Sampling Cerebrospinal Fluid and Blood from Lateral Tail Vein in Rats During EEG Recordings
Summary
The protocol shows repeated cerebrospinal fluid and blood collections from epileptic rats performed in parallel with continuous video-electroencephalogram (EEG) monitoring. These are instrumental for exploring possible links between changes in various body fluid molecules and seizure activity.
Abstract
Because the composition of body fluids reflects many physiological and pathological dynamics, biological liquid samples are commonly obtained in many experimental contexts to measure molecules of interest, such as hormones, growth factors, proteins, or small non-coding RNAs. A specific example is the sampling of biological liquids in the research of biomarkers for epilepsy. In these studies, it is desirable to compare the levels of molecules in cerebrospinal fluid (CSF) and in plasma, by withdrawing CSF and plasma in parallel and considering the time distance of the sampling from and to seizures. The combined CSF and plasma sampling, coupled with video-EEG monitoring in epileptic animals, is a promising approach for the validation of putative diagnostic and prognostic biomarkers. Here, a procedure of combined CSF withdrawal from cisterna magna and blood sampling from the lateral tail vein in epileptic rats that are continuously video-EEG monitored is described. This procedure offers significant advantages over other commonly used techniques. It permits rapid sampling with minimal pain or invasiveness, and reduced time of anesthesia. Additionally, it can be used to obtain CSF and plasma samples in both tethered and telemetry EEG recorded rats, and it may be used repeatedly across multiple days of experiment. By minimizing the stress due to sampling by shortening isoflurane anesthesia, measures are expected to reflect more accurately the true levels of investigated molecules in biofluids. Depending on the availability of an appropriate analytical assay, this technique may be used to measure the levels of multiple, different molecules while performing EEG recording at the same time.
Introduction
Cerebrospinal fluid (CSF) and blood sampling are important to identify and validate biomarkers of epilepsy, in both preclinical and clinical research1,2. Nowadays, the diagnosis of epilepsy and most of the research on epilepsy biomarkers focus on EEG and neuroimaging3,4,5. These approaches, however, present several limitations. Apart from routine scalp measurements, in many cases, EEG requires invasive techniques like depth electrodes6. Brain imaging methods have poor temporal and spatial resolution and are relatively expensive and time-consuming7,8. For this reason, the identification of non-invasive, low-cost, and biofluid-based biomarkers would provide a very attractive alternative. In addition, these biofluid biomarkers could be combined with available diagnostic approaches to sharpen their predictiveness.
Patients diagnosed with epilepsy are routinely submitted to EEG9,10 and blood sampling11,12,13,14, and many also to CSF withdrawal to exclude life-threatening causes (i.e., acute infections, autoimmune encephalitis)15. These blood and CSF samples can be used in clinical research aiming to identify biomarkers for epilepsy. For example, Hogg and co-workers have found that an increase in three plasma tRNA fragments precedes seizure occurrence in human epilepsy14. Similarly, interleukin-1beta (IL-1β) levels in human CSF and serum, expressed as ratio of IL-1β levels in CSF over serum, can predict post-traumatic epilepsy development after traumatic brain injury16. These studies highlight the importance of biofluids sampling for epilepsy biomarkers research, but they face multiple limitations intrinsic to clinical trials, e.g., the cofounding factor of anti-epileptic drugs (AEDs) in blood, the frequent lack of etiology information, inadequate controls, modest numbers of patients, and others17,18.
Pre-clinical research offers other opportunities for investigating molecules in biofluids as potential biomarkers for epilepsy. In fact, it is possible to withdraw plasma and/or CSF from animals while performing EEG recordings. Moreover, sampling can be performed repeatedly across multiple days of the experiment, and a number of age, sex, and epileptic insult-matched controls can be used to improve the study's robustness. Here, a flexible technique to obtain CSF from cisterna magna with parallel withdrawal of plasma from the tail vein in EEG-monitored rats is described in detail. The presented technique has several advantages over alternative methods. By using a butterfly needle approach, it is possible to collect CSF several times without compromising the function of EEG electrodes or similar head implants. This represents a refinement of intrathecal catheter withdrawal procedures, which are associated with a relatively high risk of infection. In addition, the reported free fall dropping approach used for blood collection is superior to other approaches of tail vein blood withdrawal because of the highly reduced risk of hemolysis, due to the fact that blood does not pass through tubing and no vacuum pressure is applied. If performed under strict germ-free conditions, there is a particularly low risk of infection for animals. In addition, by starting the blood withdrawals at the very end of the animals' tails, sampling can be repeated several times. Such techniques are easy to master and can be applied in many preclinical studies of central nervous system disorders.
Protocol
All experimental procedures have been approved by the University of Ferrara Institutional Animal Care and Use Committee and by the Italian Ministry of Health (authorization: D.M. 603/2022-PR) in accordance with guidelines outlined in the European Communities Council Directive of 24 November 1986 (86/609/EEC) on the protection of animals used for experimental and other scientific purposes. This protocol is specifically adjusted for further quantitative polymerase chain reaction (qPCR) analyses of small non-coding ribonucleic acid (sncRNAs) in the rat CSF and plasma obtained under EEG control in epileptic animals. At its option, please see the related JoVE video for a better understanding and improvements of the surgery19,20,21.
1. Preparation of animals for surgical implantation of electrodes or telemeters
NOTE: The stereotaxic surgery technique varies according to the EEG system used. The following method section provides a description of steps that are in common for the two types of surgeries.
- Use Sprague-Dawley (SD) rats (males, 7-8 weeks old, weighing 250-290 g) maintained in accordance with local laws for the Care and Use of Laboratory Animals. House the animals under standard conditions with free access to food and drinking water.
- Proceed with handling the rats for a few days before the surgery and the experimental protocol procedures.
- Use a stereotaxic apparatus for electrodes or telemeters implantation. Follow the contemporary standards for aseptic surgeries. Use sterile and good-working electrodes and transmitters.
- Shave the animal's head with the electric razor. Induce anesthesia in the animal with the mixture of ketamine (45 mg/kg) and xylazine (7.5 mg/kg) administered intraperitoneal (i.p.), then add the isoflurane anesthesia (1.4% in air; 1.2 mL/min) delivered through a face mask and fix the animal's head into the stereotaxic apparatus.
- Ensure adequate depth of anesthesia by testing pedal withdrawal reflex after foot pad pinch on both animals' hind feet and maintain isoflurane anesthesia till the end of the surgery. Regularly check the anesthetic depth for the entire duration of the procedure.
- Apply a sufficient amount of eye ointment on both animals' eyes to prevent corneal damage due to loss of blink reflex caused by anesthesia.
- Thoroughly clean the animal's scalp with liquid disinfectant and make a longitudinal 2 cm long cut using a sterilized scalpel. Open the scalp and apply surgical clips on the skin flaps to keep it open.
- Gently, detach the periosteum to expose the bregma and the area of the skull through which the recording electrode will be inserted.
2. Surgical implantation of tethered electrodes
NOTE: Before establishing the puncture CSF withdrawal procedure of this protocol (see step 9 for details), repeated CSF withdrawals via guide cannula in a few freely moving non-anesthetized rats were performed. Cannulated animals implanted with tethered electrodes were used in order to evaluate the impact of double-head implants on long-term EEG recording coupled with multiple CSF sampling. In these specific experiments, rats were implanted with a dummy guide cannula placed in the cisterna magna, the tip of which was inserted 7 mm into it stereotactically, according to previously published protocols22. Double implant surgery approaches were similar to those adopted by some workers in the past for microdialysis guide cannulas and tethered electrode implantation23,24.
- Use one arm of the stereotaxic frame, mount the electrode holder on it, and put the electrode upright into the holder. Move the electrode tip exactly above the bregma. Write down the anteroposterior and mediolateral coordinates of bregma and translate them to the desired coordinates.
- Lower the electrode until its tip almost touches the skull. Mark the drilling spot and drill the skull on the marked spot. Be careful not to damage the brain and meninges.
- Make four or more holes for anchoring screws and screw them into the skull. Pay attention to the size of the electrode when making the holes for anchoring screws. The electrode size should not interfere with the positioned anchoring screws.
- Slowly lower the stereotactic arm carrying the electrode on the dorsoventral axis, enter the brain tissue, and stop at the desired position. Fix the electrode ground wire around one of the screws screwed into the skull.
- Put the methacrylic cement onto the electrode and screws covering about half of the electrode pedestal height. Leave the upper half part of the electrode, which fits the tethering wire's ending, bare.
- Release the electrode from the holder and raise the stereotactic arm. Release the animal from the stereotaxic apparatus and give it postoperative care.
- Place the animal in a separate recovery cage and observe it every 10 minutes until awake and ambulatory. Return the animal to its standard housing cage and to another not anesthetized animals' company only after fully recovered.
3. Surgical implantation of the telemeters
NOTE: Use only sterile telemeters. If telemeters are reused, clean and sterilize them before the surgery according to their manufacturer instructions. In this protocol, a data science International (DSI) telemeters for EEG recording was used.
- Turn on the telemetry transmitter using a magnet and test the signal with an AM frequency radio. Check for a strong and clear signal before the surgery. If needed, discard the bad working telemeters.
- Prepare the telemeter leads by shortening these to optimal lengths for adult rats. Peel back the silicone coating on two (negative and positive) leads, exposing approximately 5 mm of the helical steel lead. Create a looped handle of about 2 mm in length and 1 mm in width at the tip of the leads.
- Shave the animal's flank under and behind the left shoulder. Disinfect the area of surgery with disinfectant based on stabilized peroxides and quaternary ammonium activity. Let the disinfectant act for 15 min.
- Make about 2 cm lateral incision just behind the shoulder of the animal and make a subcutaneous pocket of about 5 cm3 space for the transmitter placement. Place the transmitter parallel to the long axis of the body, with the leads directed to the rostral direction.
- Fix the transmitter to the inner wall of the subcutaneous pocket with a 3-0 cotton suture. Using blunt-ended scissors, create the subcutaneous tunnel (roughly 2.5 cm in length) through the flank and neck of the animal, thus connecting the transmitter pocket with the midline incision made on the animal's scalp at step 1.6.
- Take both telemeters leads with forceps and pull them up through the tunnel, so they are protruding from the scalp incision outlet. Hold the leads out of the scalp incision with wound clips.
- Use the stereotaxic frame to individuate the desired coordinates for positive (red) lead and drill the hole into the skull for the positive lead tip (similar to step 2.1.). Make one more hole for anchoring the screw upward to the rostrum and screw it into the skull.
- Insert the tip of positive lead under the skullcap and dura and lay it down on the brain surface. Hook up the looped tip of negative (white) lead on the screw. Put a small amount of methacrylic cement around the positive lead outlet and the negative lead to immobilize them on the skull.
- Fix the leads to the inner wall of the skull skin with a 3-0 cotton suture. Make sure the fixed leads and fixing suture will not interfere with the site of CSF withdrawal (i.e., a depressible surface with the appearance of a rhombus between the occipital protuberance and the spine of the atlas).
- Close the scalp incision and flank incision using a 3-0 cotton suture. Apply disinfectants on the wounds. Only after these have dried, apply an antibiotic cream on the sutured wounds.
4. Post-operative care
- Monitor the animals for about 1 h thereafter the surgery till upright and moving around the cage. Keep them on a warming pad to prevent hypothermia. Administer animals with a systemic antibiotic to prevent infection and a systemic analgesic to prevent post-surgical pain for 2-3 days.
- Allow rats to recover for at least 7 days after surgical procedures. Monitor the animals at least once daily for 3 days for signs of pain or distress.
5. Status epilepticus induction in rats
NOTE: For a detailed protocol of status epilepticus (SE) induction needed to reproduce the mesial temporal lobe epilepsy (mTLE) in rats, refer to Guarino et al.25.
- After a week of post-surgical recovery, assign the animals randomly to groups: (i) control animals receiving vehicle and (ii) epileptic animals which will receive pilocarpine. Use a proportionally higher number of animals for the epileptic group, since not all the pilocarpine-administered rats will survive or develop SE.
- The day before the SE induction, give animals a single dose of 127 mg/kg lithium chloride dissolved in 0.9% saline (3M) as a volume of 1 mL/kg by gastric gavage. Administer the animal's lithium, which increases the pilocarpine efficacy26, approximately 14 h before the induction of SE in order to decrease variability in time to SE onset.
- About 14 h after lithium administration, give the rats a single injection of methyl scopolamine (1 mg/kg, subcutaneously).
- Exactly 30 min after methyl scopolamine administration, give the rats a single injection of pilocarpine (50 mg/kg, i.p.) to induce the SE. Give methyl scopolamine and vehicle (0.9% NaCl solution) to control rats.
- Pilocarpine injection induces typical behavior in animals: early partial seizures (movements of vibrissae and head nods within 5 min after pilocarpine administration) evolving into recurrent generalized convulsions (SE) within 25-30 min. For rats that do not develop SE within 30 min, administer an additional dose of pilocarpine (25 mg/kg, i.p.), and if they still do not develop SE, exclude them from the study (SE non-responders).
- Observe and score the seizure behavior in rats every 5 min beginning immediately after the pilocarpine injection. Use the Racine scale for scoring27.
- Interrupt the SE 2h after the onset by i.p. administration of a cocktail of drugs: diazepam (10 mg/kg), phenobarbital (25 mg/kg), and scopolamine (1 mg/kg).
- Give the rats this cocktail again after 4 h. Finally, after another 4 h, give the rats i.p. the last mixture of drugs (diazepam 10 mg/kg plus scopolamine 1 mg/kg) in order to completely stop the seizure activity.
- Inject i.p. the animals with saline (1 mL of 0.9% NaCl solution, pH adjusted to 7.0) and feed them with a 10% sucrose solution for 2-3 days after SE to favor recovery from the body weight loss which follows SE.
- Assign post-SE surviving animals randomly to different experimental groups according to the requirements of the specific experimental protocol. Use the following inclusion/exclusion criteria for further experiments in epileptic rats: development of convulsive SE within 1 h after pilocarpine administration, weight gain in the first week after SE, and the correct positioning of the electrode in the brain area of interest for EEG registrations25.
6. Tethered video-EEG in epileptic rats and analyses of seizure activity
NOTE: This section describes the experimental procedure to record EEG signals in single-housed, freely moving rats under standard conditions. The cage should not contain objects where the animal or the recording cable can get stuck. Depending on the scientific question to be addressed, several parameters can be analyzed. In the case of epilepsy research, the EEG traces are screened to recognize electrical and motor seizures. The most common parameters used to identify a seizure are the amplitude, frequency, and duration of paroxysmal electrical activity.
- Place the animal in a clean cage in the recording room to allow habituation and reduce the stress induced by the new environment. Place the animal's cage into a Faraday cage to avoid contamination of the EGG signal with the environmental electromagnetic field.
- Connect one end of the recording cable to the recording device. Use a voltmeter to measure the electric potential and differentiate between ground and reference electrodes.
- Connect the other end of the recording cable to the electrode fixed on the head of the rat. For this aim, hold the cement cover on the animal's head when inserting the cable plug into the electrode connector and avoid applying any pressure on the rat's head.
- Counterbalance the weight of the recording cable to allow free movement of the animal while preventing the risk of twisting the cable. To do this, use a counterbalancing arm or commutators. Even if the recording cable is counterbalanced, put the food inside the cage instead of in a feeding holder, in which the animals have to rise to reach the food.
- Before starting the registration, check all the settings used to acquire and process the data from the EEG.
NOTE: This protocol does not provide an introduction to the apparatus required to perform the EEG recording, however, adequate filtering and sampling rates should be applied to avoid artifacts and noise in EEG signals.- For a routine experiment, set the sample rate at 500 Hz, and the amplification gain at 5000x. Filter the signal using a 0.005 Hz filter in addition to a notch filter to discard the surrounding electrical activity in the 50 Hz band (specific for European countries).
- Start both video and EEG recordings and ensure that the traces match an expected EEG signal by checking the power in specific frequency bands across the time. Record a baseline period before starting the interventions in the animals.
- Check animals and EEG traces periodically. It is not rare that a recording cable detaches from the electrode connector in the rat head. If this happens, reconnect the electrode in the rat head to a recording cable again and check for a clear EEG signal.
- Analyze the EEG signal manually or automatically using commercially available software. If analyzing manually, screen through EEG recording to identify seizure-like activities. If software is going to be used, configure key parameters of a seizure event, like amplitude, frequency, and duration of electrical activity.
NOTE: Single seizure may be characterized by an electrical activity with an amplitude 3x higher than baseline, frequency equal to or higher than 5 Hz, and duration of at least 5 s22. - Regardless of the method used, confirm the potential convulsive seizures by checking the synchronized video recording collected simultaneously with EEG.
7. Telemetry video-EEG in epileptic rats and analysis of seizure activity
NOTE: This section describes the experimental procedure to record radiotelemetry EEG signals in single-housed, freely moving rats under standard conditions. The protocol is based on a commercially available telemetry system. However, several telemetry systems differ slightly in their functional and technical specifications. The system should be chosen depending on lab requirements and research goals.
- Place the animal home cage above the signal receiver. Connect the signal receiver to the data acquisition system and this to a computer with the acquisition software.
- Turn on the radiofrequency telemetry implant by placing a magnet in proximity to the telemeter inserted in the rat flank. Test the signal using a radio device. Use a universal radio device to test the telemeters and hear a clear beep indicates the telemeter is activated, whereas a sizzling sound indicates an inactivated telemeter.
NOTE: The lifespan of the radiofrequency transmitter battery should be taken into account before defining the duration of the recordings. - Set up the acquisition software and synchronize the telemetry signal and video system to simultaneously acquire EEG and video data. Assign one signal receiver to each implanted transmitter and set the calibration values of the transmitter. Set the sample rate at 1000 Hz, noise detection between -500 mV and +500 mV, and the integration interval at 100 ms. Do not use low and high pass filters.
- Start the telemetry and video recordings. Perform long-term baseline recording before acquiring the epileptic-like activity. Analyze the EEG signal as described in steps 6.8 and 6.9.
8. Procedure of blood collection from the tail vein
NOTE. The vacuum blood collection system consists of a butterfly needle (23 G x ¾ x 12 (0.8 mm x 19 mm x 305 mm). The blood collection technique can be easily performed by one operator and the procedure takes about 5 min.
- Coat the butterfly needle and its tubing with 1% K2EDTA in distilled water shortly before the withdrawals, i.e., draw in and expel the K2EDTA solution from the system using the 1 mL syringe. Cut the tubing just behind the needle in order to collect blood drop by drop, without aspiration (Figure 1A).
- Place the rat in an induction chamber and anesthetize it with isoflurane (1.4% in air; 1.2 mL/min). Switch to the stereotaxic frame and maintain anesthesia through a face mask. Put the heating pad under the animal, keeping a part of its tail in direct contact with the pad.
- Move gently the animal's back to the side so that the lateral tail vein is kept at the top.
- Dip the tail in warm water (42 °C) for 2 min to dilate the lateral vein. Wipe the tail with 70% ethanol to make the vein more visible. Put warm light onto the tail using a regular incandescent bulb.
- Insert the 21G butterfly needle into the lateral tail vein with a depth of 5 mm and an angle of 20°. Collect blood in a 500 µL vacuum collection tube that contains 5 mg K2EDTA as an anticoagulant (Figure 1B,C).
- Remove the needle and stop the flow of the blood by putting pressure on the puncture site. Return the rat to its home cage.
- Gently invert the tube 10x to mix anticoagulant in the blood. Make holes of about 1.5 cm depth into the ice to accommodate the collection tubes. Gently and vertically put the sample on ice.
- Centrifuge the blood sample in a refrigerated centrifuge (4 °C) at 1300 x g for 10 min to separate plasma. Perform this procedure within 1 h at most.
- Take about 200 µL of plasma, avoiding the red and white blood cell layer. Put the withdrawn plasma into the 0.2 mL sterile microtube. If needed, store at 4 °C for up to 1 h after centrifugation.
- Put aside 5 µL of the sample for quality control. Store the sample at -80 °C until analysis.
NOTE: Do not use the vacuum even if recommended by some researchers28 or milk the tail during the procedures described in step 8.5 to obtain more blood, as it decreases the sample quality for the next sncRNA quantification analyses (please see the Representative Results for details). Keep in mind that in rat, the maximum amount of blood that can be withdrawn at one time is <10% of its total blood volume (i.e., about 1.6-1.9 mL in a 250-300 g rat) and <15% of the total blood volume (about 2.64 mL) in 1 month29. In this protocol, a maximal 500 µL of blood collection on a single occasion for up to five times in a single animal is used30,31.
9. CSF collection procedure
NOTE. The technique can be easily performed by a single operator, and the procedure requires around 2-4 min. The materials used for the collection of CSF are low-cost, single-use vacuum butterfly needles and extraction tubes. In this protocol, a butterfly-winged infusion set connected to a sterile syringe is used in order to create the vacuum (Figure 2A).
- Prepare the 23G butterfly needle, cutting its plastic sleeve protection so that the end of the bare needle is exposed by 7 mm to prevent it from penetrating more than 7 mm deep into the cisterna magna during withdrawal (Figure 2B).
- Connect the butterfly needle equipped with polymer tubing to a 1 mL syringe.
- Place the rat in an induction chamber and anesthetize it with isoflurane (1.4% in the air; 1.2 mL/min). Switch the isoflurane flow to the stereotaxic frame and maintain anesthesia delivered through a face mask. Remove the fur on the rat's rear head and neck with a razor.
- Fix the head of the rat with ear bars. Lower the animal's head down approximately 45° vertically, moving down the nose bar of the stereotaxic frame (Figure 2C). Inspect the animal's rear head and find a slightly depressed surface with the aspect of a rhombus, between the occipital protuberance and the atlas spine.
- Rub this surface with 70% ethanol in order to make it more visible and disinfect it.
- Insert the butterfly needle vertically into the center of the rhombus-shaped depressed surface into the cisterna magna for CSF collection till the movement is blocked by cutting to size plastic sleeve protection of the needle (Figure 2D). Draw back gently the 1 mL syringe piston in order to let the CSF slowly flow through the needle.
- Collect about 100 µL of the CSF into polymer tubing (Figure 2D). Avoid entering blood or any other visible contamination. Pinch the polymer tubing very close to the butterfly needle and cut the tubing at this point.
- Draw the clear (uncontaminated) sample into the syringe. Discard the contaminated sample, if visible contamination enters the collecting tubing.
- Expel the sample into the sterile 0.2 mL microtube and store on ice for up to 1 h.
- Disinfect the site of CSF withdrawal on the animal's head. Remove the rat from stereotaxic frame and put it back into its cage.
- Put aside 2 µL of the sample for quality control. Store the rest of the sample at -80 °C for further analysis.
NOTE: In rats, if multiple CSF withdrawals are performed repeatedly, the recommended volume to be withdrawn at each collection is 100 µL32. In this protocol, a maximum of 100 µL of CSF on a single occasion with 5x maximal withdrawal in 15 days in a single animal was done.
10. Spectrophotometry analysis of the sample's quality
NOTE: After proper collection of CSF and plasma samples, the samples are ready for spectrophotometer analyses and do not require any specific handling. Measure the hemoglobin absorbance by UV spectrophotometry at 414 nm to evaluate the hemolysis risk in samples. Use a cut-off absorbance value of 0.25 in rat samples. The choice of this limit may depend on subsequent qPCR analysis and its specific requirements for sncRNAs quantification.
- Switch on the UV spectrophotometer. Select the method for the absorbance measurement at a single wavelength of 414 nm for PLASMA or CSF. Click Next.
- Rinse the 1 mm cuvette with purified water. Put 5 µL of 70% ethanol onto the cuvette measuring spot. Dry with a paper towel and rub with lint-free tissue paper. Check if it is perfectly transparent.
- Put 1.5 µL of purified water into the 1 mm cuvette and close it. Insert the cuvette into the measuring chamber of the spectrophotometer and measure the blank sample absorbance by clicking on Blank button. Check that the absorbance value at 414 nm is 0.000.
- Dry the cuvette with a paper towel and clean it with lint-free tissue paper. Check if it is perfectly transparent.
- Put 1.5 µL of the sample into the 1 mm cuvette and close it. Insert the cuvette into the measuring chamber of the spectrophotometer. Measure the sample, clicking on the Sample button. Check the absorbance of the sample at 414 nm and annotate it.
- Proceed quantifying hemoglobin absorbance in all available samples. Measure the blank sample ahead of any plasma or CSF sample, by clicking alternatively on the Blank and Sample buttons.
- Preserve the samples with A414 nm < 0.25 at -80 °C and discard the samples if A414 nm > 0.25.
- Rinse the 1 mm cuvette with purified water and 70% ethanol, sequentially. Dry the cuvette.
- Close the empty cuvette into the measuring chamber to prevent its dusting. Switch off the spectrophotometer.
Representative Results
The outcome of different CSF and blood withdrawal procedures performed in 9 control and 18 chronic epileptic rats, all implanted with electrodes at 1-month post-SE, is reported in terms of success rate. After implantation, all rats were video-EEG monitored for 1 month, during which the CSF plus blood was withdrawn 5x every 3 days during the two last weeks of the experiment (i.e., at days 52, 55, 58, 61, and 64 post-SE; dpSE). Data from multiple withdrawals in different animals were used to compare the success rate of CSF collection in the double-head implant-endowed rats (cannulated for CSF withdrawal) with the success rate of CSF collection (carried out by cisterna magna puncture) in only tethered or telemetry electrodes implanted animals (Table 1). In different animals, the impact of vacuum blood collection or tail milking on the quality of plasma samples was evaluated (Table 2). For this purpose, UV spectrophotometry analysis at 414 nm was used for the detection of free hemoglobin. For statistical analyses, commercial software was used, and Kruskal-Wallis or one-way ANOVA with post-hoc Tukey's multiple comparison tests were used (p<0.05 considered statistically significant). The data are expressed as a mean ± SEM.
Success rate of multiple CSF sampling in cannulated and punctured rats
CSF has been sampled 5x within 2 weeks in 3 groups of rats: (i) cannulated and tethered electrode implanted rats (CT group of animals); in these, the CSF withdrawal was performed via dummy guide cannula and PTFE tubing joint to 1 mL syringe when they were non-anesthetized and freely moving under video-EEG; (ii) punctured (step 9) and tethered electrode implanted rats (PT group); (iii) punctured and telemetry electrode implanted rats (PTe group). A total of 9 animals per group (6 epileptic and 3 control rats) were used. The number of successful collections over 5 times was evaluated. The success rate was similar in punctured rats: 86.7% ± 5.8% in tethered and 88.9% ± 4.8% in telemetry electrode implanted animals. Instead, in the cannulated rats, the rate was reduced even if not significantly different (71.1% ± 8.9%, Table 1). Such results indicate that the cannula on animals' heads may interfere with repeated CSF sampling and compromise longitudinal studies. The puncture technique is more suitable for multiple CSF withdrawals in electrodes implanted animals.
Impact of vacuum and tail milking on the plasma collection method
Blood was collected 5x from 9 rats (6 epileptic and 3 control rats) at days 52, 55, 58, 61, and 64 post-SE and the plasma quality was evaluated for hemolysis visually and by UV spectrophotometry at 414 nm. To obtain the first sample in each rat, the vacuum withdrawal via a 21G butterfly needle attached to a 1 mL syringe was employed. With the second sample, the drop withdrawal and 21G butterfly needle system were employed when milking the tail simultaneously. To get the 3rd-5th sample, the drop withdrawal procedure without milking the tail (described in step 9) was used.
When employing a vacuum, the plasma was pink colored under the visual inspection, and the mean absorbance value of 9 rats' samples was 0.647 ± 0.067 (Table 2, Figure 3). Similar results were obtained if employing the tail milking during the procedure: pink-colored plasma with 0.620 ± 0.043 mean absorbance (Table 2, Figure 3). In contrast, with the gravity-enabled drop withdrawal and 21G butterfly needle system, the mean plasma absorbance values were significantly reduced (0.226 ± 0.017 at 58 dpSE; 0.223 ± -0.09 at 61 dpSE; 0.226 ± 0.018 at 64 dpSE; Table 2, Figure 3) with respect to vacuum or tail milking method. Moreover, the drop plasma samples were mainly transparent. Higher values of absorbance (52 and 55 dpSE) correlated with the pink color of samples (data not shown). These results may suggest that the last method is the best to get samples of very high quality for analyses.
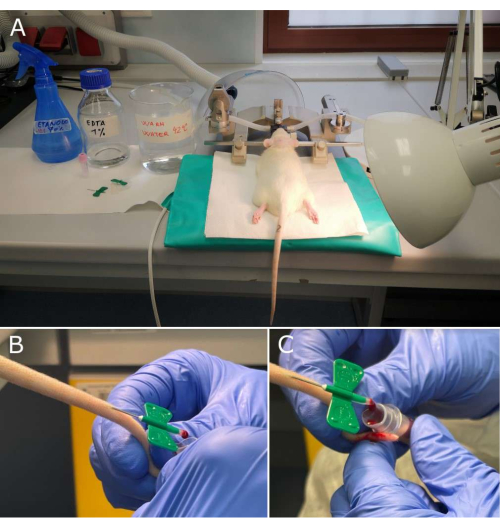
Figure 1: Key steps of plasma sampling workflow. (A) Materials necessary for blood withdrawal and rat in the stereotaxic frame, ready for collection; (B, C) Magnifications of the tail with 21G butterfly needle inserted into the lateral tail vein and the blood drop falling down the walls of the collection tube with an anticoagulant. Please click here to view a larger version of this figure.
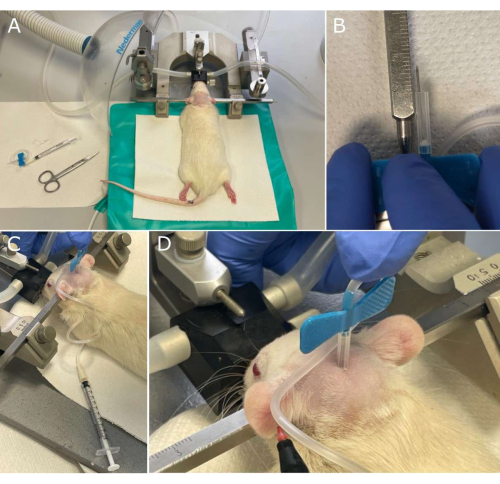
Figure 2: Key steps of cerebrospinal fluid (CSF) sampling workflow. (A) Materials necessary for the CSF withdrawal and rat in the stereotaxic frame, shortly before collection; (B) The 23G butterfly needle preparation by cutting its plastic sleeve protection so that the end of the bare needle is exposed for 7 mm to ensure correct penetration into the cisterna magna; (C) The rat head is inclined downwards by 45° during withdrawal. (D) Magnification on the rhomboid site with a butterfly needle inserted in the cisterna magna. Note the CSF that rises in the tubing, indicated by the tip of the marker. Please click here to view a larger version of this figure.
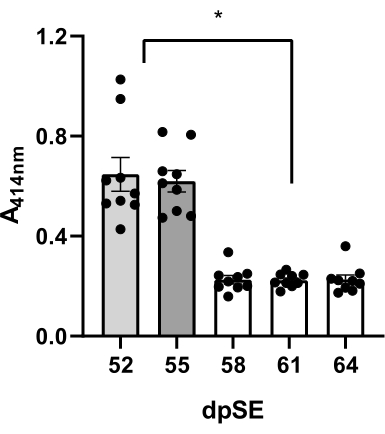
Figure 3: Quality evaluation of plasma samples. Degree of hemolysis measured at 414 nm for free hemoglobin by UV spectroscopy in plasma samples of 9 animals at 5 time-points (52, 55, 58, 61, and 64 days' post status epilepticus, dpSE) using different methods: day 52 – the vacuum technique; day 55 – the tail milking; days 58-64 the drop techniques were employed. The decrease in free hemoglobin in plasma obtained by drop technique compared to vacuum and tail milking methods was significant (*p <0.05 according to one-way ANOVA and post-hoc Tukey's multiple comparison test). Please click here to view a larger version of this figure.
Table 1: Success rates of CSF withdrawals. Comparison of the success rates of repeated CSF withdrawal in three experimental groups of animals expressed as a percentage of successful withdrawals across 5 days. The value 1 was assigned to successful withdrawal of > 100 µL of clear CSF; the zero value was assigned to withdrawals < 100 µL and/or of unclear CSF. Abbreviations: N/A – the absence of collection due to the loss of cannula during the sampling procedure (CT animals only); CT – cannulated tethered; PT – punctured tethered; PTe – punctured telemetry electrodes implanted. Please click here to download this Table.
Table 2: Evaluation of hemolysis in plasma samples. Results of the hemolysis measurements at 5 time-points using three different methods of blood sampling: day 52 – the vacuum technique; day 55 – the tail milking; days 58-64 the drop techniques. Values >0.3 of absorbance correlated with pink color of samples. Please click here to download this Table.
Discussion
The present work illustrates an easy-to-master technique of CSF and blood collection in rats, which may be useful not only for studies in models of epilepsy but also of other neurological conditions or diseases such as Alzheimer, Parkinson, or multiple sclerosis. In epilepsy research, both sampling procedures coupled with video-EEG are ideal when a correlation between the levels of different soluble molecules and seizure activity is pursued. For this specific reason, a continuous video-EEG recording was employed: i) in order to correctly diagnose epilepsy or ii) to monitor the different phases of the disease progression, and/or iii) to correlate sampling with the occurrence of spontaneous seizures. Such sampling techniques can be performed in anesthetized rats, thus causing minimal stress.
Critical steps, troubleshooting, method limitations
The protocol has some critical technical steps. Firstly, it can be difficult to find the correct place for CSF collection at the first attempt. If the operator misses the cisterna magna at the first attempt, any subsequent trial would be blood contaminated, as the animal will bleed from the needle wound. From this point of view, the success in the collection is heavily dependent on the operator's skill. Secondly, some steps of blood withdrawal need special attention. In particular, there is a high risk of hemolysis if the operator rubs the tail too vigorously with ethanol, if the temperature of the water used for the tail vein vasodilation is higher than 42 °C, or if the blood in the collection tube is mixed with anticoagulant too energetically. Another peculiarity of blood collection in chronic epileptic animals is the influence of their bradycardia on the rate at which blood drops out of the tail vein33. If this is too slow, the blood may coagulate on the walls of the collection tube. To avoid this problem, one option is to split the sampling into two collection tubes, reducing the volume of blood/tube. Finally, there is one pitfall that is intrinsic to epilepsy studies. The stress provoked by animal manipulation before sampling may induce seizures, which in turn may interfere with the levels of molecules under the investigation34. Whenever it is possible, place the anesthesia induction chamber into the home cage and allow the animal to enter it spontaneously. As a modification of the proposed protocol, a restrainer can be used to perform blood withdrawal without isoflurane anesthesia. However, this can be done in telemetry, but not in tethered animals, because tethered rats may lose their head implants during this procedure.
Having a well-trained operator and posing maximum effort to avoid stress, the only limitation of the present protocol is the maximal volume that can be withdrawn without compromising the animal's health. According to current standards, it is recommended to collect a maximum of 100 µL of CSF for 4x over 15 days in a single animal32. Similarly, it is suggested to collect less than 10% of total blood body volume on a single sampling and less than 15% of total blood body volume in 28 days30,31.
Comparison of the method with other techniques
Proposed time-resolved CSF and plasma sampling approaches have several advantages with respect to existing alternative methods. First, a cisterna magna puncture used to sample CSF in epileptic rats has a lower risk of head implant loss in comparison to a cannulated system if coupled to tethered EEG. In contrast to puncture procedures, the cannula attached to the electrode by dental cement (bulky and heavy for animals' heads), while solicited by repeated attachments/detachments to the CSF withdrawals, is much more prone to being lost over multiple days of sampling. Indeed, the success rate results show how some cannulated animals (N/A) do not arrive at the advanced sampling time points, thus their respective samples are lost (Table 1). Additionally, the puncture method seems to be superior to cannulated approach in terms of better sterility and reduced meningeal reaction with limited increase of cell and albumin content in CSF, as has been previously documented by other22,35,36. The degree of CSF leukocytes and albumin contamination may be important for the validity of methods used for epilepsy biomarkers quantification35. Second, the free fall dropping method of blood plasma sampling used for repeated measures is superior to any other withdrawal method as it is not terminal (non-recovery), unlike the decapitation, cardiac puncture, abdominal/thoracic blood vessel or retro-orbital withdrawal and allows multiple blood sampling. It is simpler than many tail vein blood vacuum withdrawal techniques, as it does not require tubing28,37 and produces hemolysis-free plasma samples of high quality for further sncRNA analyses focused on identifying putative biomarkers of epileptogenesis38. The absence of free hemoglobin in the samples, when employing the drop sampling technique or avoiding the tail milking, was confirmed by low absorbance results of the plasma samples (Table 2) in line with previously published procedures suitable for the evaluation of sncRNA plasma content39,40.
Applications and future directions
The above-described methods may be applied to measure soluble molecules of interest in any model of neurological diseases. A specific example is the sampling of biological liquids for the identification of potential/putative epilepsy biomarkers. There is an urgent unmet medical need to discover these biomarkers for people with epilepsy, especially prognostic and susceptibility/risk biomarkers, as they do not yet exist.
In conclusion, the present protocol is feasible in rats, including epileptic rats, and is easy to actuate for trained individuals. Moreover, it permits multiple high-quality sampling in longitudinal studies in compliance with the principle of 3Rs (i.e., replacement, reduction, and refinement)41.
Divulgazioni
The authors have nothing to disclose.
Acknowledgements
This study was supported by a grant from the European Union's Horizon 2020 Work Programme (call H2020-FETOPEN-2018-2020) under grant agreement 964712 (PRIME; to M. Simonato).
Materials
| Blood collection set BD Vacutainer Safety-Lok | BD Italy SpA, Milan, Italy | 367246 | Material |
| Blood Collection tubes (Microtainer K2E) | BD Italy SpA, Milan, Italy | 365975 | Material |
| Butterfly Winged Infusion Set 23G x 3/4'' 0.6 x 19 mm | Nipro, Osaka, Japan | PSY-23-ET-ICU | Material |
| Centrifuge refrigerated ALC PK 130R | DJB Labcare Ltd, Buckinghamshire, England | 112000033 | Material |
| Cotton suture 3-0 | Ethicon, Johnson & Johnson surgical technologies, Raritan, New Jersey, USA | 7343H | Material |
| Diazepam 5 mg/2ml, Solupam | Dechra Veterinary Products, Torino, Italy | 105183014 (AIC) | Solution |
| Digital video 8-channel media recorder system of telemetry EEG set up | Data Sciences International (DSI), St Paul, MN, USA | PNM-VIDEO-008 | Equipment |
| Digital video surveillance system of tethered EEG set up | EZVIZ Network, Hangzhou, Cina | EZVIZ (V5.3.2) | Equipment |
| Disinfectant based on stabilized peroxides and quaternary ammonium activity | Laboratoire Garcin-Bactinyl, France | LB 920111 | Solution |
| Dummy guide cannula 8 mm | Agn Tho's, Lindigö, Sweden | CXD-8 | Material |
| Electrode 3-channel two-twisted | Invivo1, Plastic One, Roanoke, Virginia, USA | MS333/3-B/SPC | Material |
| Electrode holder for stereotxic surgery | Agn Tho's, Lindigö, Sweden | 1776-P1 | Equipment |
| Eppendorf BioSpectrometer basic | Eppendorf AG, Hamburg, Germany | 6137 | Equipment |
Eppendorf PCR Tubes 0.2 mL |
Eppendorf Srl, Milan, Italy | 30124332 | Material |
| Eppendorf μCuvette G1.0 | Eppendorf AG, Hamburg, Germany | 6138 | Equipment |
| Feeding needle flexible 17G for rat | Agn Tho's, Lindigö Sweden | 7206 | Material |
| Grass Technology apparatus | Grass Technologies, Natus Neurology Incorporated, Pleasanton, California, USA | M665G08 | Equipment (AS40 amplifier, head box, interconnecting cables, telefactor model RPSA S40) |
| Isoflurane 100%, IsoFlo | Zoetis, Rome, Italy | 103287025 (AIC) | Solution |
| Ketamine (Imalgene) | Merial, Toulouse, France | 221300288 (AIC) | Solution |
| Lithium chloride | Sigma-Aldrich, Milan, Italy | L9650 | Material |
| Microinjection cannula 31G 9 mm | Agn Tho's, Lindigö Sweden | CXMI-9 | Material |
| MP150 modular data acquisition and analysis system | Biopac, Goleta, California, USA | MP150WSW | Equipment |
| Ophthalmic vet ointment, Hylo night | Ursapharm, Milan, Italy | 941791927 (AIC) | Material |
| Pilocarpine hydrochloride | Sigma-Aldrich, Milan, Italy | P6503 | Material |
| PTFE Tube with joint | Agn Tho's, Lindigö, Sweden | JT-10 | Material |
| Saline | 0.9% NaCl, pH adjusted to 7.0 | Solution | |
| Scopolamine hydrobromide trihydrate | Sigma-Aldrich, Milan, Italy | S2250 | Material |
| Scopolamine methyl nitrate | Sigma-Aldrich, Milan, Italy | S1876 | Material |
| Silver sulfadiazine 1% cream | Sofar, Trezzano Rosa, Milan, Italy | 025561010 (AIC) | Material |
| Simplex rapid dental methacrylic cement | Kemdent, Associated Dental Products Ltd, Swindon, United Kingdom | ACR811 | Material |
| Stereotaxic apparatus | David Kopf Instruments, Los Angeles, CA, USA | Model 963 | Equipment |
| Sucrose solution | 10% sucrose in distilled water | Home-made | Solution |
| Syringe 1 mL | Biosigma, Cona, Venezia, Italy | 20,71,26,03,00,350 | Material |
| Telemeters | Data Sciences International (DSI), St Paul, MN, USA | CTA-F40 | Material |
| Telemetry EEG traces analyzer | Data Sciences International (DSI), St Paul, MN, USA | NeuroScore v3-0 | Equipment |
| Telemetry system | Data Sciences International (DSI), St Paul, MN, USA | Hardware plus software Ponemah core 6.51 | Equipment |
| Xylazine hydrochloride | Sigma-Aldrich, Milan, Italy | X1251 | Material |
Riferimenti
- Hanin, A., et al. Cerebrospinal fluid and blood biomarkers of status epilepticus. Epilepsia. 61 (1), 6-18 (2020).
- Pitkänen, A., et al. Advances in the development of biomarkers for epilepsy. The Lancet Neurology. 15 (8), 843-856 (2016).
- Dlugos, D., et al. Childhood Absence Epilepsy Study Team (2013). Pretreatment EEG in childhood absence epilepsy: associations with attention and treatment outcome. Neurology. 81 (2), 150-156 (2013).
- Lorenzo, N. Y., et al. Intractable frontal lobe epilepsy: pathological and MRI features. Epilepsy research. 20 (2), 171-178 (1995).
- van Dellen, E., et al. Epilepsy surgery outcome and functional network alterations in longitudinal MEG: a minimum spanning tree analysis. NeuroImage. 86, 354-363 (2014).
- Shah, A. K., Mittal, S. Invasive electroencephalography monitoring: Indications and presurgical planning. Annals of Indian Academy of Neurology. 17 (Suppl 1), S89-S94 (2014).
- Whiting, P., et al. A systematic review of the effectiveness and cost-effectiveness of neuroimaging assessments used to visualise the seizure focus in people with refractory epilepsy being considered for surgery. Health technology assessment. 10 (4), 1-iv (2006).
- Lenkov, D. N., Volnova, A. B., Pope, A. R., Tsytsarev, V. Advantages and limitations of brain imaging methods in the research of absence epilepsy in humans and animal models. Journal of neuroscience methods. 212 (2), 195-202 (2013).
- Leach, J. P., Stephen, L. J., Salveta, C., Brodie, M. J. Which electroencephalography (EEG) for epilepsy? The relative usefulness of different EEG protocols in patients with possible epilepsy. Journal of neurology, neurosurgery, and psychiatry. 77 (9), 1040-1042 (2006).
- Huppertz, H. J., et al. Localization of interictal delta and epileptiform EEG activity associated with focal epileptogenic brain lesions. NeuroImage. 13 (1), 15-28 (2001).
- Linder, C., et al. Comparison between dried blood spot and plasma sampling for therapeutic drug monitoring of antiepileptic drugs in children with epilepsy: A step towards home sampling. Clinical biochemistry. 50 (7-8), 418-424 (2017).
- Wegner, I., Wilhelm, A. J., Lambrechts, D. A., Sander, J. W., Lindhout, D. Effect of oral contraceptives on lamotrigine levels depends on comedication. Acta neurologica Scandinavica. 129 (6), 393-398 (2014).
- Palmio, J., et al. CSF and plasma adipokines after tonic-clonic seizures. Seizure. 39, 10-12 (2016).
- Hogg, M. C., et al. Elevation in plasma tRNA fragments precede seizures in human epilepsy. Journal of Clinical Investigation. 129 (7), 2946-2951 (2019).
- Ellul, M., Solomon, T. Acute encephalitis – diagnosis and management. Clinical medicine. 18 (2), 155-159 (2018).
- Diamond, M. L., et al. IL-1β associations with posttraumatic epilepsy development: a genetics and biomarker cohort study. Epilepsia. 55 (7), 1109-1119 (2014).
- Auvin, S., et al. Prospective clinical trials to investigate clinical and molecular biomarkers. Epilepsia. 58 (Suppl 3), 20-26 (2017).
- Weber, Y. G., Nies, A. T., Schwab, M., Lerche, H. Genetic biomarkers in epilepsy. Neurotherapeutics. 11 (2), 324-333 (2014).
- Fornari, R. V., et al. Rodent stereotaxic surgery and animal welfare outcome improvements for behavioral neuroscience. Journal of Visualized Experiments. (59), e3528 (2012).
- Geiger, B. M., Frank, L. E., Caldera-Siu, A. D., Pothos, E. N. Survivable stereotaxic surgery in rodents. Journal of Visualized Experiments. (20), e880 (2008).
- Gardiner, T. W., Toth, L. A. Stereotactic Surgery and Long-Term Maintenance of Cranial Implants in Research Animals. Contemporary Topics in Laboratory Animal Science. 38 (1), 56-63 (1999).
- Westergren, I., Johansson, B. B. Changes in physiological parameters of rat cerebrospinal fluid during chronic sampling: evaluation of two sampling methods. Brain Research Bulletin. 27 (2), 283-286 (1991).
- Soukupová, M., et al. Impairment of GABA release in the hippocampus at the time of the first spontaneous seizure in the pilocarpine model of temporal lobe epilepsy. Experimental Neurology. 257, 39-49 (2014).
- Soukupová, M., et al. Microdialysis of Excitatory Amino Acids During EEG Recordings in Freely Moving Rats. Journal of Visualized Experiments. (141), e58455 (2018).
- Guarino, A., et al. Low-dose 7,8-Dihydroxyflavone Administration After Status Epilepticus Prevents Epilepsy Development. Neurotherapeutics. 19 (6), 1951-1965 (2022).
- Curia, G., Longo, D., Biagini, G., Jones, R. S. G., Avoli, M. The pilocarpine model of temporal lobe epilepsy. Journal of Neuroscience Methods. 172 (2), 143-157 (2008).
- Racine, R. J. Modification of seizure activity by electrical stimulation: II. Motor seizure. Electroencephalography and Clinical Neurophysiology. 32 (3), 281-294 (1972).
- Zou, W., et al. Repeated Blood Collection from Tail Vein of Non-Anesthetized Rats with a Vacuum Blood Collection System. Journal of Visualized Experiments. (130), e55852 (2017).
- . Blood sampling: Rat Available from: https://nc3rs.org.uk/3rs-resources/blood-sampling/blood-sampling-rat (2022)
- Powles-Glover, N., Kirk, S., Wilkinson, C., Robinson, S., Stewart, J. Assessment of toxicological effects of blood microsampling in the vehicle dosed adult rat. Regulatory Toxicology and Pharmacology. 68 (3), 325-331 (2014).
- Zeller, W., Weber, H., Panoussis, B., Bürge, T., Bergmann, R. Refinement of blood sampling from the sublingual vein of rats. Laboratory Animal. 32 (4), 369-376 (1998).
- Wang, D., Zhao, Y., Yang, Y., Xie, H. Safety assessment of multiple repeated percutaneous punctures for the collection of cerebrospinal fluid in rats. Brazilian Journal of Medical and Biological Research. 54 (6), e10032 (2021).
- Möller, C., et al. Impact of repeated kindled seizures on heart rate rhythms, heart rate variability, and locomotor activity in rats. Epilepsy & Behavior. 92, 36-44 (2019).
- Espinosa-Garcia, C., Zeleke, H., Rojas, A. Impact of Stress on Epilepsy: Focus on Neuroinflammation-A Mini Review. International Journal of Molecular Sciences. 22 (8), 4061 (2021).
- Cassar, S. C., et al. Comparing levels of biochemical markers in CSF from cannulated and non-cannulated rats. Journal of Neuroscience Methods. 192 (2), 249-253 (2010).
- Huang, Y. L., Säljö, A., Suneson, A., Hansson, H. A. Comparison among different approaches for sampling cerebrospinal fluid in rats. Brain Research Bulletin. 41 (5), 273-279 (1996).
- Hattori, N., Takumi, A., Saito, K., Saito, Y. Effects of serial cervical or tail blood sampling on toxicity and toxicokinetic evaluation in rats. Journal of Toxicological Sciences. 45 (10), 599-609 (2020).
- Roncon, P., et al. MicroRNA profiles in hippocampal granule cells and plasma of rats with pilocarpine-induced epilepsy–comparison with human epileptic samples. Scientific Reports. 5, 14143 (2015).
- van Vliet, E. A., et al. Standardization procedure for plasma biomarker analysis in rat models of epileptogenesis: Focus on circulating microRNAs. Epilepsia. 58 (12), 2013-2024 (2017).
- Kirschner, M. B., et al. Haemolysis during sample preparation alters microRNA content of plasma. PLoS One. 6 (9), e24145 (2011).
- Grimm, H., et al. Advancing the 3Rs: innovation, implementation, ethics and society. Frontiers in Veterinary Science. 10, 1185706 (2023).

