Isolation of Primary Porcine Retinal Pigment Epithelial Cells for In Vitro Modeling
Summary
This protocol outlines the procedure for obtaining and culturing primary retinal pigment epithelial (RPE) cells from locally sourced porcine eyes. These cells serve as a high-quality alternative to stem cells and are suitable for in vitro retinal research.
Abstract
The retinal pigment epithelium (RPE) is a crucial monolayer in the outer retina responsible for supporting photoreceptors. RPE degeneration commonly occurs in diseases marked by progressive vision loss, such as age-related macular degeneration (AMD). Research on AMD often relies on human donor eyes or induced pluripotent stem cells (iPSCs) to represent the RPE. However, these RPE sources require extended differentiation periods and substantial expertise for culturing. Additionally, some research institutions, particularly those in rural areas, lack easy access to donor eyes. While a commercially available immortalized RPE cell line (ARPE-19) exists, it lacks essential in vivo RPE features and is not widely accepted in many ophthalmology research publications. There is a pressing need to obtain representative primary RPE cells from a more readily available and cost-effective source. This protocol elucidates the isolation and subculture of primary RPE cells obtained post-mortem from porcine eyes, which can be sourced locally from commercial or academic suppliers. This protocol necessitates common materials typically found in tissue culture labs. The result is a primary, differentiated, and cost-effective alternative to iPSCs, human donor eyes, and ARPE-19 cells.
Introduction
The retinal pigment epithelium (RPE) is a monolayer located in the outer retina between Bruch's membrane and the photoreceptors1. RPE cells form tight junctions with proteins such as zonula occludens-1 (ZO-1) and possess a distinctive phenotype characterized by pigmentation and hexagonal morphology2,3. These cells contribute to the blood-retinal barrier, thereby supporting photoreceptor health and maintaining retinal homeostasis4,5. Additionally, RPE cells play a critical role in vision by absorbing light and recycling essential components for the photoreceptors6. For instance, RPE65, a protein highly expressed in RPE cells, converts all-trans retinyl esters to 11-cis retinol7,8. Given the multitude of functions performed by RPE cells, their dysfunction is implicated in various diseases, including age-related macular degeneration and diabetic retinopathy9,10. To enhance the understanding of retinal pathologies and develop new treatments, in vitro models of the retina are frequently employed.
To generate representative models of healthy or diseased retinas, it is imperative to use a mimetic RPE cell type. The commercially available ARPE-19 cell line lacks native phenotypes, such as pigmentation, while iPSCs can take months to differentiate11,12,13. Although human donor eyes may be ideal, they are often not readily available to many research labs.
Here, we have devised a method to utilize porcine eyes, which share many similarities with human eyes14, for obtaining primary RPE cells. These primary porcine RPE cells have been utilized in multiple retinal models15,16. Not only are these cells cost-effective, but they also require less time to acquire than iPSCs or donor eyes. Additionally, they exhibit native characteristics, such as pigmentation and microvilli. While similar protocols for porcine RPE extraction exist17,18,19, this straightforward and detailed technique further validates enzymatic dissociation and employs materials commonly found in most cell culture laboratories.
Protocol
The eyes used in this procedure are obtained post-mortem from a local, USDA-inspected butcher shop, and no work is performed using live animals. After the animals have been sacrificed, approximately 2 h pass before the eyes are enucleated. As tissue decay may begin to occur, it is important to keep the eyes cool during transport to prevent further decay. In this procedure, the eyes are immediately placed into a refrigerator after enucleation. Subsequently, the bag of eyes is positioned inside a 1000 mL polypropylene beaker and surrounded by ice within an 8 L cooler. It is important not to place the eyes directly on the ice. Once the eyes arrive at the laboratory, the isolation of RPE cells is carried out within a biosafety cabinet. It is crucial to complete this process within 3-5 h. This protocol has been optimized for processing 10 eyes.
1. Preparing DNase stock aliquots
NOTE: It is recommended that this step is performed before the isolation.
- Prepare a 5 mM calcium chloride solution with sterile deionized water.
CAUTION: Calcium chloride powder can cause severe eye irritation or damage. Wear appropriate PPE. - Add 5 mM calcium chloride solution to DNase powder (see Table of Materials) to get a final DNase concentration of 3 mg/mL. Ensure the solution is mixed thoroughly.
CAUTION: DNase is a respiratory sensitization hazard. Do not inhale powder/substance. - Aliquot small volumes, for example 1 mL, into microcentrifuge tubes.
- Freeze aliquots at -20 °C until use.
2. Setting up the dissection area
- Transfer the sterile dissection instruments and cell culture equipment into the biosafety cabinet: surgical drape, Petri dishes, cell strainers, well plates, gauze, tweezers, scalpel handle, scalpel blade, and iris scissors (see Table of Materials).
- Using tweezers, lay out the surgical drape. Place one 6-well plate atop the surgical drape and remove the lid.
- Place an aliquot of sterile Dulbecco's Phosphate Buffered Saline (DPBS) on ice and into the biosafety cabinet.
- Fill one well of a 6-well plate halfway full (roughly 6 mL) of 10% povidone-iodine solution.
- Fill the remaining wells with chilled DPBS.
- Remove the lid from one of the Petri dishes and place it, inverted, atop the surgical drape. Set aside the Petri dish base as a waste container.
- Place an aliquot of cell culture media (Dulbecco's Modified Eagle Medium (DMEM), 10% Fetal Bovine Serum (FBS), 1% antibiotics/antimycotics) and an aliquot of 0.25% trypsin/EDTA into a 37 °C water bath.
- Remove the DNase stock solution from the freezer (see rest of protocol for more details).
3. Exterior tissue removal
NOTE: Exterior tissue removal can be performed outside of the biosafety cabinet. Inspect the eye prior to cutting to ensure that the sclera has not been punctured, the pupil is not foggy, and the optic nerve is present. Discard eyes not meeting these criteria.
- While holding the muscle attached to the eye with one hand, use the other hand to gently cut away the tissue surrounding the sclera with a scalpel. Be careful not to crush the eye or accidentally cut through the sclera. If the sclera is cut through and exposes the black interior, discard the eye.
CAUTION: A cut resistant glove is highly recommended for the hand holding the muscle to protect against accidental cuts. - Trim the optic nerve with curved iris scissors to a length of no greater than 3 mm. If the optic nerve is too long, the eye cup will not sit properly in the cell strainer later.
- Place the eye onto ice with the cornea down.
- Repeat steps 3.1-3.3 until the exterior tissue is removed from each eye.
4. Eye dissection
- Use tweezers to transfer an eye into the biosafety cabinet and into the well containing 10% povidone-iodine solution and rotate the eye to ensure that it is fully coated in the iodine solution. To keep the tools as sterile as possible, these tweezers should only be used for touching the exterior of the eye.
- While the eye is sitting in iodine solution, place a sterile gauze onto the shallow, inverted lid of the Petri dish prepared earlier. In a separate Petri dish, place a cell strainer. Only use the cell strainer to support the eye cup, not for cell separation.
- After allowing the eye to sit in iodine solution for roughly 30 s, wash the eye by dipping it into the wells containing DPBS for roughly 5 s each, moving from well to well to progressively dilute the iodine solution, and transfer the eye onto the sterile gauze.
- Make a small incision using a scalpel below the ora serrata, or roughly 1/3 down the globe from the iris edge.
- Following the incision, use iris scissors to cut evenly along the circumference of the globe, parallel to the cornea. When maneuvering the eye, only use the tweezers to touch the outside of the eye or the gauze to maintain sterility.
- Use the tweezers to grab the optic nerve and gently lift the globe away from the gauze. The vitreous should fall out of the globe. If needed, gently shake the eye to help dislodge the vitreous.
NOTE: If the vitreous is very difficult to remove, try cutting lower on the globe. - Gently place the eye cup into the prepared cell strainer, with the interior of the eye cup facing up. If uneven, use tweezers to adjust the eye cup to get the incision as level as possible.
- Gently add cooled DPBS to the eye cup so the fluid level is just below the lowest point of the incision. This DPBS will be removed following neural retina removal.
NOTE: The volume of DPBS added will vary depending on eye size and incision location. - Repeat steps 4.1-4.8 until all eyes are dissected.
5. Neural retina removal and RPE dissociation
NOTE: The following section is easier to perform in stages. In this set up, this process is performed on one batch (usually 3 eye cups) at a time. For steps 5.1-5.7, each step should be performed on the entire batch before moving to the next step.
- Under a flashlight, find any areas where the neural retina (white/pink) is starting to detach from the RPE (black). Then, use blunt tweezers to gently grab the lifted neural retina and peel it away.
NOTE: If there are no areas where the neural retina is detaching, use the tweezers to gently grab it near the top of the eye cup. Using this method, if damage is done to the RPE/choroid, those cells will not be dissociated during RPE collection. - Gently collect the neural retina near the optic disc.
- Aspirate out the neural retina using a Pasteur pipette attached to a vacuum aspiration system. It is recommended to feather the aspiration to help in the removal process.
NOTE: A pipette tip can be placed on the end of a Pasteur pipette to reduce suction. - Carefully add additional cooled DPBS to replace what was aspirated.
- Remove remaining neural retina by aspirating once more. This time, remove as much DPBS as possible while not disturbing the exposed RPE.
- Gently add trypsin to the eye cup. Keep the volume level below the incision line and any sections of damaged RPE to reduce contamination.
CAUTION: Trypsin is an enzyme that will cause skin and eye irritation on contact. Wear appropriate PPE. - After trypsin is added to each eye, ensure that the Petri dish lid is replaced. Next, transfer the Petri dish to a humidified incubator at 37 °C and 5% CO2 for 30 min.
- Repeat steps 5.1-5.7 for each batch until all eye cups have been processed.
6. RPE collection
- Collect the RPE cells from each batch of eyes (2-3 eyes) and place in a single 15 mL centrifuge tube to make the seeding process straightforward.
- Under a flashlight, gently triturate using a 1000 µL pipette to dislodge the RPE cells. If the RPE cells are not detaching, add fresh trypsin and incubate for an additional 10-15 min. Be careful not to scrape the retina.
- Collect the RPE cells in a 15 mL centrifuge tube.
- Wash the eye cup with cell culture media to collect any remaining cells and neutralize the trypsin. Ensure there is at least twice the volume of media to trypsin in the 15 mL centrifuge tube.
- Repeat step 6.1 until RPE cells have been collected from all eye cups.
- Centrifuge the cell suspensions at 250 x g for 5 min at room temperature.
7. Preparing DNase working solution
- Calculate the volume of 3 mg/mL DNase stock solution (prepared in step 1) needed to add to cell culture media for a final concentration of 100 µg/mL and final volume of 3 mL per tube of cells (i.e., for three centrifuge tubes of cells, 9 mL of 100 µg/mL DNase solution is required).
- Combine the appropriate volumes of DNase stock solution and cell culture media. Using DNase in the working solution helps prevent cell clumping and allows cells to be seeded evenly.
8. RPE cell seeding
- Following centrifugation in step 6.3, remove supernatants from each 15 mL centrifuge tube, careful not to disrupt the cell pellet.
- Resuspend each cell pellet in 3 mL of DNase working solution.
- Place the 15 mL centrifuge tubes containing the resuspended cells into a humidified incubator at 37 °C and 5% CO2 for 15 min.
- Following incubation, centrifuge the cell suspensions at 150 x g for 5 min at room temperature.
- Remove the supernatants, careful not to disrupt the cell pellets.
- Resuspend each cell pellet in 3 mL of fresh cell culture media.
- For each centrifuge tube containing 2-3 eyes worth of RPE cells, seed one well of a 6-well plate (passage 0).
- Carefully, transfer the seeded 6-well plate into a humidified incubator at 37 °C and 5% CO2.
9. Primary porcine RPE cell culture
- Allow the newly seeded RPE cells to attach for 48-72 h before moving the plate. During the first media change, a wash with fresh cell culture media can be performed to help remove excess cell debris.
- Replace the cell culture media every 48 h.
- When cells reach confluency, transition to a cell culture media with an FBS concentration of 2% and maintain until experimentation.
10. RPE cell validation
- Immunocytochemical (ICC) staining
- Fix cells in 4% formaldehyde in DPBS for 10 min at room temperature.
- Wash twice with DPBS.
- Permeabilize cells (if needed) using 0.2% Triton-X 100 in DPBS for 10 min at room temperature.
- Wash twice with DPBS.
- Apply blocking solution of 3% (w/v) bovine serum albumin (BSA) in DPBS for 1 h at room temperature.
- Add primary antibody at 1:100 dilution (or recommended manufacturer concentration) (see Table of Materials) and let sit for 1 h at room temperature or overnight at 4 °C.
- Wash three times with DPBS for 5 min each.
- Add secondary antibody (if needed) at 2 µg/mL (or recommended manufacturer concentration) (see Table of Materials) and let sit for 1 h at room temperature.
- Wash three times with DPBS for 5 min each if secondary antibody is applied.
- Add nuclear staining probe at manufacturer's concentration (see Table of Materials) and let sit for 25 min at room temperature.
- Wash three times with PBS for 5 min each.
- If on a cell culture insert, mount to microscope slide with a coverslip using mounting medium as outlined by Rickabaugh and Weatherston et al.20. Otherwise, image cells in DPBS.
- Scanning electron microscopy (SEM)
- Follow the procedure outlined by Harris et al.21.
- Trans Epithelial Electrical Resistance (TEER)
- Follow manufacturer's protocol for TEER measurement (see Table of Materials) with 1 mL of culture medium in the basal chamber15.
- Enzyme-linked immunosorbent assay (ELISA)
- Perform ELISA15 using a multiplex human enzyme-linked immunosorbent assay kit following the manufacturer's protocol (see Table of Materials).
Representative Results
Using this procedure, primary RPE cells were successfully isolated from porcine eyes. Figure 1A shows RPE cells 3 days post isolation with characteristic pigmentation. After 1 week of growth, cells were fully confluent and formed a healthy monolayer (Figure 1B). Cells were then transferred to cell culture inserts where they maintained their pigmentation and morphology (Figure 1C), further supporting the efficacy of the isolation procedure. Cultures that did not exhibit these characteristics and instead showed variant morphology and excessive pigmentation (Figure 1D) were suspected of cellular contamination and not used in experiments. RPE cells isolated in this manuscript express RPE65, a protein highly expressed in RPE cells (Figure 2). The areas where RPE65 expression appears lower are likely due to pigmentation blocking the fluorescence, as those areas correspond to more heavily pigmented cells (Figure 2A). Another characteristic protein expressed by the isolated RPE cells was VEGF. After 10 days, VEGF was expressed more on the basal side of the RPE monolayer with a concentration of 2612.5 ± 28.0 pg/mL compared to the apical side with a concentration of 2141.2 ± 53.5 pg/mL (Figure 3). Primary porcine RPE cells also have microvilli and cobblestone morphology (Figure 4). Additionally, when grown on cell culture inserts, TEER readings increased from 166.1 ± 75.9 Ω∙cm2 at 7 days to 363.4 ± 9.0 Ω∙cm2 after 14 days (Figure 5), indicating consistent, healthy tight junction formation over time22. To further investigate junction formation, RPE cells were analyzed for ZO-1 expression, which was localized to cell boundaries, implying healthy junction formation and monolayer integrity (Figure 6).
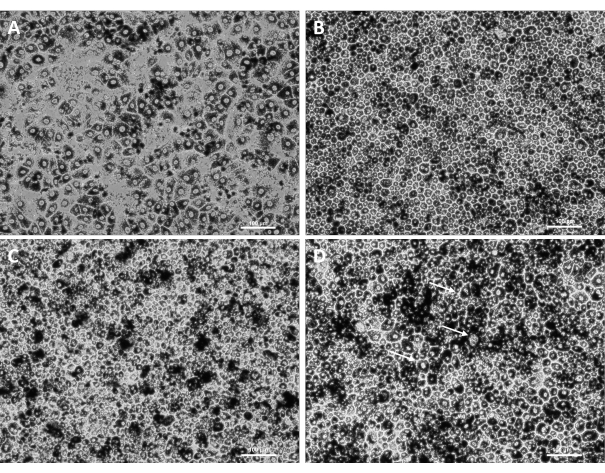
Figure 1: Primary RPE after being seeded onto well plates and subcultured. Healthy RPE cells 3 days post-isolation (A), fully confluent monolayer 7 days post-isolation (B), RPE successfully passaged onto a culture insert (C), and areas of potential cellular contamination (arrows) on an RPE culture (D). Scale bar = 100 µm. Please click here to view a larger version of this figure.
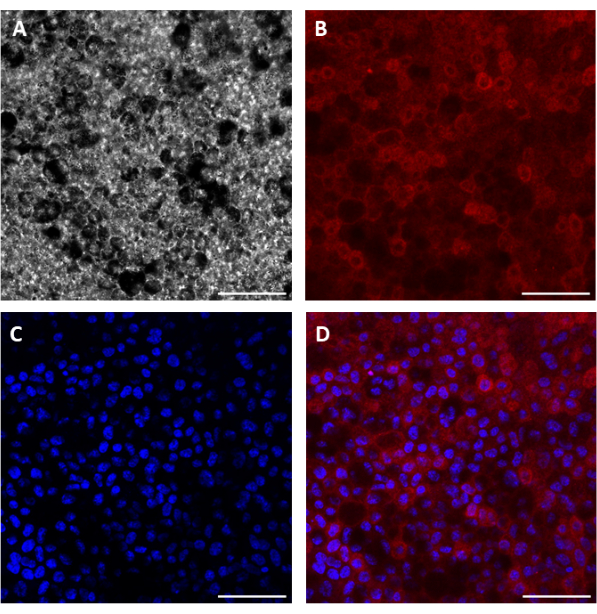
Figure 2: Immunocytochemical images showing RPE65 expression in porcine RPE cells. Brightfield (A), RPE65 (B), nuclei (C), and merged (D) images of cells 15 days post seeding. Note, the grainy background in the brightfield image is the porous culture insert membrane. Scale bar = 50 µm. Please click here to view a larger version of this figure.
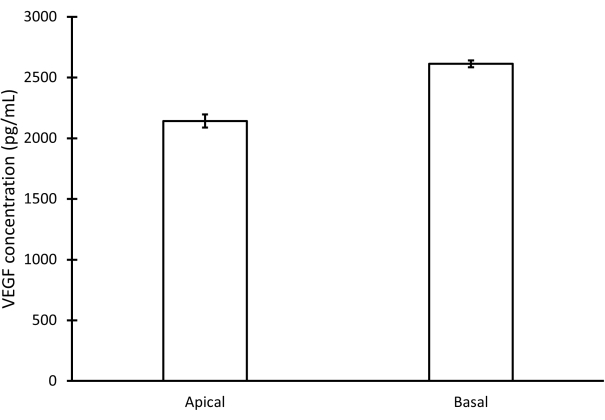
Figure 3: VEGF expression in apical and basal sides of the RPE monolayer. VEGF concentration was determined by an ELISA performed after 10 days of cell growth on culture inserts. Error bars represent standard deviation with n = 2. Please click here to view a larger version of this figure.
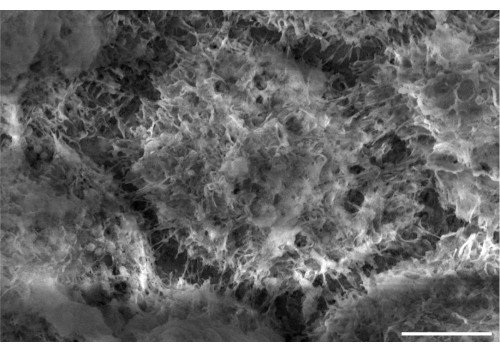
Figure 4: Scanning electron microscopy (SEM) image of primary porcine RPE cells. Cells were grown for 30 days before imaging. Scale bar = 5 µm. Please click here to view a larger version of this figure.
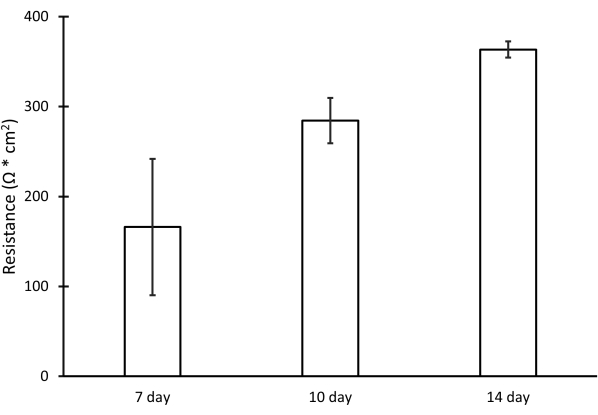
Figure 5: TEER readings performed on RPE cells cultured on cell culture inserts. Resistance values after 7, 10, and 14 days of growth. Error bars represent standard deviation with n = 3. Please click here to view a larger version of this figure.
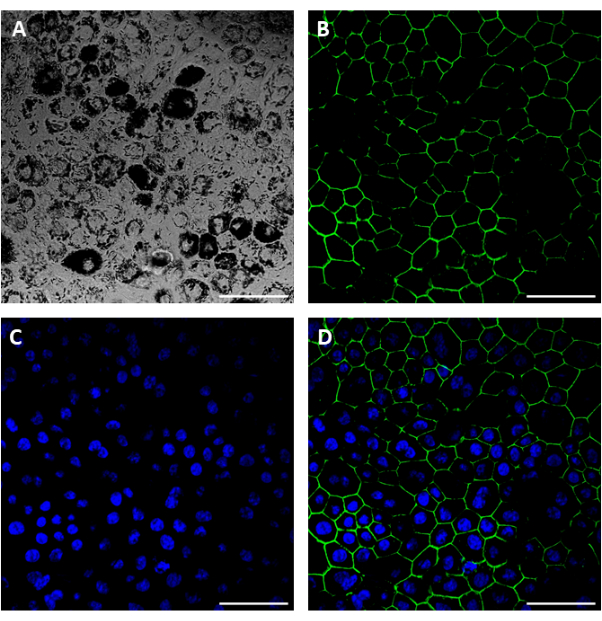
Figure 6: Immunocytochemical images showing ZO-1 expression in porcine RPE cells. Brightfield (A) ZO-1 (B), nuclei (C), and merged (D) images of cells 6 days post seeding on glass bottom well plates. Scale bar = 50 µm. Please click here to view a larger version of this figure.
Discussion
This protocol describes how to isolate RPE cells from porcine eyes. Pigmentation and cobblestone morphology are seen within 7 days of isolation (Figure 1B). Furthermore, TEER data indicate tight junction formation22 and a healthy monolayer (Figure 5). These results show that RPE cells isolated with this method are similar to human RPE and can be beneficial in retinal cell culture models.
The eyes used in this manuscript are obtained post-mortem from a local butcher shop, but could also be obtained from other sources, such as academic settings with collaborators using porcine products. It is important to begin the procedure as soon as possible after animal death and to keep the eyes cool prior to the procedure. During the isolation, it is essential to not disrupt the RPE until triturating after trypsinization. This care is most critical when peeling the neural retina, as a lack of caution may lead to grabbing Bruch's membrane1 with the tweezers and introducing other cell types into the solution.
While this procedure has been optimized for our conditions, some changes can be made to the methodology. For example, if specific seeding densities are desired, cells can be counted after the DNase incubation. Additionally, if vacuum aspiration is not working, the neural retina can be removed by carefully trimming with small, curved scissors. When seeding for experiments, a high seeding density such as 2.5 x 105 cells/cm2 can be used to prevent excess cellular division. Furthermore, repeated passaging is not recommended as the cells can begin to lose their native phenotypes. While this study has used passage 1 cells for experiments, additional passages could potentially be used if RPE phenotype and characteristics are maintained. Lastly, a concentration of 1% FBS can be used for subculture or experimentation instead of 2%, if desired.
There are a few drawbacks to this method. First, it is difficult to replicate or obtain animal age, sex, breed, and time of death, which are common problems in primary cell isolation. Second, over trypsinization can quickly result in abnormal cells, which has also been noted by Fietz et al17. However, ultimately, this method offers an inexpensive and efficient way to obtain primary RPE cells. These cells do not require lengthy differentiation times like iPSCs. These porcine RPE also innately have key structures, like microvilli and pigmentation15,16, unlike their non-differentiated counterparts. As such, these cells offer a more representative cell type for retinal studies. Lastly, this method could be very beneficial for more rural areas or institutions that do not have access to human donor eyes to produce retinal models analogous to human models to understand vision and disease.
Divulgazioni
The authors have nothing to disclose.
Acknowledgements
The authors would like to thank Farhad Farjood for help with porcine RPE cell culture and isolation and Thomas Harris for help with SEM. The authors acknowledge the support from the Microscopy Core Facility at Utah State University for the SEM analysis. The facility maintains a scanning electron microscope acquired through a National Science Foundation Major Research Instrumentation Grant (CMMI-1337932). Funding for this study was provided by a National Institutes of Health through Grant 1R15EY028732 (Vargis) and a BrightFocus Foundation Grant M2019109 (Vargis). Additional funding was provided by an Undergraduate Research and Creative Opportunities Grant (Weatherston) from Utah State University's Office of Research and a seed grant (Vargis) from Utah State University's Alzheimer's Disease and Dementia Research Center.
Materials
| 6 Micro-well glass bottom plate with 14 mm micro-well #1 cover glass | Cellvis | P06-14-1-N | |
| Antibiotic-Antimycotic (100x) | Gibco | 15240062 | |
| Biosafety Cabinet | |||
| Calcium Chloride, Dried, Powder, 97% | Alfa Aesar | L13191.30 | |
| Cell Strainer | Fisher Scientific | 22-363-548 | one per eye |
| Centrifuge | |||
| Centrifuge Tubes, 15 mL | Fisher Scientific | 05-539-12 | |
| Cooler, 8 L | Igloo | 32529 | |
| Corning Transwell Multiple Well Plate with Permeable Polyester Membrane Inserts | Fisher Scientific | 07-200-154 | Culture inserts |
| Cut Resistant Glove | Dowellife | 712971375857 | |
| Cytiva HyClone Dulbecco's Phosphate Buffered Saline, Solution | Fisher Scientific | SH3026401 | for ICC dilutions only |
| Deionized Water | |||
| DMEM, 1x with 4.5 g/L glucose, L-glutamine & sodium pyruvate | Corning | 10-013-CV | |
| DNase I from Bovine Pancreas | Sigma Aldrich | DN25 | |
| DPBS/Modified – calcium – magnesium | Cytiva | SH3002B.02 | stored at 4 °C |
| ELISA kit, Q-Plex Human Angiogenesis (9-Plex) | Quansys Biosciences, Logan, UT | ||
| ENDOHM 6 TEER device | World Precision Instruments | ||
| Fetal Bovine Serum (FBS) | Avantor | 232B20 | |
| Fisher BioReagents Bovine Serum Albumin (BSA) DNase- and Protease-free Powder | Fisher Scientific | BP9706100 | |
| Flashlight | |||
| Formaldehyde, ACS Grade, 36.5% (w/w) to 38.0% (w/w), LabChem | Fisher Scientific | LC146501 | |
| Gauze Sponges | Fisher Scientific | 22-415-504 | One per eye |
| Goat anti-Mouse IgG (H+L) Highly Cross-Adsorbed Secondary Antibody, Alexa Fluor Plus 647, Invitrogen | Thermo Scientific | A32728 | RPE65 secondary antibody |
| Ice | Crushed prefered | ||
| Inverted Phase Contrast Microscope | |||
| Invitrogen NucBlue Live ReadyProbes Reagent (Hoechst 33342) | Fisher Scientific | R37605 | |
| Iris Fine Tip Scissors, Standard Grade, Curved, 4.5" | Cole-Palmer | EW-10818-05 | |
| Iris Scissors, 11 cm, Straight, Tungsten Carbide | Fisher Scientific | 50-822-379 | |
| LSM-710 Confocal Microscope | Zeiss | ||
| Petri Dish, 100 mm x 20 mm | Corning | 430167 | one per 2-3 eyes and one for dissection surface/waste |
| Povidone-Iondine Solution, 10% | Equate | 49035-050-34 | |
| RPE65 Monoclonal Antibody (401.8B11.3D9), Invitrogen | Thermo Scientific | MA116578 | RPE65 primary antibody |
| Scalpel Blades Size 10 | Fisher Scientific | 22-079-683 | |
| Scalpel Handles Style 3 | Fisher Scientific | 50-118-4164 | |
| Surgical Drape, 18 x 26" | Fisher Scientific | 50-209-1792 | |
| Tissue Culture Incubator | 37 °C, 5% CO2, 95% Humidity | ||
| Tissue Culture Plates, 6 Wells | VWR | 10062-892 | One for eye wash and one for seeding |
| Tri-Cornered Polypropylene Beaker, 1000 mL | Fisher Scientific | 14-955-111F | |
| Triton X-100 | Sigma Aldrich | T8787 | |
| Trypsin 0.25%, 2.21 mM EDTA in HBSS; w/o Ca, Mg, Sodium Bicarbonate | Corning | 25053Cl | |
| Tweezers Style 20A | Fisher Scientific | 17-467-231 | |
| Tweezers Style 2A | Fisher Scientific | 50-238-47 | for removing neural retina |
| Tweezers Style 5-SA-PI | Fisher Scientific | 17-467-168 | |
| Vacuum Aspiration System | |||
| Water Bath, 37 °C | |||
| ZO-1 Monoclonal Antibody (ZO1-1A12), FITC, Invitrogen | Fisher Scientific | 33-911-1 | ZO-1 conjugated primary antibody |
Riferimenti
- Booij, J. C., Baas, D. C., Beisekeeva, J., Gorgels, T. G. M. F., Bergen, A. A. B. The dynamic nature of Bruch’s membrane. Progress in Retinal and Eye Research. 29 (1), 1-18 (2010).
- Caceres, P. S., Rodriguez-Boulan, E. Retinal pigment epithelium polarity in health and blinding diseases. Current Opinion in Cell Biology. 62, 37-45 (2020).
- Georgiadis, A., et al. The tight junction associated signalling proteins ZO-1 and ZONAB regulate retinal pigment epithelium homeostasis in mice. PLOS One. 5 (12), e15730 (2010).
- Sparrow, J. R., Hicks, D., Hamel, C. P. The retinal pigment epithelium in health and disease. Current Molecular Medicine. 10 (9), 802-823 (2010).
- Strauss, O. The retinal pigment epithelium in visual function. Physiological Reviews. 85 (3), 845-881 (2005).
- Yang, S., Zhou, J., Li, D. Functions and diseases of the retinal pigment epithelium. Frontiers in Pharmacology. 12, 727870 (2021).
- Moiseyev, G., Chen, Y., Takahashi, Y., Wu, B. X., Ma, J. RPE65 is the isomerohydrolase in the retinoid visual cycle. Proceedings of the National Academy of Sciences. 102 (35), 12413-12418 (2005).
- Uppal, S., Liu, T., Poliakov, E., Gentleman, S., Redmond, T. M. The dual roles of RPE65 S-palmitoylation in membrane association and visual cycle function. Scientific Reports. 9 (1), 5218 (2019).
- Somasundaran, S., Constable, I. J., Mellough, C. B., Carvalho, L. S. Retinal pigment epithelium and age-related macular degeneration: A review of major disease mechanisms. Clinical & Experimental Ophthalmology. 48 (8), 1043-1056 (2020).
- Xu, H. Z., Song, Z., Fu, S., Zhu, M., Le, Y. Z. RPE barrier breakdown in diabetic retinopathy: seeing is believing. Journal of Ocular Biology, Diseases, and Informatics. 4 (1-2), 83-92 (2011).
- Hellinen, L., et al. Characterization of artificially re-pigmented ARPE-19 retinal pigment epithelial cell model. Scientific Reports. 9 (1), 13761 (2019).
- Hazim, R. A., Volland, S., Yen, A., Burgess, B. L., Williams, D. S. Rapid differentiation of the human RPE cell line, ARPE-19, induced by nicotinamide. Experimental Eye Research. 179, 18-24 (2019).
- Samuel, W., et al. Appropriately differentiated ARPE-19 cells regain phenotype and gene expression profiles similar to those of native RPE cells. Molecular Vision. 23, 60-89 (2017).
- Middleton, S. Porcine ophthalmology. Veterinary Clinics of North America: Food Animal Practice. 26 (3), 557-572 (2010).
- Farjood, F., Ahmadpour, A., Ostvar, S., Vargis, E. Acute mechanical stress in primary porcine RPE cells induces angiogenic factor expression and in vitro angiogenesis. Journal of Biological Engineering. 14, 13 (2020).
- Fietz, A., Hurst, J., Joachim, S. C., Schnichels, S. Establishment of a primary porcine retinal pigment epithelium monolayer to complement retinal ex vivo cultures. STAR Protocols. 4 (3), 102443 (2023).
- Hood, E. M. S., Curcio, C. A., Lipinski, D. Isolation, culture, and cryosectioning of primary porcine retinal pigment epithelium on transwell cell culture inserts. STAR Protocols. 3 (4), 101758 (2022).
- Toops, K. A., Tan, L. X., Lakkaraju, A. A detailed three-step protocol for live imaging of intracellular traffic in polarized primary porcine RPE monolayers. Experimental Eye Research. 124, 74-85 (2014).
- Rickabaugh, E., Weatherston, D., Harris, T. I., Jones, J. A., Vargis, E. Engineering a biomimetic in vitro model of bruch’s membrane using hagfish slime intermediate filament proteins. ACS Biomaterials Science & Engineering. 9 (8), 5051-5061 (2023).
- Harris, T. I., et al. Utilizing recombinant spider silk proteins to develop a synthetic bruch’s membrane for modeling the retinal pigment epithelium. ACS Biomaterials Science & Engineering. 5 (8), 4023-4036 (2019).
- Zou, X. L., et al. Protection of tight junction between RPE cells with tissue factor targeting peptide. International Journal of Ophthalmology. 11 (10), 1594-1599 (2018).

