Generating 3D Co-culture Spheres of Astrocytes and Neurons to Induce Synapse Formation
Abstract
Source: Cvetkovic, C., et al. Synaptic Microcircuit Modeling with 3D Cocultures of Astrocytes and Neurons from Human Pluripotent Stem Cells. J. Vis. Exp. (2018).
This video demonstrates a procedure for co-culturing the astrocytes and neurons to form 3D spheres. These 3D spheres provide a distinct platform for examining synapse formation and neural network integration, thereby serving as a potential model for research in developmental neuroscience, neurodegenerative diseases, and neuropharmacological interventions.
Protocol
1. Preparation and Maintenance of 3D Sphere Cocultures
- Dissociate hAstros from 3D aggregates in suspension.
- Gently dissociate human astrocyte (hAstro) aggregates with detachment solution when dark centers appear and remove spheres that spontaneously attach. Break spheres gently once a week to maintain sphere health and to avoid necrotic cores.
NOTE: Do not exceed 5 min of treatment with detachment solution.
- Gently dissociate human astrocyte (hAstro) aggregates with detachment solution when dark centers appear and remove spheres that spontaneously attach. Break spheres gently once a week to maintain sphere health and to avoid necrotic cores.
- Dissociate hNeurons or iNeurons from a 2D monolayer.
- Remove media from the 6-well plate and add 500 µL of detachment solution to each well-containing monolayer culture of hNeuron (human neuron) or iNeurons (inducible neurons). Incubate at 37 °C for 5 min. Gently add 2-3 mL DMEM/F12 basal medium to remove attached cells.
- Collect cells and media in a 15 mL conical tube and centrifuge at 300 x g for 1 min. Aspirate the supernatant, add 1 mL of fresh media, and pipette up and down gently with a 1,000 µL micropipette to achieve a single-cell suspension.
- Form 3D spheres using microwell culture plates.
- Prepare the plate by adding 0.5 mL of anti-adherence rinsing solution to each well of the microwell plate. Centrifuge plate at 2,000 x g for 5 min. Aspirate rinsing solution, add 1 mL of DMEM/F12 basal medium to each well to wash, and aspirate again.
NOTE: Always ensure that the centrifuge is balanced during use. - Count each cell type using a hemocytometer or automated cell counter. Add desired ratio of dissociated hAstros and hNeurons or iNeurons to each well in a total volume of 2 mL of neural medium (NM) + Y (include Dox if iNeurons are utilized). Centrifuge plate at 100 x g for 3 min and return to the incubator.
NOTE: 24-well microwell plates (see the Table of Materials) contain 300 microwells per well. Add a minimum of 6 x 105 cells (for spheres of 2 x 103 cells each) and a maximum of 6 x 106 cells (for spheres of 2 x 104 cells each) in 2 mL of media per well. For viable spheres of a specific density within this range, use a defined quantity of cells and divide by 300 to calculate the number of cells per sphere. Alternative sphere-forming methods and tools may also be utilized if desired. Follow the manufacturer's instructions for acceptable ranges of cell densities. - Allow cells to self-assemble into densely packed spheres within microwell plates over the next 2 days (Figure 1A). Replace 50% of media if the culture is yellowing.
NOTE: Exchange only half media and pipette gently when removing and adding media in order not to disturb spheres. Spheres may lift up from microwells and fuse together with higher force. - After 2 days, gently remove spheres from microwells with a 1,000 µL micropipette (Figure 2D). Lightly apply force to the bottom of microwells with media to remove any additional adhered spheres. Let spheres settle in a 15 mL conical tube, aspirate the old media, and add fresh media.
- Prepare the plate by adding 0.5 mL of anti-adherence rinsing solution to each well of the microwell plate. Centrifuge plate at 2,000 x g for 5 min. Aspirate rinsing solution, add 1 mL of DMEM/F12 basal medium to each well to wash, and aspirate again.
- Set up the spinner flask bioreactor system for forming 3D spheres.
- Clean the surface of a magnetic stir plate with 70% ethanol, and place it into a cell culture incubator. Autoclave individual spinner flasks to sterilize.
- Add spheres with a minimum of 50–60 mL media to each spinner flask (Figure 2D, inset). Place the flask on the magnetic stir plate set at 60 rpm. Culture spheres in spinner flasks until they are ready for data collection.
NOTE: A minimum of 3 weeks in culture is needed for synapse formation.
Representative Results
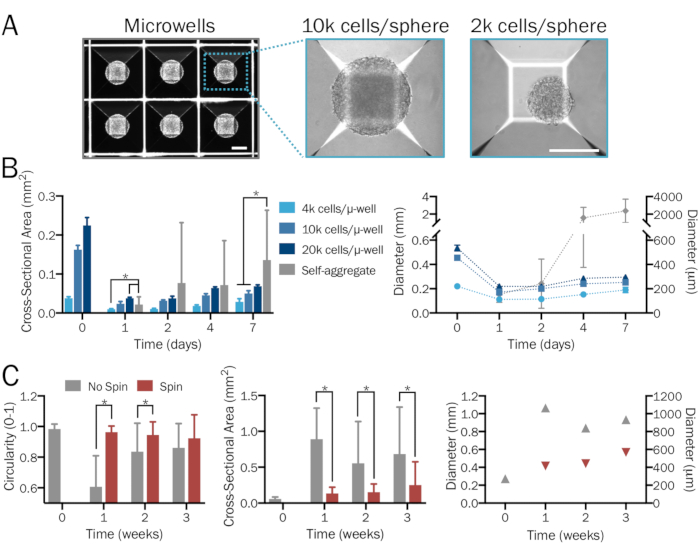
Figure 1: Systematic and reproducible generation of bioengineered 3D neural spheres. (A) Microwell (µ-well) culture plates are used to form 3D neural spheres of desired density and neuron-to-astrocyte ratios in a systematic and user-specified manner, yielding consistent size and shape. Images are shown 1 day after sphere formation. Scale bars = 200 µm. (B) A range of starting cell densities (from 4 x 103 to 2 x 104 cells per microwell) produces spheres of varying sizes. In the absence of microwell plates, hPSCs combine to form significantly larger and nonuniform aggregates whose diameters surpass the limit of diffusion after a week (n = 6-14 spheres per group and time point). (C) iNeuron spheres cultured in a spinner flask at 80 rpm exhibited less fusion and thus were significantly smaller, more uniform, and exhibited greater circularity compared to those in stationary culture over a period of three weeks (n = 6-51 spheres per group and time point). Plots represent mean ± SD; * indicates significance (p < 0.05) between groups, determined using two-tailed t-tests.
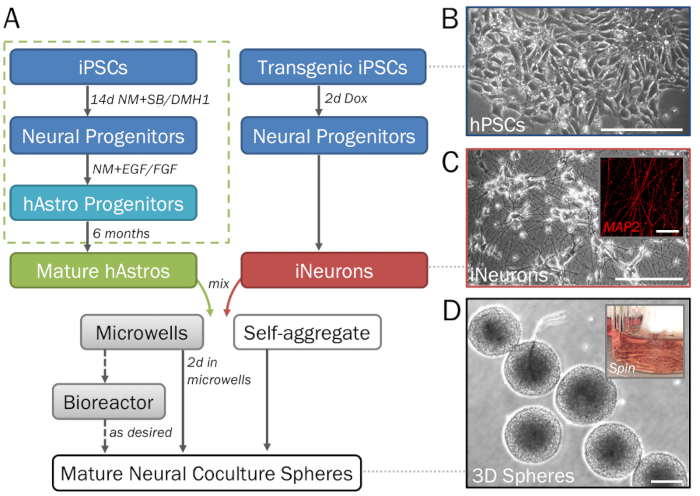
Figure 2: Stepwise depiction of the differentiation and formation of 3D neural spheres derived from hPSCs. (A) Timeline of key steps in the protocol. (B) Pure populations of neural cells can be generated from human induced pluripotent stem cells (hPSCs). For the generation of astrocyte progenitors (dotted green box). (C) Inducible neurons (iNeurons) generated from transgenic hPSCs via induced overexpression of neurogenin 2 demonstrate neuronal morphology on 2D ECM (day 7) and are positive for MAP2 (inset). (D) Spheres removed from microwell plates demonstrate consistent size for high-throughput screening. Spheres may be cultured in a spinner flask bioreactor (inset; see step 1.4) if desired to prevent fusion. Scale bar = 50 µm (C, inset). Scale bars = 200 µm (A, D).
Divulgazioni
The authors have nothing to disclose.
Materials
| 6 well plate | Fisher Scientific | 08-772-1B | |
| 15 ml conical tubes | Olympus Plastics | 28-101 | |
| Accutase | Sigma | A6964-100ML | Detachment solution |
| AggreWell plate | Stemcell Technologies | 34850 | |
| Anti-Adherence Rinsing Solution | Stemcell Technologies | 7010 | Prevent cell adhesion to microwell plates |
| Anti/anti | Thermofisher | 15240062 | |
| B27 | Thermofisher | 17504044 | Media Supplement |
| BrainPhys neuronal medium | Stemcell Technologies | 5790 | Neurophysiological basal medium alternative |
| DMEM/F12 | Thermofisher | 10565-042 | With GlutaMAX supplement |
| DMH-1 | Stemcell Technologies | 73634 | HAZARD: Toxic if swallowed. Working concentration: 2 uM |
| Donkey serum | Lampire Biological Laboratories | 7332100 | Working concentration: 5% in primary blocking buffer, 1% in secondary blocking buffer |
| Doxycycline Hydrochloride (Dox) | Sigma | D3072-1ml | HAZARD: Toxic for pregnant women. Working concentration: 2 ug/mL |
| Epidermal growth factor (EGF) | Peprotech | AF-100-15 | Working concentration: 10 ng/mL |
| Fibroblast growth factor-2 (FGF) | Peprotech | 100-18B | Working concentration: 10 ng/mL |
| Hemacytometer or automatic cell counter | Life Technologies | AMQAX1000 | |
| Matrigel membrane matrix | Corning | 354230 | ECM coating solution. Working concentration: 80 ug/ml. Prepare on ice and ensure that pipettes, tubes, and media are pre-chilled. |
| MEA 2100 System | Multichannel Systems | MEA2100 | |
| Rho-Kinase Inhibitor Y27632- (Y) | Tocris | 1254 | Working concentration: 10 uM |
| SB431542 | Stemcell Technologies | 72234 | Working concentration: 2 uM |
| Spinner flasks | Fisher Scientific | 4500-125 | |
| T25 Culture Flask | Olympus Plastics | 25-207 | Vented caps |
| Trypan blue | Invitrogen | T10282 |

