout the treatment when the animal bed is fixed on the FUS system, using the fixtures provided on the platform.
7. MRI procedures
- Take the MRI bed with the animal stereotactically fixed and place it in the MRI cradle previously connected to the MRI scanner (see Table of Materials), by slotting the MRI bed hole at its end to the peg on the MRI cradle, as shown in Figure 3. Attach the inlet and outlet anesthesia tubes to the corresponding tubes in the MRI machine.
- Slide the MRI cradle containing the animal into the MRI bore, making sure to keep the same positioning as where the phantom was placed.
- Perform a localizer to see the location of the animal brain and then a post-contrast T1-weighted MRI scan covering the entire brain using the MRI settings from Table 2.
- Export the MRI scan as a set of uncompressed DICOM files, one for each slice.
8. Focused ultrasound treatment (Figure 4)
- Remove the animal from the MRI cradle after completion of the scan and place it on the platform by slotting the front of the bed into its corresponding peg at the back of the platform, and slotting the back of the bed into its corresponding peg.
- Connect the inlet tube of the MRI bed to a source of isoflurane for anesthesia and set the gas flow rate to 1.0 mL/min and the vaporizer to 2.0% for maintenance of anesthesia. Connect the outlet tube to a charcoal filter canister for anesthesia absorption.
- Transfer the set of DICOM files to the computer and place them in a folder in the main working directory of the current study.
NOTE: Refer to step 5.9 for file requirements. - In the software, go to the main initialization page and turn on Jog Mode by clicking on Guided Focus Finding and then click on Jog Mode On.
- Place a dab of centrifuged ultrasound gel on the animal's head, with enough gel to cover the entire scalp above the skull.
- Pour DI and degassed water into the water bath up to 80%, and then slot the water bath onto its corresponding columns on the platform.
- Lower the water bath filled with DI water until the bottom membrane touches the ultrasound gel on the animal's head, forming a coupling surface between the water and the gel. Make sure that the ultrasound gel covers the entire head of the animal to the water bath and that there are no air bubbles in the ultrasound gel between the water bath and the mouse's scalp.
- Submerge the ultrasound transducer into the water bath, ensuring no air bubbles are forming on the transducer surface.
- Lower the transducer arm using Jog Mode Down toward the partly submerged transducer, and couple it to the transducer by aligning the magnetic slots with each other while the transducer surface remains submerged.
- Turn off Jog Mode, and then Exit Focused Finding, as done during the initialization step. Make sure to confirm the home position as done before.
- Go to the treatment tab, and then upload the post-contrast T1-weighted MRI files by clicking the folder icon in the top middle of the screen. Select the folder containing the DICOM files that were uploaded to the computer previously and open them. This step should automatically load all DICOM files in the top right panel.
- Next, in the Sonication Mode tab, select the proper treatment mode by choosing either Burst or Continuous Wave. If on burst mode, in the Sonication Settings tab, enter the burst length, burst period, and number of periods. These parameters will correspond to each sonication location. If in continuous wave mode, only enter the sonication duration.
NOTE: In this study, continuous wave mode was used for a 120 s duration. - Click on the target icon in the top middle of the page and select the proper locations on the correct MRI slice where the FUS focal region is to be aimed. A light red square with a red circle will be present where clicked. More than one selection can be chosen.
- Left of the MRI image is a table, which will populate with the 3D coordinates of the focal regions selected. In the last column of the table, enter the power level to be sonicated at each focal spot, which can be calculated separately for each transducer used to obtain the desired pressure or intensity levels. Click on each focal spot in the table to confirm, which will result in the coordinates being highlighted, and the corresponding focal region on the image will turn blue.
NOTE: Refer to the transducer datasheet to determine how to translate the power level entered into the table into pressure or intensity, as these values are transducer specific. - Once satisfied with all focal regions, select Motion Test. This will prompt the software to move the transducer to all of the focal spots to ensure the movements are possible. After confirmation, the software will indicate Motion Test Complete. If an error occurs, adjust the focal regions so that they can fit within the 3D axes of the transducer motors.
- Once ready, select Begin Sonication to begin the sonication protocol, moving the transducer and applying the correct FUS parameters at each focal region selected.
- Once the sonication protocol is finished, uncouple the transducer from the transducer arm, remove the water bath from the platform, and wipe off the ultrasound gel from the animal's scalp.
- Unscrew the bite and ear bars, remove the animal from the MRI bed, and then place the animal on a warm pad until the animal wakes up from anesthesia. At this point, return the animal to its cage.
- For subsequent animals, repeat the same protocol, starting from section 6.
- Once the desired animals are treated, clean all surfaces touched by animals with 70% ethanol (see Table of Materials).
- Quit out of the software, and then shut down and turn off each piece of equipment.
9. Post-treatment steps
- Repeat the in vivo imaging system (IVIS) protocol from section 1 for bioluminescence measuring 24 h post-treatment. To analyze treatment effectiveness, compare the bioluminescence recordings before and after treatment to determine the growth rate for both treated and untreated groups.
- Perform follow-up MRI scans using the same settings from step 6.4. Using contrast-enhanced scans, compare the mean greyscale intensity of the tumor region or calculate the total volume that is covered by the contrast agent to determine differences in tumor volume pre- and post-treatment.
Representative Results
Tumor size declines in animals treated with SDT 24 h post-treatment.
On the day of SDT treatment, the original average bioluminescence signal for the control and treatment groups (N = 4 each) was 2.0 x 106 ± 3.1 x 106 and 2.3 x 106 ± 1.3 x 106 p/s/cm2/sr, respectively. The average bioluminescence values corresponding to tumor size before treatment between the two groups were not statistically significant (p = 0.89). The average bioluminescence signal of the treatment group was 3.57 x 106 ± 2.3 x 106 24 h following SDT, while the bioluminescent signal of the control group was increased to 5.5 x 106 ± 8.2 x 106 p/s/cm2/sr. As shown in Figure 5, this corresponds to a growth rate of 83.4% ± 78% and 172% ± 34%, respectively, assuming exponential growth (p = 0.08). Of the four treated animals, three had lower growth rates post-treatment compared to controls. There was one outlier in the treatment group that showed comparable growth to controls, skewing the deviations.
Additionally, the animals underwent subsequent contrast-enhanced MR imaging the day following treatment for pre- and post-treatment comparison of the tumor. The average grayscale intensity of contrast agent in tumors was conducted across each MRI slice for each animal to measure how much contrast agent was entering tumors following treatment, as an estimate of tumor size. Pre-treatment, the average tumor grayscale intensity between control and treated groups was similar. On average, this greyscale intensity increased in the control group to a larger magnitude than in treated groups, although this was not significant (p = 0.47). This data can be seen in Table 2. The high variability of these results is potentially due to the fact that MRIs were taken only 24 h post-treatment, at which time the therapeutic potential of SDT is only beginning to occur. Even so, Figure 6 shows an example of the lesions created by SDT.

Figure 1: FUS system setup. (Top) MRgFUS System with labeled components. (Front) 1. Platform. 2. Axis motorized transducer arm. 3. FUS transducer. 4. Water bath. 5. MRI bed. 6. Monitor. 7. Transducer impedance matching box. 8. Power amplifier box. 9. Function generator. 10. Function generator channel 1 BNC port. 11. Desktop computer. (Bottom) MRgFUS system with color-coded wiring schematic with the following connections. (Back) 1. Power cords. 2. Monitor HDMI to desktop HDMI. 3. Port B USB B to desktop USB A. 4. Oscilloscope LAN ethernet to desktop ethernet. 5. Motion interface ethernet to desktop ethernet. 6. Oscilloscope aux in/out BNC to AWG input BNC. 7. Oscilloscope channel 1 (Front) BNC to SYNC BNC. 8. Matching box output BNC to RF input coaxial. 9. RF output coaxial to matching box coaxial. Please click here to view a larger version of this figure.
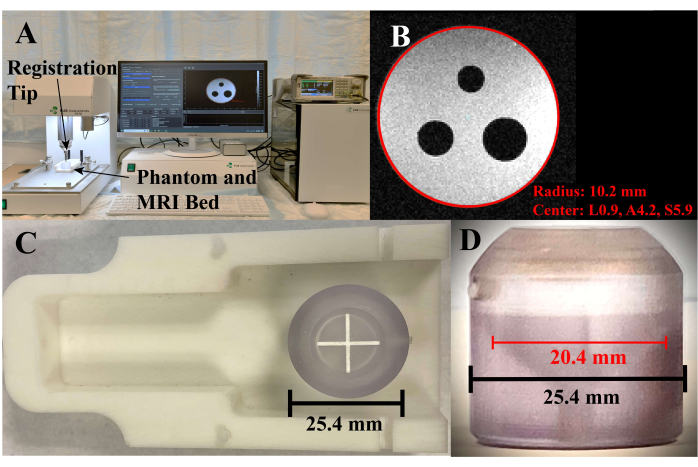
Figure 2: Phantom registration. (A) System setup and software during phantom registration. (B) Screenshot of the phantom registration screen, where the red circle is the selected circumference of the axial cross section. (C) Phantom placed on the MRI bed, top view. (D) Side view of the phantom, where the red line is in the axial slice corresponding to the circle in C. Please click here to view a larger version of this figure.
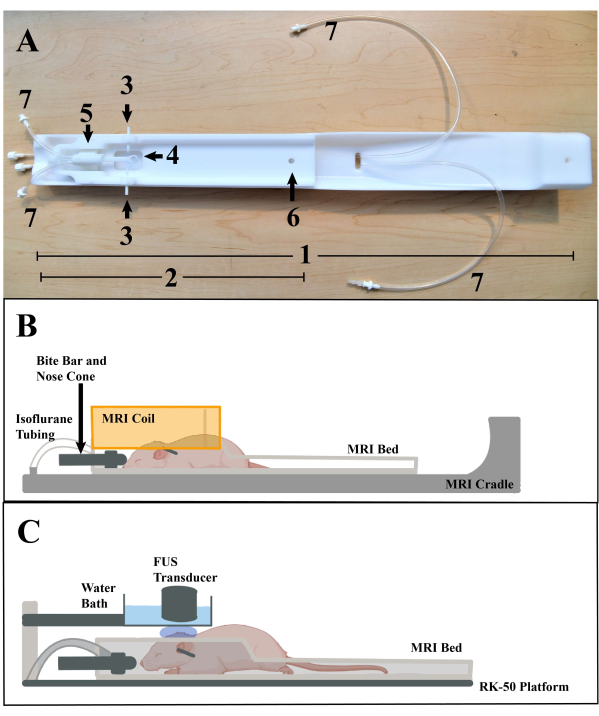
Figure 3: Animal placement. (A) MRI bed and cradle, with various parts labeled: 1. MRI cradle. 2. Stereotactic MRI bed. 3. Ear bars. 4. Bite bar. 5. Nose cone. 6. MRI bed peg hole. 7. Isoflurane anesthesia tubes. (B) Illustration representing the placement of the mouse on the MRI bed and placement on the cradle, with the RF coil (Orange) (illustration modified using Biorender 2022 template). (C) Illustration representing the placement of the mouse on the MRI bed during a FUS treatment (illustration modified using Biorender 2022 template). Please click here to view a larger version of this figure.
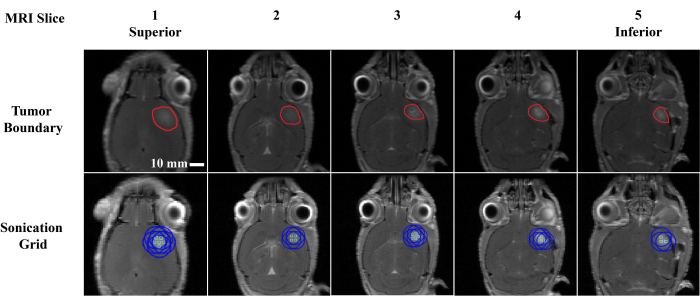
Figure 4: Focal point selection. Example of a sonication point selection in a single animal. Each column represents a T1-weighted post-contrast MRI slice where each slice is 0.5 mm in the proximal (slice 1) to distal (slice 5) direction. The tumor boundary was manually segmented and is outlined in red (row 1), and the corresponding sonication locations (row 2) are represented by a light green square (focal max center) and blue circle (half max focal circumference). Each location was sonicated for 2 min. Please click here to view a larger version of this figure.
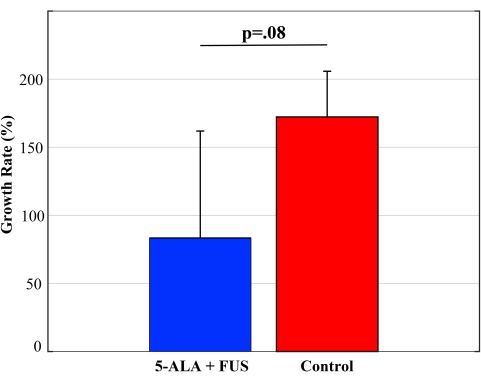
Figure 5: Growth rate following SDT. The growth rate of GBM tumors from pre- to 24 h post-SDT treatment in treated and untreated (control) animals with intracranial M59 tumors based on the measured luminescence. Error bars indicate standard deviation. A two-sample student's T-test was performed to determine significance. Please click here to view a larger version of this figure.
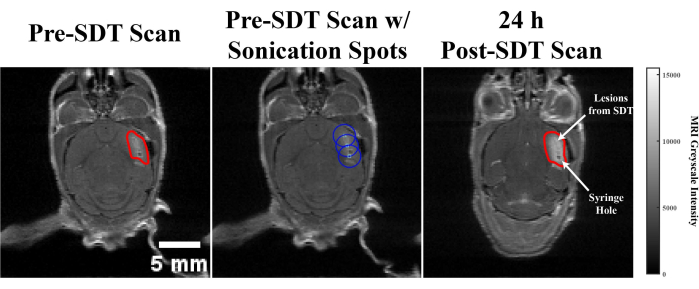
Figure 6: SDT generated lesion. Pre- and post-contrast enhance T1-weighted MRI scans from an animal model featuring a representative axial slice showing a lesion in the tumor created by SDT. (Left) MRI scan taken prior to SDT treatment, with the tumor outlined in red. (Middle) FUS focal point selection where the maximum pressure is represented by light blue circles and the half-maximum pressure regions are represented by blue circles. (Right) Post-SDT MRI scans, where the tumor is outlined in red. SDT-created lesions and the syringe hole for implantation are shown. Please click here to view a larger version of this figure.
| Sequence | T1 |
| Repetition time | 3000 ms |
| Echo time | 30 ms |
| Slice thickness | 0.5 mm |
| Number of slices | 25 |
| Pixel spacing | 0.187 mm x 0.187 mm |
| Acquisition matrix | 133 x 133 |
| Averages | 4 |
Table 1: MRI settings.
| Control | SDT Group | P-Value | |
| Pre-Treatment | 7.49 x 103 ± 2.2 x 103 | 7.48 x 103 ± 1 x 103 | 0.99 |
| Post-Treatment | 8.79 x 103 ± 7.7 x 102 | 7.95 x 103 ± 1.1 x 103 | 0.33 |
| Percent Difference | 16% ± 16% | 7% ± 12% | 0.47 |
Table 2: Post-contrast enhanced T1-weighted MRI greyscale.
Discussion
New therapeutic and efficacious treatment options are necessary for patients with GBM. This protocol has outlined a preclinical FUS-mediated treatment for GBM that is currently undergoing extensive investigation for clinical translation. Although SDT has exciting potential, there is still much to understand and optimize in the preclinical setting.
One of the most important components of this protocol is utilizing MR-guided FUS to target the tumor for maximal efficacy. Using a phantom, a 3D coordinate space can be created, where each pixel of axial MRI slices can be assigned a coordinate. Then, a simple procedure of selecting the sonication location on the MR image informs the transducer where to aim. The preclinical FUS system used is highly versatile and applicable when needing to target locations of specific pathology such as a tumor, including deeper seated tumors which would be hard to target without imaging confirmation. Using gadolinium as a contrast agent, there is clear visualization of the tumor, allowing the user to make informed decisions when choosing targets. The advantage that SDT has over many other treatments is that it is a tumor-specific therapy. Low-intensity FUS should only target the tumor tissue, while leaving the healthy brain parenchyma relatively untouched3,8.
The results of this experiment highlight how the advantages of this protocol can lead to therapeutic results that are similar to other findings in the literature for SDT. Figure 5 shows that within as little as 24 h following the day of treatment, there was a slowdown of tumor growth in the treated cohort. Although insignificant using this small sample size, significance might result with a larger number of animals. This delay in tumor growth is similar to what was shown in the pioneering paper on this subject by Wu et al. (2019), which exhibited slowed tumor growth over time in treated animals, as well as increased survival times9.
Considerations that were made when designing this protocol included animal strain, tumor type, and sonosensitizing agent selection. Athymic nude mice were chosen for this protocol for multiple reasons. First, the nude mouse is easier to sonicate as the lack of hair prevents any attenuation. Also, the lack of an immune system allows for the implantation of patient-derived xenografts (PDXs) so that the tumor model more closely resembles the clinical situation. The downside of using an athymic model is that the immune system cannot be characterized, so any SDT-generated immune response will not be measured in these studies10. The tumor line chosen is an aggressive and fast-growing PDX line. The time of treatment is very important because the establishment of the tumor must be verified, but the tumor burden should not fill the cranial hemisphere. Different cell lines require different incubation times to achieve an optimally sized tumor for preclinical experimentation. In this protocol, 5-ALA was used as the sonosensitizer because of its preferential uptake in GBM tumors, which has been confirmed in vitro for this cell line in previous experiments (unpublished data). Other sonosensitizers can be substituted and tested to determine the compound most suitable for efficacy and safety. Finally, treatment was commenced 3 h post 5-ALA injection, as previous literature has shown that this is the optimal time with that injection dosage5.
The FUS parameters chosen in this protocol (10 W/cm2 for 2 min at 515 kHz at each target location) were decided based on a review of previous literature and initial experiments4,9. A grid of sonication points covering the entire tumor was chosen in order to generate the ROS effect throughout the entire tumor. The intensity used here is higher than other publications, but at a short time span, this is not expected to lead to any adverse temperature-related effects, as intensities up to 25 W/cm2 have been successfully used in a mouse model without significant side effects11. Importantly, no standardized or optimized set of FUS parameters has been published in the literature. Therefore, the specific values that are reported here can be adjusted to determine the optimal set of parameters, leading to the maximal reduction of tumor tissue while maintaining safety. Additionally, as different cell lines have varying levels of vascularization and hypoxia, this treatment may need to be adjusted. We have shown overall decreased tumor growth (Figure 5) within 24 h of SDT treatment, although the parameters need to be optimized and more animals need to be tested to determine the maximal effect of this treatment. Post-treatment MRI scans show no appearance of lesions created by FUS treatment in healthy tissue, with the effect localized to tumor tissue (Figure 6). There is also the opportunity to combine SDT with other FUS techniques, such as transiently permeabilizing the blood-brain barrier, to maximize 5-ALA uptake in the tumor12. This protocol can be further supplemented by performing various histology techniques to check for safety and efficacy at the structural level. A hematoxylin and eosin (H&E) stain can be performed to check for structural or tumor damage13, while a terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) stain can be performed to check for cellular apoptosis14. Regardless, this protocol presents a safe and tumor-specific treatment where changes are noticeable even 24 h post-treatment, which is apparent by comparing the growth rate of tumors treated with SDT and untreated tumors, as well as comparing tumor slices before and after sonication.
With any protocol, there are always disadvantages or limitations that need to be weighed. The main limitation of the current protocol is time and expense. Meanwhile, one of the advantages of this protocol is its automated focused aim. To accomplish this focused procedure, MRI scans need to be taken for each individual animal to ensure that the targeting of the tumor is correct, a process which can be both time-consuming and expensive. Additionally, depending on the number of focal spots desired, the amount of time to perform this protocol could be hours for even just a few animals, resulting in low experimental animal numbers. Despite these drawbacks, this targeted noninvasive protocol remains a feasible preference when compared to open surgery options.
In conclusion, this protocol showed the ability of SDT treatment to decrease tumor growth in the brain within 24 h of treatment while maintaining healthy neural tissue in a preclinical mouse model. Studies of the effectiveness of SDT and optimizing the various parameters to increase ROS production are necessary to make this treatment clinically suitable. New avenues should be explored for the use of SDT as a noninvasive therapeutic model.
Divulgazioni
The authors have nothing to disclose.
Acknowledgements
The authors acknowledge funding support from the National Science Foundation (NSF) STTR Phase 1 Award (#: 1938939), by ASME Defense Advanced Research Projects Agency (DARPA) Award (#: N660012024075), and Johns Hopkins Institute for Clinical and Translational Research's (ICTR's) Clinical Research Scholars Program (KL2), administered by the National Institutes of Health (NIH) National Center for Advancing Translational Sciences (NCATS). The cells were purchased from and provided by the Mayo Foundation for Medical Education and Research.
Materials
| 0.5% Trypsin-EDTA | Thermo Fisher Scientific | 15400054 | |
| 1 mL Syringes | BD | 309597 | |
| 10 µL Hamilton syringe | Hamilton Company | 49AL65 | |
| 10 µL Pipette tips | USAScientific | ||
| 1000 mL Flask | Corning | MP-34514-25 | |
| 15 mL conical tubes | Corning | CLS430791 | |
| 200 Proof ethanol | PharmCo | 111000200 | |
| 5 mL pipettes | Falcon | 357543 | |
| 50 mL Conical tubes | Corning | 430290 | |
| 500 mL filter | Corning | 431097 | |
| 5-Aminolevulinic acid hydrochloride | Research Products International | A11250 | |
| 7T PET-MR system | Bruker | Biospec 70/30 | |
| Aluminum foil | Reynolds Brand | ||
| Amplifier | FUS Instruments | 2175 | |
| Athymic nude mice | Charles River Laboratories | Strain Code 490 | |
| Bone drill | Foredom | HP4-917 | |
| Centrifuge | Thermo Fisher Scientific | 75004261 | |
| Charcoal isoflourane waste container | Patterson scientific | 78909457 | |
| Computer | FUS Instruments | 2269 | |
| Cover glass | Fisherbrand | 12-545J | |
| Desktop monitor | ASUS | VZ239H | |
| D-Luciferin | Gold Biotechnology | LUCK-1G | |
| DMEM | Thermo Fisher Scientific | 11965092 | |
| Electronic shaver | Wahl | 93235-002 | |
| Eppendorf tubes | Posi-Click | 1149K01 | |
| Fetal bovine serum | Thermo Fisher Scientific | 16000044 | |
| Formalin | Thermo Fisher Scientific | SF100-20 | |
| Function generator | Siglent | QS0201X-E01B | |
| Gadolinium contrast agent (Gadavist) | McKesson Corporation | 2068062 | |
| Gauze | Henry Schein | 101-4336 | |
| Heat lamp | |||
| Heat pad | Kent Scientific | RT-0501 | |
| Hemocytometer | Electron Microscopy Sciences | 63514-12 | |
| Induction chamber | Patterson scientific | 78933388 | |
| Isofluorane vaporizer | Patterson scientific | 78916954 | |
| Isoflurane | Covetrus | 29405 | |
| Isoflurane system | Patterson Scientific | 78935903 | |
| IVIS spectrum | Perkin Elmer | 124262 | |
| Lightfield microscope | BioTek | Cytation 5 | |
| Nair | Church and Dwight Co. | 42010553 | |
| Ophthalmic ointment | Puralube vet ointment | ||
| P-20 pippette | Rainin | 17008650 | |
| Patient derived xenographs | Mayo Clinic | M59 | |
| Penicillin/Streptomyosin | Thermo Fisher Scientific | 10378016 | |
| Phosphate buffered saline | Thermo Fisher Scientific | 70-011-069 | |
| Pippetter | Drummond | 4-000-101 | |
| Povidone-iodine | Covetrus | PI050CV | |
| RK-50 MRgFUS system | FUS Instruments | 2182 | |
| Scale | |||
| Scalpel blade | Covetrus | 7319 | |
| Scalpel handle | Fine Science Tools | 91003-12 | |
| Screwdriver set | Jakemy | JM-8160 | |
| Skin marker | Time Out | D538,851 | |
| Staple remover | MikRon | ACR9MM | |
| Stapler | MikRon | ACA9MM | |
| Staples | Clay Adams | 427631 | |
| Stereotactic frame | Kopf Instruments | 5000 | |
| Stereotactic MRI prototype plastic imaging fixture | FUS Instruments | ||
| T-25 culture flask | Corning | 430641U | |
| Transducer and matching box | FUS Instruments | T515H750-118 | |
| Ultrasonic degasser | FUS Instruments | 2259 | |
| Ultrasound gel | ParkerLabs | 01-08 | |
| Water bath | FUS Instruments | ||
| Xylazine | Covetrus | 1XYL006 |
Riferimenti
- Tan, A. C., et al. Management of glioblastoma: State of the art and future directions. CA: A Cancer Journal for Clinicians. 70 (4), 299-312 (2020).
- Stupp, R., et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. The New England Journal of Medicine. 352 (10), 987-996 (2005).
- McHale, A. P., Callan, J. F., Nomikou, N., Fowley, C., Callan, B. Sonodynamic therapy: concept, mechanism and application to cancer treatment. Advances in Experimental Medicine and Biology. 880, 429-450 (2016).
- Ohmura, T., et al. Sonodynamic therapy with 5-aminolevulinic acid and focused ultrasound for deep-seated intracranial glioma in rat. Anticancer Research. 31 (7), 2527-2533 (2011).
- Shono, K., et al. Elevated cellular PpIX potentiates sonodynamic therapy in a mouse glioma stem cell-bearing glioma model by downregulating the Akt/NF-κB/MDR1 pathway. Scientific Reports. 11 (1), 15105 (2021).
- Ozawa, T., James, C. D. Establishing intracranial brain tumor xenografts with subsequent analysis of tumor growth and response to therapy using bioluminescence imaging. Journal of Visualized Experiments. (41), e1986 (2010).
- Poussard, A., et al. In vivo imaging systems (IVIS) detection of a neuro-invasive encephalitic virus. Journal of Visualized Experiments. (70), e4429 (2012).
- Borah, B. M., et al. Sonodynamic therapy in combination with photodynamic therapy shows enhanced long-term cure of brain tumor. Scientific Reports. 10 (1), 21791 (2020).
- Wu, S. -. K., Santos, M. A., Marcus, S. L., Hynynen, K. MR-guided focused ultrasound facilitates sonodynamic therapy with 5-Aminolevulinic acid in a rat glioma model. Scientific Reports. 9 (1), 10465 (2019).
- Zhang, Q., et al. Sonodynamic therapy-assisted immunotherapy: A novel modality for cancer treatment. Cancer Science. 109 (5), 1330-1345 (2018).
- Nonaka, M., et al. Sonodynamic therapy consisting of focused ultrasound and a photosensitizer causes a selective antitumor effect in a rat intracranial glioma model. Anticancer Research. 29 (3), 943-950 (2009).
- Pi, Z., et al. Sonodynamic therapy on intracranial glioblastoma xenografts using sinoporphyrin sodium delivered by ultrasound with microbubbles. Annals of Biomedical Engineering. 47 (2), 549-562 (2019).
- Rodgers, G., et al. Virtual histology of an entire mouse brain from formalin fixation to paraffin embedding. Part 1: Data acquisition, anatomical feature segmentation, tracking global volume and density changes. Journal of Neuroscience Methods. 364, 109354 (2021).
- Chimata, A. V., Deshpande, P., Mehta, A. S., Singh, A. Protocol to study cell death using TUNEL assay in Drosophila imaginal discs. STAR Protocols. 3 (1), 101140 (2022).

