Summary
This manuscript presents a protocol for surgically removing the postganglionic lumbar sympathetic neurons from a mouse. This procedure will facilitate a multitude of studies aimed at investigating the role of sympathetic innervation in distal tissue targets.
Abstract
Peripheral nerve injuries are common, and full functional recovery after injury is achieved in only 10% of patients. The sympathetic nervous system plays many critical roles in maintaining bodily homeostasis, but it has rarely been studied in the context of peripheral nerve injury. The extent of postganglionic sympathetic neuronal functions in distal targets in the periphery is currently unclear. To better explore the role of sympathetic innervation of peripheral targets, a surgical "knock-out" model provides an alternative approach. Although this can be achieved chemically, chemical destruction of postganglionic sympathetic neurons can be nonspecific and dose-dependent. The use of a surgical lumbar sympathectomy in mice, once thought to be "virtually not practicable" in small animals, allows for specific targeting of postganglionic sympathetic neurons that innervate the hind limbs. This manuscript describes how to surgically remove the L2-L5 lumbar sympathetic ganglia from a mouse as a survival surgery, which reliably decreases the hind paw sweat response and the number of sympathetic axons in the sciatic nerve.
Introduction
Peripheral nerve injuries (PNIs) can lead to motor, sensory, and sympathetic deficits in distal tissue targets that rarely fully functionally recover1. PNI research has often focused on the motor and sensory regeneration; however, nearly one-quarter of the rat sciatic nerve consists of unmyelinated sympathetic axons2. The role of sympathetic innervation in the peripheral tissues, nevertheless, is not fully understood3. The sympathetic nervous system plays a major role in maintaining bodily homeostasis, participating in immune regulation, thermoregulation, vascular tone, mitochondrial biogenesis, and more4,5,6,7,8,9,10,11. When sympathetic innervation at the neuromuscular junction is lost, persistent muscle weakness and synaptic instability are observed despite the maintenance of motoneuron innervation12. This sympathetic regulation of synaptic transmission at the neuromuscular junction has been shown to decline with aging13,14, which contributes to sarcopenia, defined as the age-dependent reduction in muscle mass, force, and power15. A better understanding of the role of sympathetic innervation of peripheral tissues is necessary for the development of therapies that will optimize functional outcomes for patients with PNIs and other forms of sympathetic dysfunction.
A sympathectomy is a powerful experimental tool that will allow for investigations of the role of sympathetic innervation in distal target tissues. Specifically, removal of the L2-L5 level sympathetic ganglia removes a majority of the sympathetic innervation to the lower limbs, which is especially useful for investigators interested in the sciatic nerve.
This protocol details the removal of L2-L5 level postganglionic sympathetic neurons from a mouse as a survival surgery. This procedure requires rodent microsurgical skills and familiarity with mouse anatomy, and when performed effectively, does not cause any visible phenotypic differences. A surgical lumbar sympathectomy has been used in rodent research, more so in rats than in mice16,17,18,19,20,21; however, a detailed protocol describing the protocol does not currently exist. Previous studies utilizing the lumbar sympathectomy have primarily focused on the role of sympathetic innervation in the pain response, which is generally attenuated by sympathectomy in various nerve injury models. Fewer studies have used this technique in mice22, likely due to the smaller size of anatomic landmarks, as the use of surgical sympathectomy was believed to be "virtually not practicable" in small animals23,24. Localized sympathectomies in the form of microsympathectomies have also been utilized in rodent models, also mostly in the context of pain behaviors25,26,27. The microsympathectomy, in contrast to the total lumbar sympathectomy, utilizes a dorsal approach through which a segment of the gray ramus to a specific spinal nerve is disconnected and removed, allowing for a very targeted sympathectomy that will avoid wider spread side effects.
Because mouse models are critical for many studies requiring genetic manipulation, this procedure will have versatile applications beyond the breadth of peripheral nerve injuries as well. Using a transabdominal approach, the lumbar sympathetic ganglia can be reliably visualized and resected from the mouse with no apparent adverse effects. Although protocols for the chemical destruction of postganglionic sympathetic neurons are available, such as the use of 6-hydroxydopamine (6-OHDA)23,24, this surgical procedure allows for anatomically specific targeting of the postganglionic lumbar sympathetic ganglia. The use of a surgical sympathectomy also avoids the nonspecific and dose-dependent concerns related to pharmacological methods28,29.
The use of chemical sympathectomies via administration of 6-OHDA was described in 1967 as a simple way to achieve selective destruction of adrenergic nerve endings since surgical sympathectomies in small animals were not favored23,24. 6-OHDA is a catecholaminergic neurotoxin that is endogenously formed in patients with Parkinson's disease, and its toxicity is derived from its ability to form free radicals and inhibit the electron transport chain in mitochondria30,31. Through norepinephrine uptake-1 transport mechanisms, 6-OHDA is able to accumulate within noradrenergic neurons, such as postganglionic sympathetic neurons28. Eventually, the neuron is destroyed by 6-OHDA; however, terminals in the peripheral nervous system do regenerate, with the restoration of functional activity even when the amine levels are still reduced. Different dosage thresholds are also present for different organs in response to 6-OHDA, and higher doses of 6-OHDA have been shown to exhibit more nonspecific effects, extending its neurotoxic consequences to non-catecholamine-containing neurons and even non-neuronal cells. Aside from noradrenergic neurons, dopaminergic neurons are affected by 6-OHDA as well29, making the chemical sympathectomy ultimately less specific to postganglionic sympathetic neurons than the surgical sympathectomy.
Therefore, a surgical lumbar sympathectomy enables the targeted ablation of the sympathetic innervation to the lower limbs, which can be combined with a variety of experimental techniques and genetic manipulations in the mouse to study how the sympathetic nervous system contributes to various injury and disease states.
Protocol
All experiments were approved by the Institutional Animal Care and Use Committee (IACUC) of Emory University (under the IACUC protocol number PROTO201700371). Four adult female wild-type C57BL/6J mice, aged 14 weeks and weighing between 16-21 g, were used in this study. The details of the reagents and equipment used here are listed in the Table of Materials.
1. Presurgical preparation
- Autoclave the surgical tools: 1 pair of sharp scissors, 2 fine-tipped tweezers, 1 needle driver.
- Warm a heating pad to 37 °C and place it under the surgical tabletop.
- Spray the surgical area, including the anesthesia induction chamber, with disinfectant and wipe it down thoroughly with paper towels.
- Ensure proper gowning and hand washing prior to handling the mouse.
- Feed the mouse with meloxicam at 5 mg/kg body weight, and allow plenty of time for the mouse to completely swallow the medicine to avoid aspiration during induction.
- Induce the mouse under anesthesia with 3% isoflurane in 1 L/min of oxygen (following institutionally approved protocols).
- Once the mouse is anesthetized (no reaction to toe pinch), place the mouse on the surgical tabletop, supine, with their nose in a fitted nose cone.
- Once the mouse is properly fitted in the nose cone, adjust the isoflurane to 2% for maintenance of anesthesia.
- Place eye gel on the mouse's eyes to prevent keratoconjunctivitis sicca (dry eye).
- Ensure the mouse is properly secured with surgical tape to the surgical tabletop.
- Monitor continuously for ease of breathing and adequate breathing rate.
- Shave the fur from the mouse's abdomen from the level of the genitals to below the ribs.
- Once the fur is removed, first wipe the surgical area in a circular motion, from central to peripheral, with 70% ethanol. Then, wipe the surgical area with betadine in the same circular motion. Repeat both the ethanol and betadine steps a total of 3 times.
- Drape the mouse with a sterile surgical drape with an appropriately sized hole cut out of the middle to visualize the surgical field. The rhomboid shape can be made by folding the drape in half and cutting an isosceles triangle with a height of ~10 mm and a base of ~15 mm.
2. Incisions
- Using a pair of sharp scissors and fine-tipped tweezers, create a midline incision from ~1 mm above the level of the pubic symphysis to ~2 mm below the ribs.
- Identify the midline fascia (linea alba) between the bilateral rectus abdominis muscles. Using the tweezers, lift the abdominal muscles away from the underlying organs and cut along the linea alba to enter the abdominal cavity.
NOTE: The linea alba can be identified by gently pulling the bilateral rectus abdominus muscles laterally to reveal a thinner fascia line that lies longitudinally along the midline32. Making the incision through the linea alba allows the muscle to be closed more easily. - Retract the abdominal muscles and skin with 5-0 sutures laterally to visualize the next steps properly.
3. Identification of the L2-L5 lumbar sympathetic ganglia
- The lumbar sympathetic ganglia lie posterior to the abdominal aorta and inferior vena cava. To visualize the aorta, partially remove the intestines from the abdominal cavity.
NOTE: The L2-L5 level sympathetic ganglia traverse from the level of the aortic bifurcation into the iliac vessels to the level of the left renal artery21,33.- Lay a sterile cotton square saturated in sterile saline on the drape, lying superiorly and to the left of the incision. Carefully, with 2 sterile cotton-tipped applicators, push the colon and small intestine partially out onto the saline-soaked cotton square.
NOTE: Ensure that the cecum and appendix are out of the abdominal cavity and that the descending colon can be visualized. - Cover the exposed intestines with another saline-saturated cotton square.
- Periodically monitor peristalsis throughout the surgery, which can be identified by rhythmic movement of the intestines.
- Lay a sterile cotton square saturated in sterile saline on the drape, lying superiorly and to the left of the incision. Carefully, with 2 sterile cotton-tipped applicators, push the colon and small intestine partially out onto the saline-soaked cotton square.
- Identify the descending colon, which is the portion of the intestine that is traveling towards the rectum and anus and may contain visible fecal matter, and deflect this structure to the left with closed tweezers to reveal the abdominal aorta and inferior vena cava. These two vessels are bound by connective tissue and should move as one unit.
- With a pair of tweezers, lift the abdominal vessels by their surrounding connective tissue. While this is lifted, carefully place a 5-0 nylon suture through the connective tissue.
CAUTION: Do NOT puncture the abdominal vessels with the needle: This will lead to rapid decompensation of the mouse and likely death. - Once the suture has been inserted through the connective tissue, deflect the abdominal vessels to the left. This will give a triangular window, allowing direct visualization of the bilateral psoas muscles.
NOTE: Due to the intimate connections that the ganglia have with the abdominal vessels, the sympathetic ganglia, though typically located midline, will be deflected slightly to the left (mouse's right) along with the vessels. Once the abdominal vessels are deflected, one should visualize a triangle, with the two sides on the left consisting of the deflected abdominal vessels and the right side defined by the mouse's midline (between the bilateral psoas muscles). The sympathetic ganglia lie in the middle of this triangle (Figure 1A). They travel vertically side-by-side and appear translucent. They may overlap a small vessel that dives into the mouse's midline and lies flat on its right psoas muscle. Use tweezers to bluntly dissect any overlying fascia to reveal the ganglia (Figure 1B).
- With a pair of tweezers, lift the abdominal vessels by their surrounding connective tissue. While this is lifted, carefully place a 5-0 nylon suture through the connective tissue.
- Identify the left renal artery and the left testicular or ovarian artery.
NOTE: These are large vessels that will form a quadrangle, the sides of which consist of the left renal artery superiorly, the animal's midline and abdominal aorta/inferior vena cava laterally, and the left testicular or ovarian artery inferiorly. The L2 level ganglia are large and lie within this quadrangle (Figure 1A). Carefully blunt dissect to enter this quadrangle with tweezers and identify the bilateral L2 ganglia (Figure 1B). - Once the lower and upper parts of the ganglia are identified, grab the inferior aspect of the visible bilateral ganglia with a pair of tweezers and pull upwards. Visualization of more inferiorly-located ganglia, such as L4 and L5, can be increased by gently pushing any organs obstructing the view inferiorly with the second pair of tweezers.
NOTE: Visualizing the midline perforating vessel mentioned in step 3.2.2 will ensure a more complete lumbar sympathectomy. Holding a second pair of tweezers in the other hand, pull the fascia and neuronal connections holding the ganglia in the abdomen away while simultaneously pulling up on the ganglia themselves with the first pair of tweezers. Only one pair of tweezers should hold onto the ganglia, while the other clears the fascia and neuronal connections. Once at the level of the left testicular or ovarian artery, it may be helpful for L2 identification if the lower chain ganglia are still intact, as the L2 ganglia will move when pulled by the lower chain. The L3-5 ganglia can be broken at the testicular or ovarian artery before extracting the L2 ganglia from the quadrangle with tweezers. The only ganglia present within the quadrangle are the L2 ganglia. - This procedure should have minimal blood loss. If bleeding occurs, ensure adequate hemostasis prior to closure.
4. Skin closure
- After adequate hemostasis is achieved, remove the suture holding the abdominal vessels in place.
- Using 2 sterile cotton-tipped applicators, carefully replace diverted intestines into the abdominal cavity.
- With a 5-0 absorbable suture, perform a running stitch to approximate the abdominal muscles.
- With 5-0 nylon suture, use simple interrupted stitches to close the skin.
- Apply a generous layer of antibiotic ointment, such as Neosporin, to the incision site.
- Place the mouse in a clean cage on a heating pad.
- Observe the mouse every 15 min until it is awake and ambulatory. This usually takes less than 30 min.
5. Pilocarpine sweat assay
NOTE: To assess for depletion of sympathetic functional activity following a lumbar sympathectomy, a pilocarpine sweat assay was utilized 7 days post-lumbar sympathectomy.
- Prepare a 1% pilocarpine hydrochloride solution in 0.9% NaCl alongside a mixture of 10% potato starch in castor oil.
- Using a paintbrush, cover the plantar surface of the foot with betadine. Allow this layer to dry completely.
- Once the betadine layer is dry, use a separate paintbrush to apply a layer of 10% starch in castor oil.
- Administer 0.25 µL/g body weight of 1% pilocarpine subcutaneously using a syringe into the loose skin over the neck. Start a timer immediately after pilocarpine administration.
- At 8 min post-injection, take photos of the plantar surface of the foot.
- Using Fiji, count the number of dark spots located on all six (6) footpads (Figure 2A).
6. Immunohistochemistry
NOTE: To assess the degeneration of sympathetic axons in the peripheral nerves post-lumbar sympathectomy, the bilateral sciatic nerves were harvested on postoperative day 21.
- Anesthetize the mice (following step 1 of the protocol), harvest the bilateral sciatic nerves, and then promptly euthanize the animal (following institutionally approved protocols).
- Place the sciatic nerves directly into 4% paraformaldehyde in 0.01 M phosphate-buffered saline (PBS) for 20 min, then transfer them to 20% sucrose in 0.1 M PBS for overnight cryoprotection at 4 °C.
- Section the sciatic nerves longitudinally using a cryostat at 20 µm, and place the sections on charged slides.
- Block the nerve sections with 10% normal goat serum (NGS) in tris buffered saline with 1% Tween 20 (TBST) for 1 h at room temperature.
- Remove the blocking buffer and replace it with the primary antibodies rabbit anti-tyrosine hydroxylase and chicken anti-neurofilament-heavy diluted in the blocking buffer (10% NGS in TBST) at 1:750 and 1:1000, respectively. Allow the primary antibodies to incubate overnight in a humidity chamber at room temperature.
- Wash slide with TBST 3 times, 10 min each wash, before applying the secondary antibodies goat anti-rabbit 647 and goat anti-chicken 488, both diluted at 1:200 in the blocking buffer. Allow the secondary antibodies to incubate in a humidity chamber for 2 h at room temperature.
- Wash the slide with TBST 4 times, 10 min each wash, and allow the slide to dry in a light-protected area prior to mounting.
- Image nerve sections at least 40 µm apart on a fluorescent microscope on the 10x objective.
- On Fiji, straighten the nerve sections and draw three randomly placed vertical lines spanning the width of the section. Count the number of axons crossing each vertical line and divide that by the width of the section at the vertical line. Repeat for each of the three vertical lines per section. Average the three values obtained per section, and further average the values of the three sections per nerve.
Representative Results
This protocol describes the surgical removal of postganglionic lumbar sympathetic neurons from a mouse. Two mice received lumbar sympathectomies, and two mice served as controls. To achieve a successful surgical lumbar sympathectomy, adequate visualization of at least the L2 and L3 bilateral lumbar sympathetic ganglia must be achieved, as seen in Figure 1. Removal of the L4 and L5 ganglia would achieve complete sympathetic denervation of the lower body; however, visualization of the lower ganglia may be obstructed by the urogenital organs. Previous retrograde tracing studies have shown that the majority of neurons in the L2-L5 ganglia are located in the L2 and L3 ganglia. Although only female, age-matched mice were used to achieve the representative results, this surgical procedure has been reproduced many times in both male and female mice with no significant anatomical differences observed in terms of the locations of the testicular or ovarian artery and the sympathetic ganglia.
Upon removal of the ganglia, the sweating response in response to pilocarpine rapidly decreases, with significant differences seen by postoperative day 7 (Figure 2). Postoperative day 7 was chosen due to the exponential decrease in sympathetically-mediated sweating activity within 7 days after various sciatic nerve injuries in rats34. Because axon degeneration can take up to 14 days to complete and due to potential difficulty visualizing the L4 and L5 ganglia intraoperatively (bladder and genital organs may be obstructing the view), the sweating response may not be completely ablated35,36.
The tyrosine hydroxylase-positive (TH+) axon density was significantly decreased by postoperative day 21 with no change in the neurofilament-heavy chain (NF-H) density (Figure 3). TH+ axons are unlikely to be completely depleted due to the presence of TH+ sensory axons innervating low threshold mechanoreceptors and potential difficulties visualizing the full lumbar sympathetic chain37.
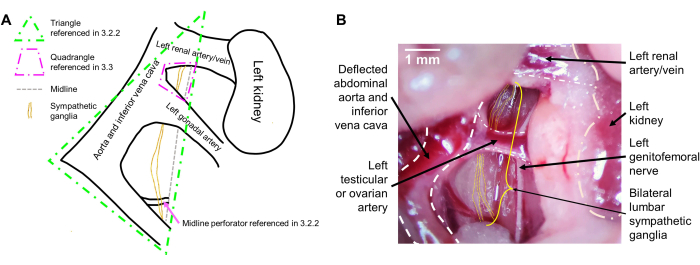
Figure 1: Intraoperative view of the lumbar sympathetic ganglia. (A) Schematic with expected landmarks. (B) Representative intraoperative image with labeled landmarks. The lumbar sympathetic ganglia are located behind the descending abdominal aorta and inferior vena cava (deflected). The ganglia are pulled to the mouse's right with the deflection of the abdominal vessels and are located on the right psoas, traversing inferiorly and superiorly. The bilateral L2 ganglia can be visualized between the left renal artery and the left testicular or ovarian artery, and the L3 ganglia are seen inferiorly to the left testicular or ovarian artery. Scale bar: 1 mm. Please click here to view a larger version of this figure.
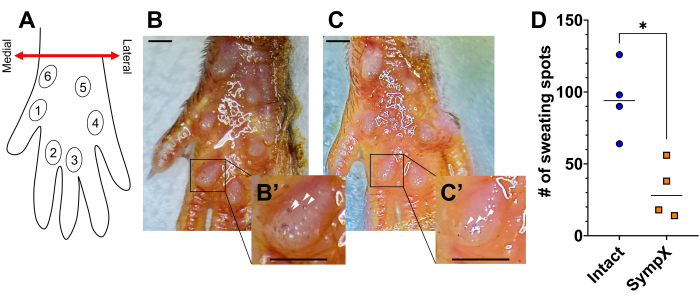
Figure 2: Surgical lumbar sympathectomy reduces the number of sweating spots on the hind paw. (A) Schematic of footpad locations on the plantar surface of the mouse's hind paw. (B) A representative image of the sweating response 8 min after the injection of pilocarpine in an intact animal. (B') Zoomed-in image with white arrows indicating a select number of sweating spots. (C) A representative image of the sweating response 8 min after the injection of pilocarpine in a sympathectomized animal on postoperative day 7. (C') Zoomed-in image with white arrows indicating a select number of sweating spots. (D) The total number of sweating spots on all six footpads of the hind paw in intact versus sympathectomized mice. Data is shown with the line representing the median. Mann-Whitney test. *p < 0.05. Scale bars: 1 mm. Please click here to view a larger version of this figure.
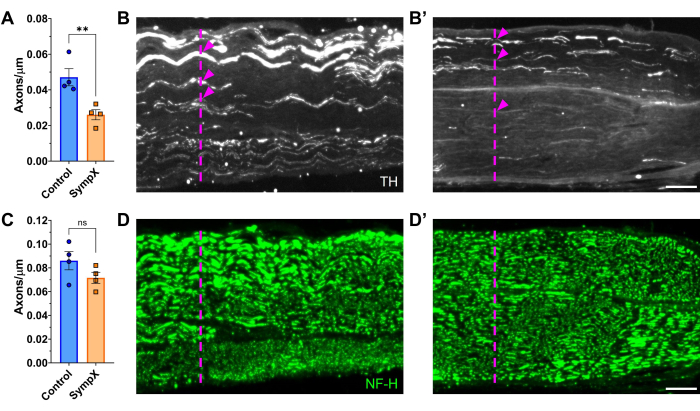
Figure 3: Surgical lumbar sympathectomy (Sympx) reduces the number of sympathetic axons in the sciatic nerve. (A) Tyrosine hydroxylase (TH)-positive axons per µm of the sciatic nerve (width of the nerve) on postoperative day 21. Representative nerve sections of TH staining in an intact (B) and sympathectomized (B') mouse. (C) Neurofilament-heavy chain (NF-H)-positive axons per µm of the sciatic nerve. Representative nerve section of NF-H staining in an intact (D) and sympathectomized (D') mouse. Magenta dashed lines represent possible randomly placed vertical lines that span the width of the nerve section used to calculate the axon densities in the nerve. Magenta arrows indicate a select number of axons that cross the randomly placed vertical lines. Data is shown as mean ± SEM. Unpaired t-test. **p < 0.01. Scale bars: 100 µm. Please click here to view a larger version of this figure.
Discussion
The lumbar sympathetic ganglia are very small structures located behind many critical abdominal organs and large vessels. Therefore, this procedure requires significant precision and accuracy. Much of the difficulty lies in identifying the sympathetic ganglia intraoperatively. It is suggested that the learner first be able to identify the ganglia in a mouse cadaver prior to attempting this procedure in a live mouse. Troubleshooting will often need to occur when identifying the sympathetic ganglia after the diversion of the intestines. To ensure adequate visualization, the descending colon must be visible. The intestinal diversion outside of the abdominal cavity must include the cecum. Additionally, the bladder may be full and enlarged, which can obstruct the view of the descending colon and, subsequently, the descending abdominal aorta and inferior vena cava. Ideally, the bladder is manually expressed once the mouse is under anesthesia prior to making the initial skin incision. However, should the bladder not be expressed and the intraoperative view be blocked, gentle expression with 2 sterile cotton-tipped applicators is possible. Take care not to damage the ureters, which are attached to the posterior aspect of the bladder. Once the abdominal aorta and inferior vena cava are identified posterior to the descending colon, adequate deflection and stabilization of the vessels is critical for visualization of the sympathetic ganglia. The fascia is relatively tough, and if the suture is placed solely through the layer of fascia and neuronal connections and not through the vessel walls, the vessels should be pulled at least 1 mm from the midline. The fascia overlying the sympathetic ganglia can be gently dissected away with tweezers. Should any small vessel break during the procedure, apply pressure with a sterile cotton-tipped applicator for at least 10 s to ensure adequate hemostasis before closure. If the large abdominal vessels are punctured during the surgery, the mouse should be quickly euthanized.
Although this method allows for direct visualization and removal of the sympathetic ganglia, thus allowing for more specific targeting of these postganglionic sympathetic neurons compared to chemical sympathectomies23, there are some limitations. This surgery results in the removal of the L2-L5 lumbar sympathetic ganglia; however, due to the visual obstruction caused by vital urogenital organs, the lowest ganglia, such as the L4-L5 level ganglia, are more difficult to visualize and remove during this procedure. The majority of the neurons innervating the lower limbs are located in the large L2 ganglia. The L5 ganglia are extremely small and contain fewer neurons, as seen in previous fluorescent retrograde tracing experiments38; however, failure to remove these neurons may distort the analysis of results from the most distal structures, such as the feet. To ensure better results when studying the distal targets in the feet, it would be advisable to check the extracted tissue to count the number of ganglia that have been removed. This can be done via a fluorescent reporter mouse in which the postganglionic sympathetic neurons can easily be visualized or via a fluorescent retrograde tracer injected into the distal target prior to the surgery. Some fluorescent reporter mice for this purpose include the ThCre:mTmG (see Table of Materials), which has extremely sparse labeling of tyrosine hydroxylase-positive neurons39, the Phox2bCre:tdTomato (see Table of Materials), which has moderate labeling of postganglionic sympathetic neurons40,41, and the ThCre:tdTomato (see Table of Materials), which has extensive labeling of the postganglionic sympathetic neurons42. This is not an exhaustive list of available lines.
Additionally, due to the large incision required for this surgical procedure, mice may need a longer recovery period before further experiments can be performed. Mice should be monitored for at least 3 days for proper defecation, urination, and feeding. Furthermore, improper hemostasis can lead to unaddressed intraabdominal bleeding, which can result in mouse fatality at unexpected time points. Therefore, ensuring that hemostasis is achieved prior to surgical closure is critical for the success of future experiments. The sympathetic neurons can also be targeted chemically, as aforementioned24.
The use of surgical sympathectomies will allow for a multitude of studies that aim to investigate the role of postganglionic sympathetic innervation in distal targets, such as the role of sympathetic innervation at the neuromuscular junction12. Additionally, fluorescent retrograde tracing techniques can be employed to quantify sympathetic reinnervation of distal tissues as this protocol can be adapted to extract the ganglia en bloc from paraformaldehyde-fixed mice38. In the context of peripheral nerve injuries, this surgical "knock-out" model will allow for better characterization of expected sympathetic functional recovery in previously innervated tissues.
Divulgazioni
The authors have nothing to disclose.
Acknowledgements
This work was supported by the NIH National Institute of Neurological Disorders and Stroke under award number K01NS124912 and in part by a developmental grant from the NIH-funded Emory Specialized Center of Research Excellence in Sex Differences U54AG062334 and the Medical Scientist Training Program of Emory University School of Medicine. Thank you to David Kim, postbaccalaureate, for sectioning sciatic nerves and to HaoMin SiMa, research specialist, for 3D printing a phone mount for our stereo microscope that allowed for the filming of the video.
Materials
| 5-0 absorable suture | CP Medical | 421A | |
| 5-0 nylon suture | Med-Vet International | MV-661 | |
| 70% ethanol | Sigma-Aldrich | E7023-4L | |
| Anesthesia Induction Chamber | Kent Scientific VetFlo | VetFlo-0530XS | |
| Anesthesia Vaporizer | Kent Scientific VetFlo | 13-005-202 | |
| Betadine | HealthyPets | BET16OZ | |
| C57BL/6J mice | Jackson Laboratory | #000664 | |
| Chicken anti-neurofilament-heavy | Abcam | ab72996 | |
| Cryostat | Leica | CM1850 | |
| Data Analysis Software | Prism | ||
| Eye lubricant | Refresh | Refresh P.M. | |
| Fine-tipped tweezers | World Precision Instruments | 500233 | |
| Fluorescent microscope | Nikon | Ti-E | |
| Goat anti-chicken 488 | Invitrogen | A32931 | |
| Goat anti-rabbit 647 | Invitrogen | A21245 | |
| Heating pad | Braintree Scientific | 39DP | |
| Image Analysis Software | Fiji | ||
| Imaging Software | Nikon | NIS-Elements | |
| Isoflurane | Med-Vet International | RXISO-250 | |
| Meloxicam | Med-Vet International | RXMELOXIDYL32 | |
| Needle driver | Roboz Surgical Store | RS-7894 | |
| Normal Goat Serum | Abcam | ab7481 | |
| Phox2bCre:tdTomato mutant mice | Jackson Laboratory | #016223, #007914 | |
| Pilocarpine hydrochloride | Sigma-Aldrich | P6503 | |
| Rabbit anti-tyrosine hydroxylase | Abcam | ab112 | |
| Small straight scissors | Fine Science Tools | 14084-09 | |
| Sterile cotton swabs 2×2 | Dynarex | 3252 | |
| Sterile cotton tipped applicators | Dynarex | 4301 | |
| Sterile drape | Med-Vet International | DR4042 | |
| Sterile saline solution | Med-Vet International | 1070988-BX | |
| ThCre:mTmG mutant mice | Mutant Mouse Resource and Research Centers | strain #017262-UCD | Jackson Laboratory, strain #007576 |
| ThCre:tdTomato mutant mice | European Mouse Mutant Archive | strain #00254 | Jackson Laboratory, strain #007914 |
Riferimenti
- Scholz, T., et al. Peripheral nerve injuries: An international survey of current treatments and future perspectives. J Reconstr Microsurg. 25 (06), 339-344 (2009).
- Schmalbruch, H. Fiber composition of the rat sciatic nerve. Anat Rec. 215 (1), 71-81 (1986).
- Tian, T., Moore, A. M., Ghareeb, P. A., Boulis, N. M., Ward, P. J. A perspective on electrical stimulation and sympathetic regeneration in peripheral nerve injuries. Neurotrauma Rep. 5 (1), 172-180 (2024).
- Gagnon, D., Crandall, C. G. Sweating as a heat loss thermoeffector. Hand Clin Neurol. 156, 211-232 (2018).
- Grassi, G. Role of the sympathetic nervous system in human hypertension. J Hypertens. 16 (12), 1979-1987 (1998).
- Dibona, G. F. Sympathetic nervous system and the kidney in hypertension. Curr Opin Nephrol Hypertens. 11 (2), 197-200 (2002).
- Elenkov, I. J., Wilder, R. L., Chrousos, G. P., Vizi, E. S. The sympathetic nerve-An integrative interface between two supersystems: The brain and the immune system. Pharmacol Rev. 52 (4), 595-638 (2000).
- Besedovsky, H. O., Del Rey, A., Sorkin, E., Da Prada, M., Keller, H. Immunoregulation mediated by the sympathetic nervous system. Cell Immunol. 48 (2), 346-355 (1979).
- Straka, T., et al. Postnatal development and distribution of sympathetic innervation in mouse skeletal muscle. Int J Mol Sci. 19 (7), 1935 (2018).
- Geng, T., et al. Pgc-1α plays a functional role in exercise-induced mitochondrial biogenesis and angiogenesis but not fiber-type transformation in mouse skeletal muscle. Am J Physiol Cell Physiol. 298 (3), C572-C579 (2010).
- Lin, J., Handschin, C., Spiegelman, B. M. Metabolic control through the pgc-1 family of transcription coactivators. Cell Metab. 1 (6), 361-370 (2005).
- Khan, M. M., et al. Sympathetic innervation controls homeostasis of neuromuscular junctions in health and disease. Proc Natl Acad Sci. 113 (3), 746-750 (2016).
- Delbono, O., Rodrigues, A. C. Z., Bonilla, H. J., Messi, M. L. The emerging role of the sympathetic nervous system in skeletal muscle motor innervation and sarcopenia. Ageing Res Rev. 67, 101305 (2021).
- Rodrigues, A. C. Z., et al. Heart and neural crest derivative 2-induced preservation of sympathetic neurons attenuates sarcopenia with aging. J Cachexia Sarcopenia Muscle. 12 (1), 91-108 (2021).
- Rosenberg, I. H. Summary comments. Am J Clin Nutr. 50 (5), 1231-1233 (1989).
- Murata, Y., Olmarker, K., Takahashi, I., Takahashi, K., Rydevik, B. Effects of lumbar sympathectomy on pain behavioral changes caused by nucleus pulposus-induced spinal nerve damage in rats. Eur Spine J. 15, 634-640 (2006).
- Xie, J., Park, S. K., Chung, K., Chung, J. M. The effect of lumbar sympathectomy in the spinal nerve ligation model of neuropathic pain. J Pain. 2 (5), 270-278 (2001).
- Lee, D. H., Katner, J., Iyengar, S., Lodge, D. The effect of lumbar sympathectomy on increased tactile sensitivity in spinal nerve ligated rats. Neurosci Lett. 298 (2), 99-102 (2001).
- Ringkamp, M., et al. Lumbar sympathectomy failed to reverse mechanical allodynia-and hyperalgesia-like behavior in rats with l5 spinal nerve injury. Pain. 79 (2-3), 143-153 (1999).
- Zhao, C., et al. Lumbar sympathectomy attenuates cold allodynia but not mechanical allodynia and hyperalgesia in rats with spared nerve injury. J Pain. 8 (12), 931-937 (2007).
- Zheng, Z. -. F., et al. Recovery of sympathetic nerve function after lumbar sympathectomy is slower in the hind limbs than in the torso. Neural Regen Res. 12 (7), 1177 (2017).
- Holmberg, K., Shi, T. -. J. S., Albers, K. M., Davis, B. M., Hökfelt, T. Effect of peripheral nerve lesion and lumbar sympathectomy on peptide regulation in dorsal root ganglia in the ngf-overexpressing mouse. Exp Neurol. 167 (2), 290-303 (2001).
- Thoenen, H., Tranzer, J. Chemical sympathectomy by selective destruction of adrenergic nerve endings with 6-hydroxydopamine. Naunyn Schmiedebergs Arch. Exp. Pathol. Pharmakol. 261, 271-288 (1968).
- Thoenen, H., Tranzer, J. P., Häusler, G. Chemical sympathectomy with 6-hydroxydopamine. New Aspects of Storage and Release Mechanisms of Catecholamines. , 130-143 (1970).
- Xie, W., et al. Localized sympathectomy reduces mechanical hypersensitivity by restoring normal immune homeostasis in rat models of inflammatory pain. J Neuroscience. 36 (33), 8712-8725 (2016).
- Zhu, X., Xie, W., Zhang, J., Strong, J. A., Zhang, J. -. M. Sympathectomy decreases pain behaviors and nerve regeneration by downregulating monocyte chemokine ccl2 in dorsal root ganglia in the rat tibial nerve crush model. Pain. 163 (1), e106-e120 (2022).
- Tonello, R., et al. Local sympathectomy promotes anti-inflammatory responses and relief of paclitaxel-induced mechanical and cold allodynia in mice. Anesthesiology. 132 (6), 1540-1553 (2020).
- Kostrzewa, R. M., Jacobowitz, D. M. Pharmacological actions of 6-hydroxydopamine. Pharmacol Rev. 26 (3), 199-288 (1974).
- Michel, P., Hefti, F. Toxicity of 6-hydroxydopamine and dopamine for dopaminergic neurons in culture. J Neuroscience Res. 26 (4), 428-435 (1990).
- Andrew, R., et al. The determination of hydroxydopamines and other trace amines in the urine of parkinsonian patients and normal controls. Neurochemical Res. 18, 1175-1177 (1993).
- Glinka, Y., Gassen, M., Youdim, M. Mechanism of 6-hydroxydopamine neurotoxicity. J Neural Transm Suppl. 5, 55-66 (1997).
- Treuting, P. M., Dintzis, S. M., Montine, K. S. . Comparative anatomy and histology: A mouse, rat, and human atlas. , (2017).
- Hweidi, S. A., Lee, S., Wolf, P. Effect of sympathectomy on microvascular anastomosis in the rat. Microsurgery. 6 (2), 9-96 (1985).
- Navarro, X., Kennedy, W. R. Sweat gland reinnervation by sudomotor regeneration after different types of lesions and graft repairs. Exp Neurol. 104 (3), 229-234 (1989).
- Gaudet, A. D., Popovich, P. G., Ramer, M. S. Wallerian degeneration: Gaining perspective on inflammatory events after peripheral nerve injury. J Neuroinflammation. 8 (1), 1-13 (2011).
- Babetto, E., et al. Targeting nmnat1 to axons and synapses transforms its neuroprotective potency in vivo. J Neuroscience. 30 (40), 13291-13304 (2010).
- Brumovsky, P. R. Dorsal root ganglion neurons and tyrosine hydroxylase-an intriguing association with implications for sensation and pain. Pain. 157 (2), 314 (2016).
- Tian, T., Harris, A., Owyoung, J., Sima, H., Ward, P. J. Conditioning electrical stimulation fails to enhance sympathetic axon regeneration. bioRxiv. , (2023).
- Tian, T., Ward, P. J. The ThCre: Mtmg mouse has sparse expression in the sympathetic nervous system. bioRxiv. , (2023).
- Ohman-Gault, L., Huang, T., Krimm, R. The transcription factor Phox2b distinguishes between oral and non-oral sensory neurons in the geniculate ganglion. J Comparative Neurol. 525 (18), 3935-3950 (2017).
- Pattyn, A., Morin, X., Cremer, H., Goridis, C., Brunet, J. -. F. The homeobox gene phox2b is essential for the development of autonomic neural crest derivatives. Nature. 399 (6734), 366-370 (1999).
- François, M., et al. Sympathetic innervation of the interscapular brown adipose tissue in mouse. Ann N Y Acad Sci. 1454 (1), 3-13 (2019).

.
