Generating Neurons by Reprogramming Human Dermal Fibroblast Cells
Abstract
Source: Shrigley, S., et al. Simple Generation of a High Yield Culture of Induced Neurons from Human Adult Skin Fibroblasts. J. Vis. Exp. (2018)
The video demonstrates the generation of neurons from adult human dermal fibroblasts (aHDFs). The aHDFs are reprogrammed using a lentiviral vector carrying genes for neuronal transcription factors and short hairpin RNAs (shRNAs). The shRNAs inhibit the expression of a neuronal transcriptional repressor, and the neuronal transcription factors activate genes to differentiate aHDFs into neurons. These reprogrammed aHDFs are cultured in an early and a late neuronal conversion medium to generate mature neurons.
Protocol
1. Preparation of Skin Fibroblasts for Reprogramming
- Using an automated cell thawing system or a 37 °C water bath, thaw the adult human dermal fibroblasts (aHDFs) and plate 200,000 per T75 flask (count with an automated cell counter) in 10 mL of fibroblast medium (see Table 1) at 37 °C in 5% CO2.
- Perform a complete medium change with fibroblast medium on the next day.
- Change the fibroblast medium every 3–4 days until the cells reach 95% confluency.
NOTE: One confluent flask will contain approximately 1,000,000 cells.
2. Plating for Reprogramming (Day −1)
NOTE: It is recommended to use a gelatin coating for short term experiments (up to 30 days); alternatively, for long term experiments it is recommended to start on a poly-L-ornithine, fibronectin and laminin (PFL) coating.
- 60 min before plating the aHDFs for reprogramming, coat a 24-well plate with 0.1% gelatin (250 µL/well) and incubate at 37 °C.
- Aspirate the fibroblast medium on the aHDFs. Wash once with Dulbecco's phosphate buffer saline (DPBS). Dissociate the cells with 0.05% trypsin (1.5 mL per T75 flask) at 37 °C for 3–5 min.
- Add fibroblast medium to neutralize the trypsin (3 mL per flush per T75 flask) and collect the detached cells in a 15 mL tube by flushing out the cells in the flask twice.
- Spin down the cells at 400 x g for 5 min. Discard supernatant and resuspend the cell pellet in 1 mL of fibroblast medium.
- Count the cells using an automated cell counter (to ensure a good quality conversion check that the cell viability is above 90% with trypan blue staining).
- For a complete 24-well plate, prepare a suspension of 1,320,000 cells in 13.2 mL of fibroblast medium to achieve a suspension of 100,000 cells/mL of medium (or 55,000 cells/well in 550 µL of fibroblast medium multiplied by the number of wells needed).
- Aspirate the gelatin from the plate and wash twice with DPBS. Add 500 µL of the cell suspension to each well and incubate overnight at 37 °C in 5% CO2.
3. Viral Transduction (Day 0)
NOTE: Working with lentiviral particles requires category 2 equipment and the use of an agent to neutralize the virus. Wearing double pairs of gloves is also strongly recommended.
- Warm up 13.2 mL of fibroblast medium to 37 °C.
- Thaw a lentiviral vector containing the transcription factors achaete-scute family bHLH transcription factor 1 (Ascl1) and Brn2 with two short hairpin RNAs (shRNA) targeting RE1-silencing transcription factor (REST) at room temperature.
- Add the necessary volume of lentivirus to infect the aHDFs at multiplicity of infection (MOI) of 20 to the medium without any transduction enhancers.
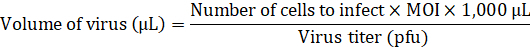
- Replace the medium in the 24-well plate with fibroblast medium containing the lentiviral vector (500 µL/well) and incubate overnight at 37 °C in 5% CO2.
- The next day, replace the medium in the wells with fresh fibroblast medium without the lentiviral vector.
NOTE: The medium is considered infectious for 7 days and as such, adequate protection and handling procedures should be used during the first week following viral transduction.
4. Maintenance of the Converting Cells
NOTE: Once conversion begins cells are susceptible to lifting; take care to tip the plate up and use a 1,000 µL pipette when removing media to avoid cells detaching.
- On day 3, remove the fibroblast medium and add 500 µL of early neuronal conversion medium (see Table 1).
- Two to three times a week, take out 225 µL of old medium from the well and add-in 250 µL of fresh early neuronal conversion medium.
- On day 18, remove all of the medium from each well and replace with 500 µL of late neuronal conversion medium (see Table 1).
- Continue to change half of the medium as above with late neuronal conversion medium every 2–3 days until day 25 or experiment endpoint (Figure 1A).
Table 1. Composition of the different media used. Full description of the composition for media needed in this protocol including fibroblast medium, early neuronal conversion medium, late neuronal conversion medium.
| Stock Concentration | Working Concentration | |
| Fibroblast medium | ||
| Basal medium | N/A | N/A |
| Penicillin/Streptomycin | 10,000 U/mL | 100 mg/mL |
| FBS | N/A | 10% |
| Early neuronal conversion medium (ENM) | ||
| Neural differentiation medium | N/A | N/A |
| Penicillin/Streptomycin | 10,000 U/mL | 100 mg/mL |
| CHIR99021 | 10 mM | 2 µM |
| SB-431542 | 20 mM | 10 µM |
| Noggin | 100 µg/mL | 0.5 µg/mL |
| LDN-1931189 | 10 mM | 0.5 µM |
| VPA | 1 M | 1 mM |
| LM-22A4 | 20 mM | 2 µM |
| GDNF | 20 µg/mL | 2 ng/mL |
| NT3 | 10 µg/mL | 10 ng/µL |
| db-cAMP | 50 mM | 0.5 mM |
| Late neuronal conversion medium (LNM) | ||
| Neural differentiation medium | N/A | N/A |
| Penicillin/Streptomycin | 10,000 U/mL | 100 mg/mL |
| LM-22A4 | 20 mM | 2 µM |
| GDNF | 20 µg/mL | 2 ng/mL |
| NT3 | 10 µg/mL | 10 ng/µL |
| db-cAMP | 50 mM | 0.5 mM |
Representative Results
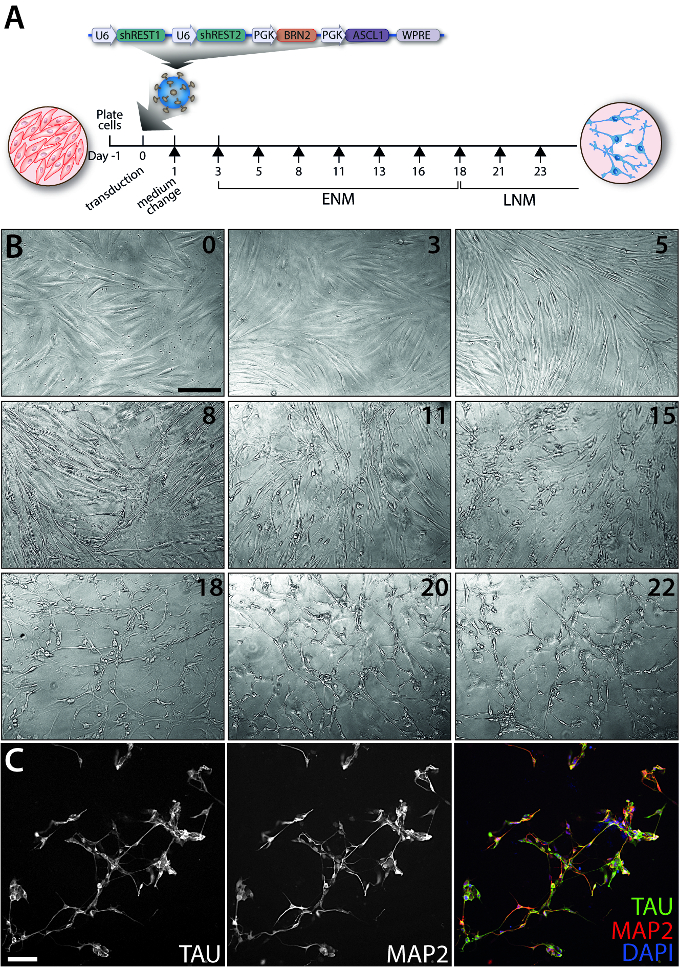
Figure 1: Evolution of the iN conversion over time. (A) Timeline of the experiment and map of the construct packaged in a lentivirus used to reprogram the adult human dermal fibroblasts. Each black arrow represents a medium change. (B) Representative phase contrast images depicting the changes in morphology of cells during the conversion process between day 0 to day 22 (as indicated on the upper right corner of each panel). Images were taken on a phase contrast microscope using the 10X objective. (C) Immunofluorescence image of a TAU and MAP2 double staining at day 35 post-transduction. Cells were fixed in 4% paraformaldehyde and permeabilized with 0.1% Triton in DPBS for 10 min. Cells were blocked for 30 min in a 5% serum solution in DPBS. The antibodies were diluted in blocking solution and applied overnight at 4 °C. Fluorophore-conjugated secondary antibodies were diluted in blocking solution and applied for 2 h. Cells were counterstained with DAPI for 15 min followed by 3 washes with DPBS. Images were taken on an inverted fluorescence microscope using the 20X objective. Scale bars = 100 µm (B, C). Abbreviations: ENM: early neuronal medium; LNM: late neuronal medium.
Divulgazioni
The authors have nothing to disclose.
Materials
| Cell Lines | |||
| Adult human dermal fibroblasts | [C2 passage #7] Donor was a 67 year old female. Cells obtained from the Parkinson’s Disease Research and Huntington’s disease clinics at the John van Geest Centre for Brain Repair (Cambridge, UK). | ||
| Reagents for Fibroblast Culture, Transduction and Conversion | |||
| Dulbecco's phosphate-buffered saline (DPBS) [-CaCl2, -MgCl2] | Gibco | 14190094 | |
| Trypsin-EDTA [0.5%] | Gibco | 15400-054 | Dilute to 0.05% in DPBS. |
| Virkon (agent used to neutralize virus) | Viroderm | 7511 | Dilute to 1% solution with warm water. |
| Milli-Q Water | Millipore | ||
| Basal medium – Dulbecco’s Modified Eagle Medium (DMEM) + GlutaMax | Gibco | 61965059 | |
| Penicillin/Streptomycin [10,000 U/ mL] | Gibco | 15140-122 | |
| Fetal Bovine Serum (FBS) | Gibco | 10270-106 | |
| CryoMACS® dimethyl sulfoxide (DMSO) 10 | Miltenyi | 170-076-303 | |
| Neural differentiation medium – NDiff 227 | Takara-Clontech | Y40002 | |
| LM-22A4 | Tocris | 4607 | Dilute 10 mg in 1450 µL DMSO. Stock concentration: 20 mM. |
| Glial cell line-derived neurotrophic factor (GDNF) [recombinant human] | R&D systems | 212-GD-010 | Dilute 10 ug in 500 µL 0,1% BSA in DPBS. Stock concentration: 20 µg/mL. |
| NT3 [recombinant human] | R&D systems | 267-N3-025 | Dilute 25 µg in 2,5 mL 0,1% BSA in DPBS. Stock concentration: 10 µg/mL. |
| db-cAMP | Sigma Aldrich | D0627 | Dilute 1 g in 40,7 mL Milli-Q water. Filter and make 500 µL aliquots or stock tubes of 10 mL. Stock concentration: 50 mM. |
| CHIR99021 | Axon | 1386 | Dilute 2 µg in 429,8 µL DMSO. Stock concentration: 10 mM. |
| LDN-193189 | Axon | 1509 | Dilute 2 mg in 360 µL DMSO. Stock concentration: 10 mM. |
| Valproic acid sodium salt (VPA) | Merck Millipore | 676380 | Dilute 5 g in Milli-Q water to acheive a stock concentration of 1 M. CAUTION: Avoid ingestion, contact with skin, and breathing dust formation. |
| Reagents for Coatings | |||
| Gelatin | Sigma Aldrich | G2500 | Dilute to 0.1% in Milli-Q water. |
| Poly-L-ornithine | Sigma Aldrich | P3655 | Dissolve in Milli-Q water. Use at 15µg/mL. |
| Fibronectin | ThermoFisher Scientific | 33010-018 | 2 mL of Milli-Q water + 70 µL 0,25 M NaOH. Use at 5 µg/mL. |
| Laminin | ThermoFisher Scientific | 23017-015 | Store at -80°C. Thaw on ice, keep cool and aliquot 30 µL. Use at 5 µg/mL. |
| Equipment | |||
| T75 flask [Nunclon Delta Surface] | ThermoFisher Scientific | 156499 | |
| 24-well plate [Nunc] | ThermoFisher Scientific | 142485 | |
| 1.5 mL polypropylene tube | Sigma Aldrich | Z336769 | |
| 15 mL falcon tube | Sarstedt | 6,25,54,502 | |
| 50 mL falcon tube | Sarstedt | 6,25,47,254 | |
| Pippette controller | For pipetting volumes 1-25 mL. | ||
| Sterile serological pipettes: 5, 10 and 25 mL | Sarstedt | 86.1253.001, 86.1254.001, 86.1685.001 | |
| Adjustable volume pipettors: 5, 20, 200, and 1,000 µL | |||
| Sterile pipette tips | For pipetting volumes of 0.5 – 1,000 µL. | ||
| ThawSTAR Automated Cell Thawing System | BioCision | BCS-601 | |
| Countess II Automated Cell Counter | ThermoFisher Scientific | AMQAX1000 | |
| Cell counting chambers [50 slides] and trypan blue [0.4%] | ThermoFisher Scientific | C10228 | For use with Countess II Automated Cell Counter. |
| Laminar flow hood | |||
| Humidified 5% CO2 37 °C incubator | |||
| Centrifuge | Suitable for 1,5, 15 and 50 mL tubes. | ||
| Orbital shaker | |||
| Inverted fluorescence microscope | Leica | DMI6000 B |

