Isolation, Culture, and Adipogenic Induction of Stromal Vascular Fraction-derived Preadipocytes from Mouse Periaortic Adipose Tissue
Summary
Here, we describe the isolation, culture, and adipogenic induction of stromal vascular fraction-derived preadipocytes from mouse periaortic adipose tissue, allowing for the study of perivascular adipose tissue function and its relationship with vascular cells.
Abstract
Perivascular adipose tissue (PVAT) is an adipose tissue depot that surrounds blood vessels and exhibits the phenotypes of white, beige, and brown adipocytes. Recent discoveries have shed light on the central role of PVAT in regulating vascular homeostasis and participating in the pathogenesis of cardiovascular diseases. A comprehensive understanding of PVAT properties and regulation is of great importance for the development of future therapies. Primary cultures of periaortic adipocytes are valuable for studying PVAT function and the crosstalk between periaortic adipocytes and vascular cells. This paper presents an economical and feasible protocol for the isolation, culture, and adipogenic induction of stromal vascular fraction-derived preadipocytes from mouse periaortic adipose tissue, which can be useful for modeling adipogenesis or lipogenesis in vitro. The protocol outlines tissue processing and cell differentiation for culturing periaortic adipocytes from young mice. This protocol will provide the technological cornerstone at the bench side for the investigation of PVAT function.
Introduction
Perivascular adipose tissue (PVAT), a perivascular structure composed of a mixture of mature adipocytes and a stromal vascular fraction (SVF), is believed to interact with the adjacent vessel wall via its secretome paracrineally1. As a critical regulator of vascular homeostasis, PVAT dysfunction is implicated in the pathogenesis of cardiovascular diseases2,3,4. The SVF of adipocyte tissue consists of several expected cell populations, including endothelial cells, immune cells, mesothelium cells, neuronal cells, and adipose stem and progenitor cells (ASPCs)5,6. It is well known that ASPCs residing in the SVF of adipose tissue can give rise to mature adipocytes5. SVF is inferred to be a critical source of mature adipocytes in PVAT. Several studies have shown that PVAT-SVF can differentiate into mature adipocytes under specific induction conditions6,7,8.
Currently, there are two isolation systems for isolating SVF from adipose tissue, one is enzymatic digestion and the other is non-enzymatic9. Enzymatic methods typically result in a higher yield of nucleated progenitor cells10. To date, the benefits of SVF in promoting vascular regeneration and neovascularization in wound healing, urogenital, and cardiovascular diseases have been widely demonstrated11, especially in dermatology and plastic surgery12,13. However, the clinical application prospects of PVAT-derived SVF have not been well explored, which may be attributed to the lack of a standardized method for the isolation of SVF from PVAT. The objective of this protocol is to establish a standardized approach for the isolation, culture, and adipogenic induction of SVF-derived preadipocytes from mouse PVAT surrounding the thoracic aorta, enabling further investigation of PVAT function. This protocol optimizes tissue processing and cell differentiation techniques for culturing periaortic adipocytes obtained from young mice.
Protocol
The animal protocols were approved by the Institutional Animal Care and Use Committee at Shanghai Chest Hospital affiliated to Shanghai Jiao Tong University School of Medicine (approval number: KS23010) and were in compliance with relevant ethical regulations. Male and female C57BL/6 mice aged 4-8 weeks are to be preferred for this experiment.
1. Preparation of surgical tools, buffers, and culture media
- Autoclave surgical tools (e.g., surgical scissors and standard forceps) at 121 °C for 30 min. Disinfect microsurgical instruments with 75% alcohol.
- Prepare sterile phosphate-buffered saline (PBS) supplemented with 1% v/v penicillin-streptomycin and another 10 mL of PBS supplemented with 10% v/v penicillin-streptomycin.
NOTE: In the following sections, if not specifically mentioned, PBS refers to regular sterile PBS. - Prepare Krebs Ringer HEPES BSA buffer: 1% bovine serum albumin (BSA), 20 mM HEPES dissolved in Krebs Ringer solution.
- Prepare fresh digestion solution: type 1 collagenase (1 mg/mL) and dispase II (4 mg/mL) dissolved in Krebs Ringer HEPES BSA buffer. Sterilize the solution using a 0.2 µm filter.
- Prepare a collagen-coated 12-well plate.
- Dilute the collagen solution (1 mg/mL) with sterile deionized water to the concentration of 40 µg/mL. Add 1 mL of the diluted collagen solution on the surface of each well to achieve a concentration of 6-10 µg/cm2. Incubate the coating for 1 h at room temperature.
- Remove the remaining solution and rinse each well 2x with 1 mL of PBS. Use the plate immediately, or air dry the plate in a class II laminar flow hood and store the coated plate at 2-8 °C. When storing, seal the coated plates with parafilm.
- Prepare culture medium: high-glucose Dulbecco's-Modified Eagles Medium (DMEM), 10% fetal bovine serum (FBS), 1% v/v penicillin-streptomycin. Keep at 4 °C for up to 2 weeks.
- Prepare lipogenic induction medium: high-glucose DMEM, 10% FBS, 1% v/v penicillin-streptomycin, 1 nM triiodothyronine, 1 µM rosiglitazone, 1 µM insulin, 0.5 mM 3-isobutyl-1-methylxanthine (IBMX), and 1 µM dexamethasone. Keep at 4 °C for up to 2 weeks.
- Prepare maintenance medium: high-glucose DMEM, 10% FBS, 1% v/v penicillin-streptomycin, 1 nM triiodothyronine, 1 µM rosiglitazone, and 1 µM insulin. Keep at 4 °C for up to 2 weeks.
2. Dissection and isolation of perivascular adipose tissue (PVAT)
- Euthanize the mouse by cervical dislocation under 2% isoflurane anesthesia or with carbon dioxide overdose. Spray with 75% alcohol for skin disinfection.
- Place the mouse in a supine position (facing upward).
- Lift the skin, make a small incision, and bluntly separate the skin and abdominal muscles with surgical scissors along the ventral midline from the pelvis to the neck. Expose the heart and lungs by cutting the diaphragm and ribs along both sides of the midline.
- Carefully remove the liver, spleen, bowels, and kidney. Avoid cutting the intestinal wall.
- Remove the lungs and esophagus.
- Gently lift the heart with forceps in one hand and separate the aorta from the spine with surgical scissors in the other hand. Then, place the heart and aorta in a 60 mm Petri dish with PBS containing 1% v/v penicillin-streptomycin.
- Remove microsurgical forceps and microsurgical scissors from alcohol and rinse in 25 mL of PBS to remove the excess alcohol. Under a stereo microscope, remove the thymus and other tissue from the heart and aorta, then remove the heart, leaving the aorta with the PVATs.
- Transfer the aorta with the PVATs to a clean 60 mm Petri dish with PBS containing 1% v/v penicillin-streptomycin.
- Use microsurgical forceps to strip PVATs, with one pair of forceps fixing the aorta and the other pulling off the adipose tissue. Remove the vasculature tissue as much as possible while minimizing damage to the perivascular adipose tissue.
- Collect the PVAT surrounding the thoracic aorta into the 2 mL microcentrifuge tube containing high-glucose DMEM supplemented with 1% v/v penicillin-streptomycin placed on ice.
3. Isolation of stromal vascular fraction (SVF)
- Rinse the collected tissues sequentially with PBS containing 10% v/v penicillin-streptomycin and PBS containing 1% v/v penicillin-streptomycin.
NOTE: From this step onwards, all experiments must be carried out under sterile conditions in a class II laminar flow hood. - Transfer the tissues to a sterile 60 mm Petri dish, add 200 µL of digestion solution, and mince them into 1 mm3 pieces using sterile scissors.
- Transfer the mix to a 15 mL centrifuge tube using a plastic pipette tip, the end broadened by a sterile scissor cut, and add 6 mL of digestion solution to start the digestion reaction.
NOTE: One digestion reaction (3 mL of digestion solution) is sufficient for PVAT depots from six mice. - Incubate the tissues at 37 °C in an incubator with an orbital shaker (150 rpm frequency for effective mixing) for 30-45 min. Tie the tubes flat on a rack to make the tubes shake horizontally. Shake up and down vigorously by hand for 10 s every 5-10 min.
NOTE: Digestion can be discontinued when a homogeneous, yellowish digestive fluid is seen with no tissue fragments left. - Strain the digested tissues through a 70 µm cell strainer into a 50 mL centrifuge tube. Rinse the cell strainer with an equal volume of culture medium to maximize cell yield and stop the digestion.
- Transfer the filtrate to a new 15 mL centrifuge tube and centrifuge at 1,800 × g for 10 min. Invert the tube to discard the supernatant and resuspend the pellets in 5 mL of PBS. Centrifuge at 1,800 × g for 5 min.
- Discard the supernatant by inverting the tube and resuspend the pellets in an appropriate volume of culture medium.
NOTE: The cell pellets collected from every six mice are resuspended in 1 mL of culture medium and seeded into one well of a 12-well plate. - Seed the cells into a collagen-coated 12-well plate and incubate at 37 °C in a humid atmosphere with 5% CO2 overnight.
- On the next day, aspirate the culture medium, wash the cells with prewarmed (37 °C) PBS containing 1% v/v penicillin-streptomycin to remove cell debris and red blood cells, and add back 1 mL of fresh culture medium each well.
4. Adipogenic induction of SVF-derived preadipocytes from periaortic adipose tissue
- Change the culture medium every other day until the cells reach ~60-70% confluence.
NOTE: It usually takes 3-4 days for the cells to reach 60-70% confluence. - When the cells reach 60-70% confluence, aspirate the culture medium and replace it with brown adipogenic induction medium. Consider the day of induction of adipogenic differentiation as day 0 of differentiation.
- After 72 h (day 3 of differentiation), refresh the medium with maintenance medium. Change the maintenance medium every 2 days until the cells are used for experiments.
- Analyze the adipogenic cells in an appropriate way, such as Oil Red O staining14 and western blotting15.
Representative Results
Using this protocol described above, we carefully isolated PVATs surrounding mouse thoracic aortas (Figure 1A–D). After washing and mincing the PVATs into small pieces using sterile scissors (Figure 1E,F), tissue fragments were digested in a digestion solution containing type 1 collagenase (1 mg/mL) and dispase II (4 mg/mL) and incubated at 37 °C on a shaker for 30-45 min (Figure 1G). The digested tissues were strained through a 70 µm cell strainer into a 50 mL centrifuge tube (Figure 1H). Cell pellets were collected after centrifugation (Figure 1I).
To confirm the adipogenic potential of the SVF-derived preadipocytes, we induced the cells with brown adipogenic induction medium containing 1 nM triiodothyronine, 1 µM rosiglitazone, 1 µM insulin, 0.5 mM IBMX, and 1 µM dexamethasone. Mature adipocytes were observed after 7-10 days of brown adipogenic induction, characterized by the formation of Oil Red O-stained lipid-rich vacuoles (Figure 2A). Western blotting analysis further showed the increased expression levels of adipocyte-specific proteins, including adiponectin, Fabp4, Pgc1α, Pparγ, Ucp1, and mitochondrial protein Cox IV (Figure 2B,C). These data suggest that the SVF-derived preadipocytes from mouse periaortic adipose tissue exhibit strong adipogenic potential.
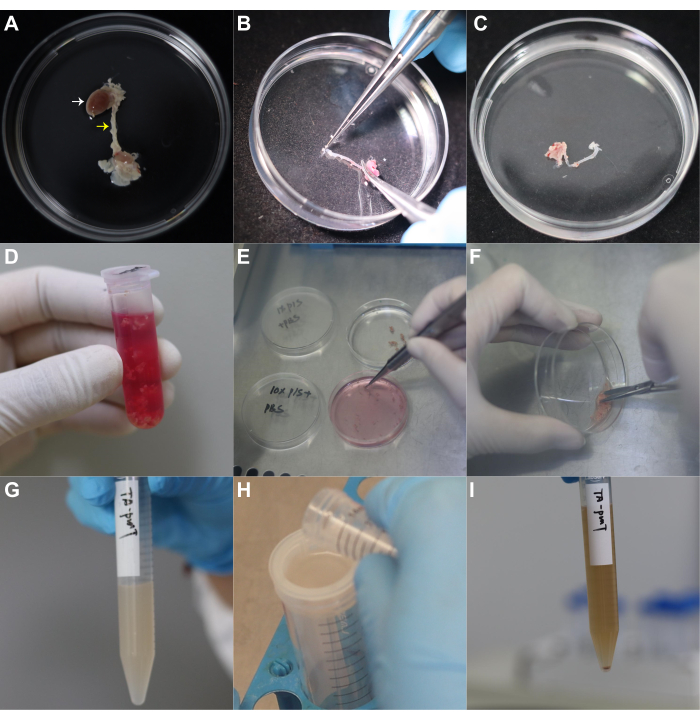
Figure 1: Isolation of stromal vascular fraction from mouse periaortic adipose tissue. (A) The heart and aorta are carefully dissected using surgical forceps and scissors. White and yellow arrowheads indicate the heart and aorta, respectively. (B) PVATs surrounding the thoracic aorta are carefully stripped off using forceps. (C) The aorta left after the removal of PVAT surrounding the thoracic aorta. (D) PVATs are collected in a 2 mL microcentrifuge tube containing high-glucose DMEM with 1% v/v penicillin-streptomycin. (E) PVATs are rinsed sequentially with PBS containing 10% v/v penicillin-streptomycin and PBS containing 1% v/v penicillin-streptomycin. (F) Mincing of the PVATs into 1 mm3 pieces using sterile scissors. (G) Transfer of minced PVATs to a 15 mL centrifuge tube containing 6 mL of digestion solution, and incubate tissues at 37 °C for 30-45 min with shaking at 150 rpm. (H) Stop digestion and filter the suspension through a 70 µm cell strainer to a 50 mL centrifuge tube. (I) Centrifuge the filtrate at 1,800 × g for 10 min; the SVF has been isolated. Abbreviations: PVAT = perivascular adipose tissue; SVF = stromal vascular fraction. Please click here to view a larger version of this figure.
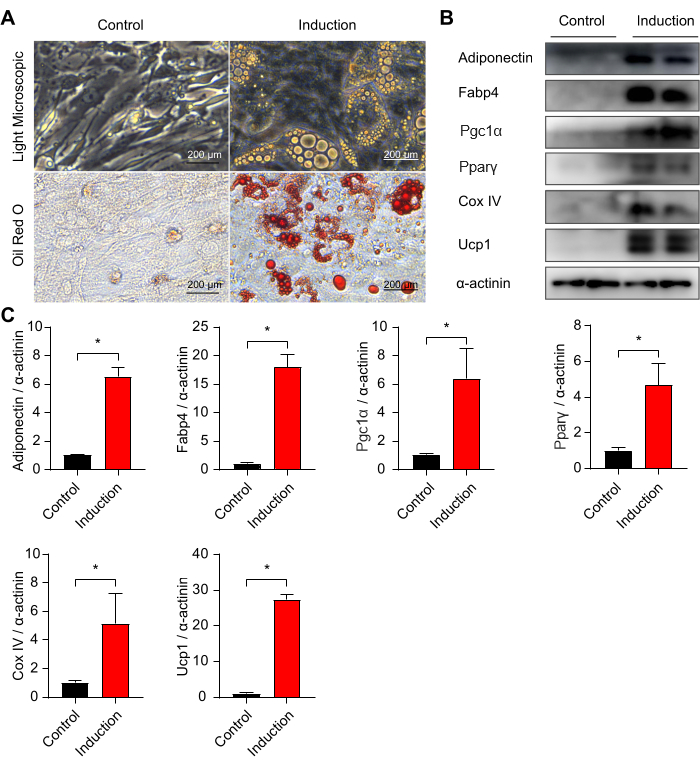
Figure 2: Adipogenic differentiation of stromal vascular fraction from mouse periaortic adipose tissue. (A) Representative images of light microscope and Oil Red O staining of primary adipocytes differentiated from periaortic preadipocytes at day 8. Scale bars = 200 µm. (B,C) Western blotting analysis of protein levels of adiponectin, Fabp4, Pgc1α, Pparγ, Cox IV, and Ucp1 for primary adipocytes differentiated from periaortic preadipocytes at day 8. Unpaired two-tailed Student's t-tests were used to calculate significant differences between the two groups. Values are mean ± SEM. Please click here to view a larger version of this figure.
Discussion
We propose a practical and feasible approach for the isolation and adipogenic induction of SVF-derived preadipocytes from mouse periaortic adipose tissue. The advantages of this protocol are that it is simple and economical. Adequate numbers of mice are critical for successful isolation, as insufficient tissue can result in low SVF density and poor growth state, ultimately affecting lipogenic efficiency. Additionally, mouse age is an important factor to consider as the adipogenic potential of SVF decreases with age. Rapid and careful separation of PVAT while minimizing contamination of vasculature is crucial. The digestion solution is also a key component as the addition of dispase II to type 1 collagenase can improve the digestion rate and yield of cells. It is important to pay close attention to the digestion process to avoid overdigestion as it may potentially affect cell viability. Following sterile techniques and maintaining appropriate culture conditions throughout the protocol is essential.
Primary cultures of periaortic adipocytes are valuable for studying PVAT properties and functions. Isolation and adipogenic induction of SVF-derived preadipocytes from periaortic adipose tissue of genetically modified mice provide a platform for exploring the important regulator of PVAT differentiation and adipogenesis in vitro. PVAT is a modulator of vasoconstriction and vascular remodeling in an outward-to-inward manner, through generating vasoactive molecules such as hydrogen peroxide, adiponectin, angiotensin 1-7, methyl palmitate, hydrogen sulfide, nitric oxide, and leptin3. This presented method allows for the further study of the crosstalk between periaortic adipocytes and endothelial cells16, vascular smooth muscle cells (VSMCs)17, adventitial fibroblasts18, or macrophages19, providing insights into the central roles of PVAT in the pathogenesis of cardiovascular diseases such as hypertension, atherosclerosis, stenosis, and aneurysms20,21,22.
In addition, the isolation and adipogenic induction of preadipocytes are the bases to study PVAT lineages and PVAT heterogeneity. PVATs are heterogeneous inter- and intravascular beds, manifesting as distinct transcriptional profiles, which may contribute to distinct physiological and pathological functional features20,23. Chang et al. reported that mice with VSMC-specific PPARγ deletion lacked PVAT in the aortic and mesenteric regions, indicating that PVAT might share the same embryonic origins with the local vascular wall24. There is evidence that PVAT from different embryonic origins may have different phenotypes25,26. The PVAT surrounding the ascending aorta and aortic arch (AA-PVAT), derived from neural crest cells of ectodermal origin, is morphologically and transcriptomically more similar to brown adipose tissue (BAT)27. Correspondingly, the SVF of PVAT derived from neural crest cells tends to differentiate into brown adipocytes more readily than white adipocytes in vitro28. The abdominal aorta PVAT, however, primarily exhibits a white adipose tissue-like phenotype29. A BAT-like phenotype of PVAT or the beiging of PVAT is associated with antiinflammatory and antipathological remodeling effects, shedding light on the treatment of cardiovascular disease by way of PVAT phenotype modulation19,30. This protocol will provide the technological cornerstone at the bench side.
Nevertheless, there are some limitations to this protocol. Due to the restricted availability of PVAT, extracting PVAT-SVF necessitates a larger number of mice. In situations where there is a high demand for SVF, it may be necessary to engage more than one person to accelerate the experimental progress. Compared to immortalized preadipocytes, SVF-derived preadipocytes require additional human and material resources.
Divulgazioni
The authors have nothing to disclose.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (82130012 and 81830010) and the Nurture projects for basic research of Shanghai Chest Hospital (Grant Number: 2022YNJCQ03).
Materials
| 0.2 μm syringe filters | PALL | 4612 | |
| 12-well plate | Labselect | 11210 | |
| 15 mL centrifuge tube | Labserv | 310109003 | |
| 3,3',5-triiodo-L-thyronine (T3) | Sigma-Aldrich | T-2877 | 1 nM |
| 50 mL centrifuge tube | Labselect | CT-002-50A | |
| anti-adiponectin | Abcam | ab22554 | 1:1,000 working concentration |
| anti-COX IV | CST | 4850 | 1:1,000 working concentration |
| anti-FABP4 | CST | 2120 | 1:1,000 working concentration |
| anti-PGC1α | Abcam | ab191838 | 1:1,000 working concentration |
| anti-PPARγ | Invitrogen | MA5-14889 | 1:1,000 working concentration |
| anti-UCP1 | Abcam | ab10983 | 1:1,000 working concentration |
| anti-α-Actinin | CST | 6487 | 1:1,000 working concentration |
| BSA | Beyotime | ST023-200g | 1% |
| C57BL/6 mice aged 4-8 weeks of both sexes | Shanghai Model Organisms Center, Inc. | ||
| Cell Strainer 70 µm, nylon | Falcon | 352350 | |
| Collagen from calf skin | Sigma-Aldrich | C8919 | |
| Collagenase, Type 1 | Worthington | LS004196 | 1 mg/mL |
| Dexamethasone | Sigma-Aldrich | D1756 | 1 μM |
| Dispase II | Sigma-Aldrich | D4693-1G | 4 mg/mL |
| Fetal bovine serum | Gibco | 16000-044 | 10% |
| HEPES | Sigma-Aldrich | H4034-25G | 20 mM |
| High glucose DMEM | Hyclone | SH30022.01 | |
| IBMX | Sigma-Aldrich | I7018 | 0.5 mM |
| Incubator with orbital shaker | Shanghai longyue Instrument Eruipment Co.,Ltd. | LYZ-103B | |
| Insulin (cattle) | Sigma-Aldrich | 11070-73-8 | 1 μM |
| Isoflurane | RWD | R510-22-10 | |
| Krebs-Ringer's Solution | Pricella | PB180347 | protect from light |
| Microsurgical forceps | Beyotime | FS233 | |
| Microsurgical scissor | Beyotime | FS217 | |
| Oil Red O | Sangon Biotech (Shanghai) Co., Ltd | A600395-0050 | |
| PBS (Phosphate-buffered saline) | Sangon Biotech (Shanghai) Co., Ltd | B548117-0500 | |
| Penicillin-Streptomycin | Gibco | 15140122 | |
| Peroxidase AffiniPure Goat Anti-Mouse IgG (H+L) | Jackson ImmunoResearch | 115-035-146 | 1:5,000 working concentration |
| Peroxidase AffiniPure Goat Anti-Rabbit IgG (H+L) | Jackson ImmunoResearch | 111-035-144 | 1:5,000 working concentration |
| Rosiglitazone | Sigma-Aldrich | R2408 | 1 μM |
| Standard forceps | Beyotime | FS225 | |
| Surgical scissor | Beyotime | FS001 |
Riferimenti
- Akoumianakis, I., Antoniades, C. The interplay between adipose tissue and the cardiovascular system: is fat always bad. Cardiovascular Research. 113 (9), 999-1008 (2017).
- Huang, C. L., et al. Thoracic perivascular adipose tissue inhibits VSMC apoptosis and aortic aneurysm formation in mice via the secretome of browning adipocytes. Acta Pharmacologica Sinica. 44 (2), 345-355 (2023).
- Xia, N., Li, H. The role of perivascular adipose tissue in obesity-induced vascular dysfunction. British Journal of Pharmacology. 174 (20), 3425-3442 (2017).
- Brown, N. K., et al. Perivascular adipose tissue in vascular function and disease: a review of current research and animal models. Arteriosclerosis, Thrombosis, and Vascular Biology. 34 (8), 1621-1630 (2014).
- Ferrero, R., Rainer, P., Deplancke, B. Toward a consensus view of mammalian adipocyte stem and progenitor cell heterogeneity. Trends in Cell Biology. 30 (12), 937-950 (2020).
- Angueira, A. R., et al. Defining the lineage of thermogenic perivascular adipose tissue. Nature Metabolism. 3 (4), 469-484 (2021).
- Boucher, J. M., et al. Rab27a regulates human perivascular adipose progenitor cell differentiation. Cardiovascular Drugs and Therapy. 32 (5), 519-530 (2018).
- Saxton, S. N., Withers, S. B., Heagerty, A. M. Emerging roles of sympathetic nerves and inflammation in perivascular adipose tissue. Cardiovascular Drugs and Therapy. 33 (2), 245-259 (2019).
- Ferroni, L., De Francesco, F., Pinton, P., Gardin, C., Zavan, B. Methods to isolate adipose tissue-derived stem cells. Methods in Cell Biology. 171, 215-228 (2022).
- Senesi, L., et al. Mechanical and enzymatic procedures to isolate the stromal vascular fraction from adipose tissue: preliminary results. Frontiers in Cell and Developmental Biology. 7, 88 (2019).
- Andia, I., Maffulli, N., Burgos-Alonso, N. Stromal vascular fraction technologies and clinical applications. Expert Opinion on Biological Therapy. 19 (12), 1289-1305 (2019).
- Suh, A., et al. Adipose-derived cellular and cell-derived regenerative therapies in dermatology and aesthetic rejuvenation. Ageing Research Reviews. 54, 100933 (2019).
- Bellei, B., Migliano, E., Picardo, M. Therapeutic potential of adipose tissue-derivatives in modern dermatology. Experimental Dermatology. 31 (12), 1837-1852 (2022).
- Kraus, N. A., et al. Quantitative assessment of adipocyte differentiation in cell culture. Adipocyte. 5 (4), 351-358 (2016).
- Figueroa, A. M., Stolzenbach, F., Tapia, P., Cortés, V. Differentiation and imaging of brown adipocytes from the stromal vascular fraction of interscapular adipose tissue from newborn mice. Journal of Visualized Experiments: JoVE. (192), (2023).
- Ma, Y., et al. Methotrexate improves perivascular adipose tissue/endothelial dysfunction via activation of AMPK/eNOS pathway. Molecular Medicine Reports. 15 (4), 2353-2359 (2017).
- Li, X., Ballantyne, L. L., Yu, Y., Funk, C. D. Perivascular adipose tissue-derived extracellular vesicle miR-221-3p mediates vascular remodeling. FASEB Journal. 33 (11), 12704-12722 (2019).
- Ruan, C. C., et al. Perivascular adipose tissue-derived complement 3 is required for adventitial fibroblast functions and adventitial remodeling in deoxycorticosterone acetate-salt hypertensive rats. Arteriosclerosis, Thrombosis, and Vascular Biology. 30 (12), 2568-2574 (2010).
- Adachi, Y., et al. Beiging of perivascular adipose tissue regulates its inflammation and vascular remodeling. Nature Communications. 13 (1), 5117 (2022).
- Ye, M., et al. Developmental and functional characteristics of the thoracic aorta perivascular adipocyte. Cellular and Molecular Life Sciences. 76 (4), 777-789 (2019).
- Stanek, A., Brożyna-Tkaczyk, K., Myśliński, W. The role of obesity-induced perivascular adipose tissue (PVAT) dysfunction in vascular homeostasis. Nutrients. 13 (11), 3843 (2021).
- Queiroz, M., Sena, C. M. Perivascular adipose tissue in age-related vascular disease. Ageing Research Reviews. 59, 101040 (2020).
- Fitzgibbons, T. P., et al. Similarity of mouse perivascular and brown adipose tissues and their resistance to diet-induced inflammation. American Journal of Physiology-Heart and Circulatory Physiology. 301 (4), H1425-H1437 (2011).
- Chang, L., et al. Loss of perivascular adipose tissue on peroxisome proliferator-activated receptor-γ deletion in smooth muscle cells impairs intravascular thermoregulation and enhances atherosclerosis. Circulation. 126 (9), 1067-1078 (2012).
- Piacentini, L., et al. Genome-wide expression profiling unveils autoimmune response signatures in the perivascular adipose tissue of abdominal aortic aneurysm. Arteriosclerosis, Thrombosis, and Vascular Biology. 39 (2), 237-249 (2019).
- Wang, Z., et al. RNA sequencing reveals perivascular adipose tissue plasticity in response to angiotensin II. Pharmacological Research. 178, 106183 (2022).
- Shi, K., et al. Ascending aortic perivascular adipose tissue inflammation associates with aortic valve disease. Journal of Cardiology. 80 (3), 240-248 (2022).
- Fu, M., et al. Neural crest cells differentiate into brown adipocytes and contribute to periaortic arch adipose tissue formation. Arteriosclerosis, Thrombosis, and Vascular Biology. 39 (8), 1629-1644 (2019).
- Gil-Ortega, M., Somoza, B., Huang, Y., Gollasch, M., Fernández-Alfonso, M. S. Regional differences in perivascular adipose tissue impacting vascular homeostasis. Trends in Endocrinology & Metabolism. 26 (7), 367-375 (2015).
- Bar, A., et al. In vivo magnetic resonance imaging-based detection of heterogeneous endothelial response in thoracic and abdominal aorta to short-term high-fat diet ascribed to differences in perivascular adipose tissue in mice. Journal of the American Heart Association. 9 (21), e016929 (2020).

