MISSION LentiPlex Pooled shRNA Library Screening in Mammalian Cells
Summary
Here we use a human LentiPlex pooled library and traditional sequencing methods to identify gene targets promoting cell survival. We demonstrate how to set up and deconvolute a LentiPlex screen and validate the results.
Abstract
RNA interference (RNAi) is an intrinsic cellular mechanism for the regulation of gene expression. Harnessing the innate power of this system enables us to knockdown gene expression levels in loss of gene function studies.
There are two main methods for performing RNAi. The first is the use of small interfering RNAs (siRNAs) that are chemically synthesized, and the second utilizes short-hairpin RNAs (shRNAs) encoded within plasmids 1. The latter can be transfected into cells directly or packaged into replication incompetent lentiviral particles. The main advantages of using lentiviral shRNAs is the ease of introduction into a wide variety of cell types, their ability to stably integrate into the genome for long term gene knockdown and selection, and their efficacy in conducting high-throughput loss of function screens. To facilitate this we have created the LentiPlex pooled shRNA library.
The MISSION LentiPlex Human shRNA Pooled Library is a genome-wide lentiviral pool produced using a proprietary process. The library consists of over 75,000 shRNA constructs from the TRC collection targeting 15,000+ human genes 2. Each library is tested for shRNA representation before product release to ensure robust library coverage. The library is provided in a ready-to-use lentiviral format at titers of at least 5 x 108 TU/ml via p24 assay and is pre-divided into ten subpools of approximately 8,000 shRNA constructs each. Amplification and sequencing primers are also provided for downstream target identification.
Previous studies established a synergistic antitumor activity of TRAIL when combined with Paclitaxel in A549 cells, a human lung carcinoma cell line 3, 4. In this study we demonstrate the application of a pooled LentiPlex shRNA library to rapidly conduct a positive selection screen for genes involved in the cytotoxicity of A549 cells when exposed to TRAIL and Paclitaxel. One barrier often encountered with high-throughput screens is the cost and difficulty in deconvolution; we also detail a cost-effective polyclonal approach utilizing traditional sequencing.
Protocol
LentiPlex Pooled Screen Setup
To identify shRNA sequences of interest, it is critical to set up the screen such that the majority of cells receive only one shRNA construct. By using a low MOI (multiplicity of infection), the probability of multiple integrants per cell is greatly decreased. However, transduction efficiencies, and therefore desired MOIs, depend strongly on the target cell type. Therefore, it is imperative that determination of the optimal MOI is carried out before starting the screen in a new cell type.
1. Transduction Of Target Cells with the Mission LentiPlex Pooled shRNA Library
- The actual MOI used will vary for different cell types. If replicates are desired then the number of plates must be increased accordingly. In addition, typically it will not be advantageous to combine the different subpools. Keeping the subpools separate will aid in the deconvolution process. Ensure proper labeling of each tissue culture dish with the subpool number that is applied during transduction. A low MOI is advised to reduce the probability of multiple integrants per cell.
- Day 1 – Seed the appropriate number of 60-mm dishes with enough cells to obtain ~70% confluency on the following day (10 pools in total, each performed in triplicate = 30 dishes total). For our study, 60 mm dishes were seeded with 6 x 105 A549 cells to achieve a confluency of 70% prior to transduction. This ensures that cells are still in the exponential growth phase. Growth rates will vary by cell type and must be determined before starting the screen.
- Allow cells to adhere by incubating overnight at 37°C in a humidified incubator in an atmosphere of 5% CO2.
- (Optional) Include two additional 60-mm dishes for transduction with negative control shRNAs. Label the dishes “Control A” for SHC001H and “Control B” for SHC002H.
- Day 2 – Transduce the cells with the LentiPlex viral particles. On the day of transduction, thaw the lentiviral particles on ice.
- Transfer the thawed particles to a laminar flow hood and keep on ice if not being used immediately.
- Calculate how much of each individual viral sub-pool to add to the cells to get the MOI of 1 across all cell culture dishes.
- Example: 1 x 106 cells/dish; Viral titer = 5 x 108 TU/ml; a desired MOI of 1. i.) 1 x 106 cells/dish x (MOI of 1) = 1 x 106 transducing units (TU) needed ii.) 1 x 106 TU/(5.0 x 108 TU/ml) obtained from the C of A = 2 μL of lentiviral stock solution should be added to the appropriate dish. Dilute the calculated amount of the virus in 100-300 μL of complete media in order to ensure better distribution of the virus when added to the cell culture dish.
- Remove media from the pre-seeded cells. Add complete media containing hexadimethrine bromide solution to each dish. The recommended final concentration of hexadimethrine bromide in media is 8 μg/ml. Use less hexadimethrine bromide if you find it to be toxic to your target cells.
- Add shRNA lentiviral particles to appropriate dishes in accordance with the predetermined experimental design. Gently swirl the plates to evenly distribute the virus across cells.
- Incubate 18-20 hours at 37°C in a humidified incubator in an atmosphere of 5% CO2.
- (Optional) Repeat 3-8 at the identical MOI with SHC001H (empty vector shRNA Control) in Control Dish A and SHC002H (scrambled shRNA control) in Control Dish B. Treat these dishes identically to the experimental dishes throughout the experiment.
- Day 3 – Change the media. Aspirate virus-containing media and add fresh complete media (without hexadimethrine bromide). Incubate the cells at 37°C overnight.
2. Treat plated cells with therapeutic component/reagent
- Day 4 – Aspirate media from wells and add fresh media containing therapeutic component at the optimal concentration. As with culture conditions, treatments will vary and proper concentrations and durations should be determined beforehand. In our study, we used 100 ng/mL of TRAIL and 1 μM of Paclitaxel. Cells were treated for 48 hours.
- Surviving cells should be re-seeded into T75 flasks and allowed to expand until nearly 100% confluent.
- (Optional) At the end of selection, inspect Control Dish A and Control Dish B. Dish A and Dish B should each have fewer colonies than at least one of the pooled library dishes. If there are an unexpectedly high number of colonies in Dish A, than either the transduction process has affected a pathway related to the desired phenotype or the selective pressure isn’t sufficiently strong and re-optimization of the assay may be required. If Dish B contains too many colonies, then expression of shRNA and engagement of the RNAi pathway may have an effect on the phenotype of interest. If this is the case repeat with SHC001H, to ensure that the effect isn’t specific to SHC002H.
3. Deconvolution from a polyclonal cell population
- Harvest the heterogeneous population of cells from each subpool and isolate genomic DNA. In our trial, cells were washed briefly in PBS and trypsinized before genomic DNA was isolated using the GenElute Mammalian Genomic Miniprep kit and supplied protocol (Sigma-Aldrich, G1N70). Alternatively, any other genomic purification kits that are currently available on the market can be used for DNA isolation. It is important to follow protocol recommendations for the starting amount of cultured cells.
- PCR amplification of targets. The shRNA template recovery procedure enables amplification of the entire pool of shRNA inserts from the selected cell population, or the retrieval of individual shRNA templates from colonies that were individually picked and expanded. After amplification of shRNA inserts from control and selected target cells, the PCR products can be sequenced.
- This PCR amplification step was optimized using Sigma ReadyMix, Catalog No. P2893. Other reagents can be used for amplification, but the cycling conditions and/or magnesium concentration may need to be modified for successful amplification. Amplification primers at the concentration 20 mM are included with the LentiPlex kit.
- Set up the PCR reaction as described in Table 1. Add the components in the order listed. The described amounts are for one 50 μL reaction. For more reactions, scale the master mix components by the desired number of reactions and include 10% overage. The amount of DNA template recommended is 50-100 ng.
- It is strongly recommended that for one reaction the template consists of the MISSION shRNA Human Positive Control Vector diluted to the appropriate concentration. Successful amplification with this template indicates that the PCR assay components and cycling conditions are adequate. NOTE: To prevent carryover contamination of experimental samples and reagents, it is suggested that the positive control be diluted in a separate location from where subsequent PCR reactions are set up.
- Save the PCR program listed in Table 2 into your thermocycler.
- For each sample, combine 3 μL PCR reaction with 1 μL of dye and electrophorese alongside 5 μL of DirectLoad™ PCR 100 bp Low Ladder, Catalog Number D3687 on a 1% agarose gel to confirm the presence of a 309 bp amplicon.
- Subcloning of PCR amplicons: PCR products were TOPO cloned using the TOPO-TA cloning kit, following the manufacturer’s protocol (Invitrogen, K4575-01). The result is that one PCR product is contained in each vector.
- 250 Individual bacterial colonies (each containing an individual PCR amplicon) were isolated and grown in 2 mL cultures of LB with 100 mg/mL ampicillin. Plasmid DNA was then extracted using the GenElute Plasmid Miniprep kit (Sigma-Aldrich, PLN70).
- Purified plasmid DNA was digested with EcoRI and electrophoresed to confirm the presence of the insert.
- Plasmid DNA samples were then sequenced and the shRNA insert identified using the LentiPlex shRNA Sequence Search database provided with the LentiPlex library.
4. Target Identification and Validation
- The shRNA sequence initiates with 5′-GAAACACCGG-3′. Enter at least the next 10 but less than 21 nucleotides as the query sequence into the Search box on the enclosed shRNA Sequence Search Access database form.
- Select the species of the pooled shRNA. Optionally, select the subpool from which the sequence was found.
- Now click on “Find Potential Hits” button to perform the search. This should identify the corresponding TRC shRNA sequence(s) that match the sequencing data. More information on the TRC shRNA sequence(s) identified and the corresponding gene targets can be readily found at Sigma-Aldrich’s Your Favorite Gene: http://www.sigma.com/yfg
- Identified target genes should be validated by repeating the original screening assay with the original TRC clone plus at least two additional shRNAs that target the same gene using a 1 shRNA to 1 well format. True hits will display the same phenotype in a majority of wells.
5. Representative Results
An example of a LentiPlex pooled screen workflow is detailed in Figure 1. Transduced cells that proliferated in the presence of TRAIL and Paclitaxel were allowed to expand until flasks were confluent. Genomic DNA was harvested and subjected to the PCR amplification and cloning as illustrated in Figure 1 A, before being submitted for sequencing and shRNA identification as described in Figure 1 C. 250 clones were sequenced, of these 25 were represented by multiple clones and the remaining 225 were represented by only 1 clone and were not pursued at this time.
As shown in Figure 2, several candidate genes including TBX3, PPP2CA, and AKT2 were represented by multiple clones. The T-box transcription factor, TBX3 appeared in a total of four clones and has been implicated in tumor migration5. PPP2CA downregulation has been demonstrated to maintain the growth of LNCaP cells cultured in androgen deficient conditions by relieving the androgen-deprivation-induced cell-cycle arrest and preventing apoptosis 6. AKT2 is a RAC-beta serine/threonine-protein kinase and putative oncogene demonstrated to play several roles in cancer development 7, 8.
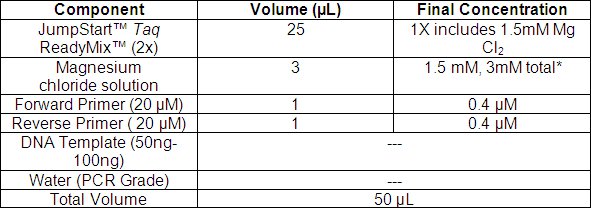
*Final concentration of Mg2+ in solution will be 3 mM; 1.5 mM is contributed from the Taq ReadyMix and 1.5 mM from the Magnesium chloride solution.
Table 1. Lentiplex PCR Reaction Conditions
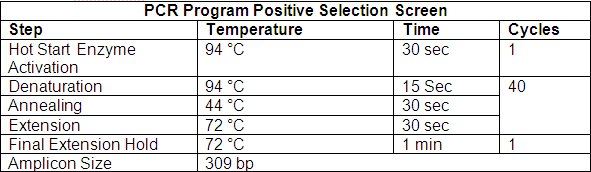
Table 2. Lentiplex PCR Amplification Program
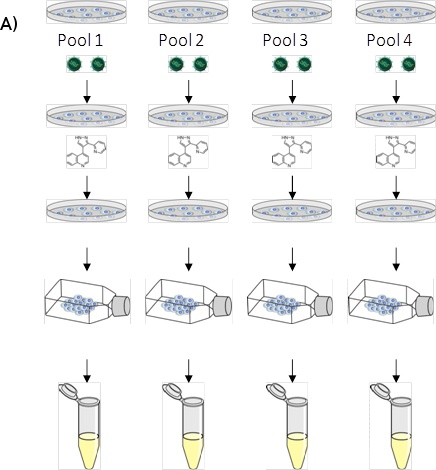
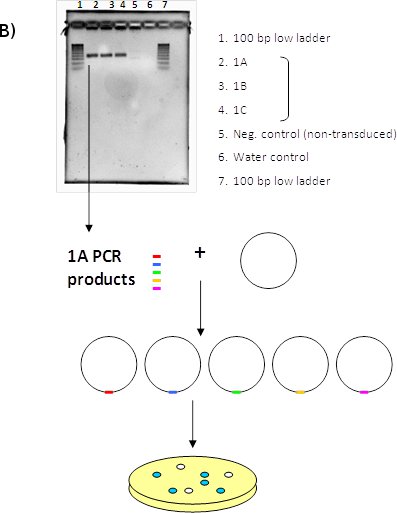
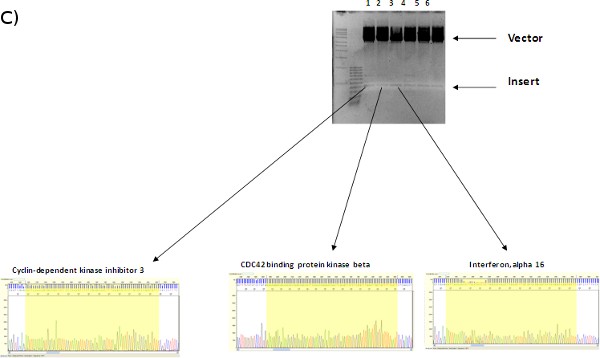
Figure 1. (A) LentiPlex pooled screen, (B) Polyclonal deconvolution workflow, and (C) identification of shRNA targets.
A. LentiPlex pooled screen. First, Seed cells, then add shRNA subpools, (10 pools in total, each performed in triplicate, for 30 dishes total). Next, treat with therapeutic component/reagent (in our case, TRAIL and Paclitaxel, respectively). Then, reseed surviving cells in flasks and allow to expand. Harvest heterogeneous population of cells from each subpool and isolate genomic DNA.
B. Polyclonal deconvolution. Perform PCR to amplify DNA containing the shRNA insert. The PCR product is uniform in size, but polyclonal in sequence since the template gDNA was also polyclonal. PCR product is cloned into the vector and then transformed into competent bacteria.
C. Identification of shRNA targets. Individual colonies are isolated and plasmid DNA is extracted. Each clone contains the cloning vector with one individual PCR fragment. Plasmid DNA is digested to confirm the presence of the insert and sequenced. The shRNA insert is identified using the LentiPlex shRNA Sequence Search database.
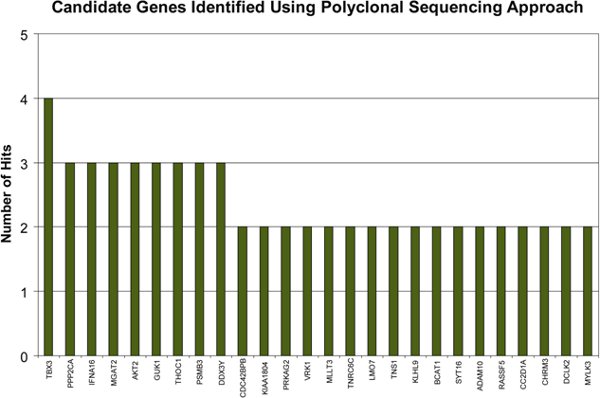
Figure 2. Identified Candidate Genes. 25 candidate genes were identified that had multiple hits. 225 candidate genes had only 1 hit and were not pursued. Please see Supplementary Table 1 for a breakdown of the individual clones per candidate gene.
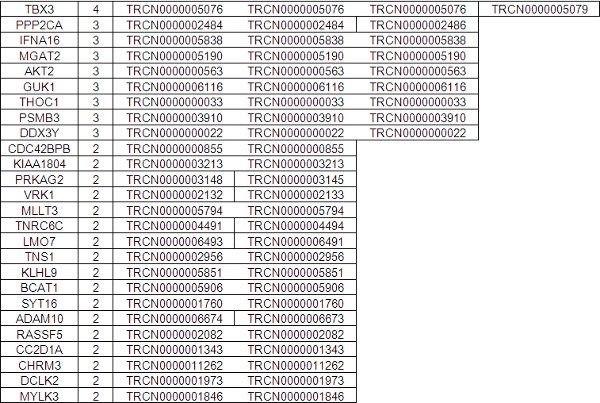
Supplementary Table 1. Individual TRC Library clones Identified From Polyclonal Sequencing Gene ID Hits Detected Clones.
Discussion
In this study, we present a genome-wide screen to identify genes involved in cytotoxicity of A549 cells following paclitaxel/TRAIL treatment. The use of a pooled approach in a genome-wide screen reduces the time, cost, and equipment investment normally associated with conventional arrayed screens. Critical to the success of the screen are optimization experiments performed beforehand. Transduction conditions and MOI must be empirically determined.
The selection method should allow for robust selection of cells displaying the desired phenotype (e.g., survival, surface protein expression, etc). Optimization of these parameters will reduce the number of false positives detected.
Here, gDNA was harvested from the bulk population of selected cells and then TOPO cloned to identify individual shRNA inserts. One possible improvement for this experiment would have been to select for live cells using FACS to ensure that only live cells are collected. The identified targets will be validated by transduction with target-specific shRNAs in a screening assay. True hits will display the phenotype in a majority of wells.
The LentiPlex Pooled Library is flexible in its application. It is compatible with a wide array of downstream applications and may be used with either positive or negative selection strategies. Depending on the screening conditions, deconvolution of the shRNA inserts is accomplished via PCR/cloning, microarray, or next-generation sequencing 9, 10.While the power and speed of these techniques to deconvolute RNAi screens is well established, one concern with microarray and next-generation sequencing methods is the availability of bioinformatics resources and knowledge needed to correctly analyze and interpret experimental results. The costs associated with these techniques can often times be a barrier to the undertaking of genome wide screens. A PCR/cloning based approach, while not as powerful as microarrays and next-generation sequencing is an effective and lower cost alternative.
Agilent now offers a custom array based on the TRC shRNA library to further aid in LentiPlex screens. These options allow researchers to use LentiPlex to study a diverse range of cellular functions.
Divulgazioni
The authors have nothing to disclose.
Acknowledgements
MISSION is a registered trademark of Sigma-Aldrich Biotechnology L.P.
LentiPlex is a trademark of Sigma-Aldrich Biotechnology L.P.
Materials
| Name of the reagent | Company | Catalog Number |
| LentiPlex Pooled Array | Sigma-Aldrich | SHPH01 |
| A549 Cells | ATCC | CCL-185 |
| TRAIL | Sigma-Aldrich | T5694 |
| Paclitaxel | Sigma-Aldrich | T7402 |
| Topo TA Cloning Kit | Invitrogen | K4575-01 |
| JumpStart Taq ReadyMix | Sigma-Aldrich | P2893 |
| GenElute Plasmid Miniprep kit | Sigma-Aldrich | PLN70 |
| EcoR1 | New England Biolabs | R0101 |
| hexadimethrine bromide | Sigma-Aldrich | H9268 |
Riferimenti
- Rao, D. siRNA vs. shRNA: similarities and differences. Adv. Drug Deliv. Rev. 61 (9), 746-746 (2009).
- Root, D. Genome-scale loss-of-function screening with a lentiviral RNAi library. Nat Methods. 3 (9), 715-715 (2006).
- Fan, Q. Synergistic antitumor activity of TRAIL combined with chemotherapeutic agents in A549 cell lines in vitro and in vivo. Cancer Chemother Pharmacol. 55, 189-189 (2005).
- Ji, D. A screen of shRNAs targeting tumor suppressor genes to identify factors involved in A549 paclitaxel sensitivity. Oncology Reports. 18, 1499-1499 (2007).
- Fillmore, C. Estrogen expands breast cancer stem-like cells through paracrine FGF/Tbx3 signaling. Proc. Natl. Acad. Sci. U.S.A. 14 (50), 21737-21737 (2010).
- Bhardwaj, A. Modulation of protein phosphatase 2A activity alters androgen-independent growth of prostate cancer cells: therapeutic implications. Mol. Cancer. Ther. 10 (5), 720-731 (2011).
- Cheng, J. AKT2, a putative oncogene encoding a member of a subfamily of protein-serine/threonine kinases, is amplified in human ovarian carcinomas. Proc. Natl. Acad. Sci. U.S.A. 89 (19), 9267-9267 (1992).
- Fernandez, A. Akt1 and Akt2: differentiating the aktion. Histol. Histopathol. 26 (5), 651-651 (2011).
- Luo, J. A genome-wide RNAi screen identifies multiple synthetic lethal interactions with the Ras oncogene. Cell. 137 (5), 835-835 (2009).
- Ketela, T. A comprehensive platform for highly multiplexed mammalian functional genetic screens. B.M.C. Genomics. 12 (1), 213-213 (2011).

