Summary
Two techniques can be used to establish this model: injection of a cancer cell suspension into the cecal wall or transplantation of a piece of subcutaneous tumor onto the cecum. This model is useful for studying the natural progression of colorectal cancer and testing new therapeutic agents against colorectal cancer.
Abstract
Protocol
I. Cell Preparation
- Colorectal cancer cells are grown in culture and harvested when subconfluent.
- A single cell suspension is prepared in phosphate buffered saline and kept on ice.
II. Tumor Preparation
- A mouse with a previously established subcutaneous colorectal tumor is euthanized.
- The subcutaneous tumor is removed using sterile technique and divided into 2-3 mm pieces
- The tumor pieces are kept in phosphate buffered saline on ice.
III. Mouse Preparation
Note: In our laboratory we use inhaled isoflurane to anesthetize the mouse; alternatively, one can use injectable anesthetics to achieve the same effect
- The depth of anesthesia is assessed using toe pinch. There should be no withdraw reflex with toe pinch.
- Antibiotics may be given at this point.
- The anesthetized mouse, which was previously shaved, is properly positioned.
- The abdomen is prepped with a betadine solution.
- The abdomen and surgical site are draped in a sterile fashion.
IV. Laparotomy
- A small nick is made in the skin
- The abdominal wall musculature is grasped and lifted up
- The abdominal cavity is entered and a single blade of the scissors is used to push the intra-abdominal contents away
- The incision is extended to 2-3 cm
V. Exposure of the Cecum
- The cecum with its blind ending pouch is identified and exteriorized
- The cecum is isolated from the rest of the mouse using a pre-cut, sterile gauze
- Warm saline is used to keep the cecum moist
VI. Injection of Cells into the Cecal Wall
- A 27 G or finer needle is used to inject a 50 µL volume of cells into the cecal wall
- The needle is removed
- The injection site is inspected to ensure no leakage
- The cecum is returned to the abdominal cavity
VII. Transplantation of Tumor onto the Cecum
Note: In addition to injecting cells into the cecal wall, an alternative approach is to transplant tumor onto the cecum
- A figure of 8 stitch is placed onto the cecum using 6-0 or 7-0 sized suture
- The cecal wall is lightly damaged
- Then, the tumor piece is positioned
- The stitch is tied down
- The cecum is returned to the abdominal cavity
VIII. Mouse Abdominal Wall Closure and Recovery
- The mouse abdominal wall is closed using three interrupted stitches using 3-0 or 4-0 sized suture
- Alternatively, one can use a simple running stitch
- Post-operative analgesics and a fluid bolus may be given at this point
- The mouse is allowed to recover from anesthesia.
Note: With inhaled anesthetics this typically takes 30 seconds
IX. Results – Primary Tumor and Liver Metastasis
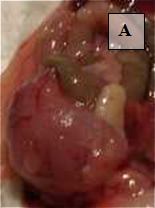
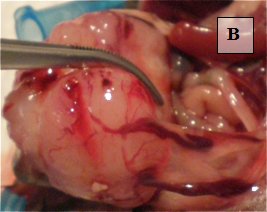
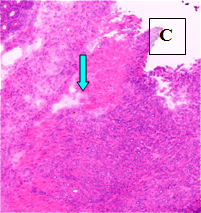
Primary Tumor – shown in situ (A) and with evidence of neovascularization (B); on H&E staining, tumors are locally invasive (C)
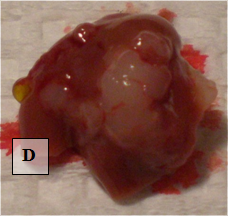
Liver Metastases – shown ex vivo (D)
Discussion
Although mouse subcutaneous tumor models are easy to establish and monitor, it is clear that this model cannot replicate the original anatomic site of colorectal cancer. Due to the difference in microenvironment, colorectal cancer cells growing under the skin have been shown to change their phenotype and almost always fail to progress and metastasize 1,2. In fact, tumor response to therapy can vary dramatically depending on whether cancer cells are implanted in an ectopic (subcutaneous) versus orthotopic location 3. Orthotopic mouse models of colorectal cancer, which feature cancer cells growing in their natural location, replicate human disease with high fidelity. The two techniques to establish this model have unique advantages and disadvantages. Injection of a cell suspension into the cecal wall introduces colorectal cancer cells which have been previously growing in an in vitro environment and are arguably homogeneous. Cells may have reduced invasive or metastatic potential after several passages in culture. Transplantation of a piece of subcutaneous tumor introduces a more heterogeneous population of cancer cells that have been established in vivo. However, tumors contain stromal cells and frequently have necrotic portions which may affect the consistency of this model (personal communication, I. Fidler, MD Anderson). From a technical standpoint, our laboratory has found that injection of a cell suspension into the cecal wall is more difficult and carries the risks of intraluminal injection and leakage post-injection. Interestingly, an orthotopic mouse model of rectal cancer has been described that does not require mouse laparotomy 4. Mice are anesthetized and the rectal mucosa is prolapsed with digital pressure. A small volume of colorectal cancer cells in single cell suspension is injected submucosally. Primary, invasive rectal cancers develop in mice as early as one week after injection; however, none of the mice develop liver metastases and the authors did not comment on the frequency of lung metasases.
Acknowledgements
Department of Surgery, University of California, San Francisco
Raymond Shaheen, M.D.
Materials
| Material Name | Tipo | Company | Catalogue Number | Comment |
|---|---|---|---|---|
| Cell culture media and components | will vary depending on cell line used | |||
| Phosphate Buffered Saline | BioWhitaker | 17-512F | also sold by a number of other vendors | |
| 15 mL centrifuge tubes, polypropylene | Corning | 430790 | ||
| 6 well plates with lid | Becton Dickinson Labware | 353046 | ||
| Iris forceps, straight, serrated tips | World Precision Instruments | 15914 | best to have a pair of these instruments | |
| Iris dissecting scissors, straight, sharp | World Precision Instruments | 14393 | ||
| Needle Holder (regular) | World Precision Instruments | 14109 | ||
| Needle Holder (Castroviejo) | World Precision Instruments | 14137 | ||
| Cidex Plus | World Precision Instruments | 7364 | instruments should be autoclaved periodically, but may also be sterilized by soaking in Cidex | |
| 6-0 or 7-0 suture | for cecum; courtesy of Department of Surgery, UCSF | |||
| 3-0 or 4-0 suture | for abd wall; courtesy of Department of Surgery, UCSF | |||
| Anesthetic machine / Isoflurane | J.A. Baulch and Associates | 3206 | also sold by a number of other vendors | |
| Heating Pad, disposable, 6″ x 6″ | Prism Technologies | 20419 | ||
| Tape, orange, 13 mm x 13 m | Fisher Scientific | 15901F | for restraining anesthetized mouse | |
| Pro-Cord/Cordless Trimmer | Oster | 78997-010 | for shaving mouse fur | |
| Betadine | Fisher Scientific | 19-027132 | may also be purchased at any medical supply store | |
| 2 x 2 gauze, 3-ply | Johnson and Johnson | 7635 | may also be purchased at any medical supply store | |
| Sterile Field, Barrier, 16″ x 29″ | Johnson and Johnson | 0905 | may also be purchased at any medical supply store | |
| Cotton tipped applicators, 6″ | Fisher Scientific | 14-960-3Q | may also be purchased at any medical supply store | |
| Syringe, 10 cc, Luer lock tip | BD | 309604 | ||
| Syringe, 1 cc, tuberculin, slip tip | BD | 309602 | ||
| 27 1/2 G needle | BD | 305109 | ||
| 30 G needle | BD | 305106 |
Riferimenti
- Heijstek, M. W., Kraneburg, O., Rinkes Borel, I. H. Mouse models of colorectal cancer and liver metastases. Dig Surg. 22, 16-25 (2005).
- Kobaek-Larsen, M., Thorup, I., Diederichsen, A., Fenger, C., Hoitinga, M. R. Review of colorectal cancer and its metastases in rodent models: comparative aspects with those in humans. Comp Med. 50, 16-26 (2000).
- Wilmanns, C., Fan, D., O'Brian, C. A., Bucana, C. D., Fidler, I. J. Orthotopic and ectopic organ environments differentially influence the sensitivity of murine colon carcinoma cells to doxorubicin and 5-fluorouracil. Int J Cancer. 52, 98-104 (1992).
- Kashtan, H., Rabau, M., Mullen, J. B., Wong, A. H. C., Roder, J. C., Shiptz, B., Stern, H. S., Gallinger, S. Intra-rectal injection of tumor cells: a novel animal model of rectal cancer. Surg Oncol. 1, 251-256 (1992).

