Inchworming: A Novel Motor Stereotypy in the BTBR T+ Itpr3tf/J Mouse Model of Autism
Summary
Inchworming is a highly repetitive synchronous digging motion displayed by BTBR T+ Itpr3tf/J (BTBR) mice when placed in a testing cage with sufficient sawdust bedding. The procedure is a modification of the juvenile social interaction protocol and is used here to assess repetitive motor stereotypies relevant to Autism Spectrum Disorder.
Abstract
Autism Spectrum Disorder (ASD) is a behaviorally defined neurodevelopmental disorder characterized by decreased reciprocal social interaction, abnormal communication, and repetitive behaviors with restricted interest. As diagnosis is based on clinical criteria, any potentially relevant rodent models of this heterogeneous disorder should ideally recapitulate these diverse behavioral traits. The BTBR T+ Itpr3tf/J (BTBR) mouse is an established animal model of ASD, displaying repetitive behaviors such as increased grooming, as well as cognitive inflexibility. With respect to social interaction and interest, the juvenile play test has been employed in multiple rodent models of ASD. Here, we show that when BTBR mice are tested in a juvenile social interaction enclosure containing sawdust bedding, they display a repetitive synchronous digging motion. This repetitive motor behavior, referred to as "inchworming," was named because of the stereotypic nature of the movements exhibited by the mice while moving horizontally across the floor. Inchworming mice must use their fore- and hind-limbs in synchrony to displace the bedding, performing a minimum of one inward and one outward motion. Although both BTBR and C56BL/6J (B6) mice exhibit this behavior, BTBR mice demonstrate a significantly higher duration and frequency of inchworming and a decreased latency to initiate inchworming when placed in a bedded enclosure. We conclude that this newly described behavior provides a measure of a repetitive motor stereotypy that can be easily measured in animal models of ASD.
Introduction
Although the pathophysiology of autism spectrum disorders (ASD) remains unclear, diagnosis depends upon the presence of three broad symptom categories: abnormal social reciprocity, impairments in communication, and repetitive/stereotypic behaviors1. Most rodent models are unable to simultaneously reproduce all three symptoms in a single animal. Thus, models that can reliably simulate some or all aspects of the disorder hold great value for the development of efficacious preventative and therapeutic strategies. One model that does display all three aspects of ASD is the BTBR mouse1,2.
In combination with ultrasonic vocalizations and social approach assays, the juvenile social interaction test is commonly used to characterize mouse models of pediatric ASD; these tests measure decreased and abnormal social behaviors which represent core features of ASD. In addition to the juvenile social interaction test, many other tests have been used to measure stereotypic behaviors in animal models of ASD. For example, the marble burying test, which examines the tendency of an animal to displace bedding material using the snout and forepaws in effort to cover an object, is thought to measure compulsive stereotypic behaviors that occur in response to aversive stimuli3,4. Accordingly, digging tests have been implemented to quantify this repetitive behavior in ASD animal models and different species of rodents3,5-7. Digging behaviors are defined as obvious directed actions of the snout or paws to displace bedding materials and have been hypothesized to be a sensitive parameter of repetitive motor behavior4.
Here, we report a novel type of digging, which we have termed "inchworming," in the BTBR strain of mice. The characterization of this novel behavior may provide a robust measure of a repetitive motor behavior that may be used for further validation of rodent models such as ASD, Huntington' Disease or obsessive compulsive behavior8,9. We define "inchworming" as at least one synchronous movement of the fore- and hind-limbs inwards followed by a synchronous movement outwards which leads to the displacement of bedding material. This differs from typical digging behavior in many ways. First, the inchworming behavior is usually observed as a combination of episodes rather than a single isolated event. In contrast, digging may occur as single coordinated movement of either the hind or fore paws3. In addition, inchworming movements tend to occur horizontally across the enclosure floor as opposed to the stationary positions that are characteristic of digging behaviors. Finally, the inchworming behavior described below occurs frequently in the BTBR inbred strain of mice but is rarely identified in the B6 control strain, whereas typical digging behaviors are common in B6 and BTBR strains of mice10. The inchworming test procedures outlined in this study represent modifications of a standard juvenile social interaction protocol1.
Given that marble burying, digging, and juvenile play have established value for measuring abnormal behavior in mouse models of ASD, the inchworming behavior complements these previously defined measurements of stereotypic behaviors. In particular, the inchworming behavior provides a useful measure of lower order motor stereotypies that are commonly identified in ASD8,11. In addition, this new inchworming procedure may provide researchers with an additional valuable tool for the analysis of repetitive motor behaviors relevant to developmental disorders such as ASD.
Protocol
All procedures were carried out in accordance with the Canadian Animal Care Committee and approved by the University of Calgary Animal Care Committee.
1. Animal Preparation
- Isolate mice overnight (12 hr) prior to testing. To do this, place all of the BTBR and B6 mice (P35-P40) needed for experimental analysis in cages by themselves, the day before testing.
- Place the isolated mice in the behavioral analysis room 30 min prior to testing so that animals will habituate to the novel environment.
2. Equipment Preparation
- Place the 30 x 30 cm Plexiglas box with a removable lid in the testing room. Place the box on a table and position the video camera to a 45° angle from the bottom; this location of the camera will permit visualization of the entire floor of the enclosure and the mouse. Use an infrared backlight to help minimize glare from the wall of the box.
- Use a video camera that possesses a high zoom function with infrared capabilities to optimize filming in a low lux environment. Focus the camera on the enclosure enlarging the image so that the mice are visualized throughout the entire box.
- Cover the entire floor of the box with sawdust bedding (should be approximately 1 inch deep).
3 Testing Procedure
- Place an intra-strain pair of mice (BTBR – BTBR or B6-B6) within the testing enclosure.
- Turn the lights off in the testing room.
- Start the video camera to record and exit the room. This will prevent experimenter influence on the behavior of the mice. Record the activity for 11 min.
- Once the recording is complete, re-enter the testing room and return the mice to their original home cages.
- Dispose of the sawdust bedding, wash the entire testing box with 70% ethanol, and place new sawdust bedding in the bottom of the testing enclosure.
- Repeat the entire procedure for the rest of the intra-stain pairs and return the mice to their original home cages.
Note: To verify the reliability of the inchworming data, the procedure may be repeated on subsequent days with different BTBR-BTBR and B6-B6 pair combinations. This will ensure that the observed behavior is not dependent upon the specific partner in the enclosure, but the inchworming individual.
4. Data Analysis
- Inchworming is defined as the synchronous inward and outward movement of the animal's fore- and hind-feet, with a minimum of one co-occurring inward and then subsequent outward movement that effectively displaces the bedding. Manually watch and score each video twice (once for each mouse) to ascertain the total duration, frequency, and latency to inchworm in the 10 min video. Manual scoring of inchworming duration, frequency and latency should be based on the following definitions:
Inchworming Duration: Is defined as the total time each mouse spends engaged in the inchworming behavior. To score this aspect of inchworming, use a timer that can be started and stopped. Start timing when the mouse begins to inchworm and stop the timer when the mouse stops inchworming. When and if the mouse begins to inchworm again, re-start and re-stop the timer. Repeat the starting and stopping of the timer for the duration of the 10 min observation period each time the mouse inchworms. This will generate an inchworming duration score that is between 0:00 and 10:00 min.
Inchworming Frequency: Is defined as the number of times the mouse engages in the inchworming behavior in the 10 min observation period. A single count is awarded each time the mouse starts and stops inchworming. The inchworming frequency score will be a number between 0 and ∞.
Latency to Inchworm: Is defined as the time that passes prior to the first onset of inchworming. Start the timer when the video starts, this will be time 0:00, and stop the timer when the mouse inchworms for the first time. If the mouse does not display the inchworming behavior the latency to inchworm will be 10:00. When analyzing latency to inchworm, a score between 0:01 and 10:00 min will be obtained. Mice that fail to inchworm for the duration of the testing period should be removed from the sample population to ensure that the results are pertinent to a population of mice that display the inchworming behavior. - Calculate the total frequency and duration of inchworming for each mouse in the video. Calculate the time required for the first mouse in the pair to inchworm for the latency to inchworm for that specific pair of mice. These parameters are based on prior methodological work related to repetitive digging behaviors in mice6.
- Calculate the average duration, frequency, and latency to dig for each experimental group in the analysis (i.e. BTBR vs. B6). Use an ANOVA or a t-test to compare data between strains/experimental groups.
Note: When the procedure is completed on a second day, use a repeated measures ANOVA to ensure that there were no subsequent trial effects or pairing effects.
Representative Results
The modified version of the juvenile play test described here can be used to observe and analyze the novel repetitive digging behavior termed inchworming. Inchworming is exhibited to a far greater extent by BTBR mice than B6 controls. In a typical 10 min session, BTBR mice will display the distinct inchworming behavior (synchronous inward and outward movement of the paws in an effort to displace the sawdust bedding) for approximately 5 min, or half of the observation period. Furthermore, the latency to inchworm is significantly shorter for BTBR mice – they typically begin inchworming within the first 10 sec of the start of the recorded session. In contrast, control mice (B6) have a mean latency to inchworm of 4 min and spend very small portions of their time actually displaying the behavior. These findings are similar to data from prior studies on repetitive digging behaviors in rodents3,6.
Figure 1 depicts the proper experimental set up for testing procedures. Figure 2 shows the average inchworming duration (A), frequency (B) and latency (C) to inchworm for the BTBR mice and the control B6 strain. All parameters were significantly different between the strains. In the experiment reported here, the inchworming test was administered over a 2 day trial period, with alternate pairings in the second day, and the results of each day were compared. No significant differences were found, thus ensuring that the effects were not dependent on either specific mouse pairings or the repetition of the test. Finally, Figure 3 contains still photos of different mice engaged in the two primary components of the inchworming behavior.

Figure 1. Equipment set up for the inchworming experiment. Equipment set up for the inchworming experiment. A 30 cm cube Plexiglas box is set on a table in front of an infrared zoom camera positioned at a 45° angle. The bottom of the enclosure is covered fully with an even layer of at least 1 inch of sawdust.
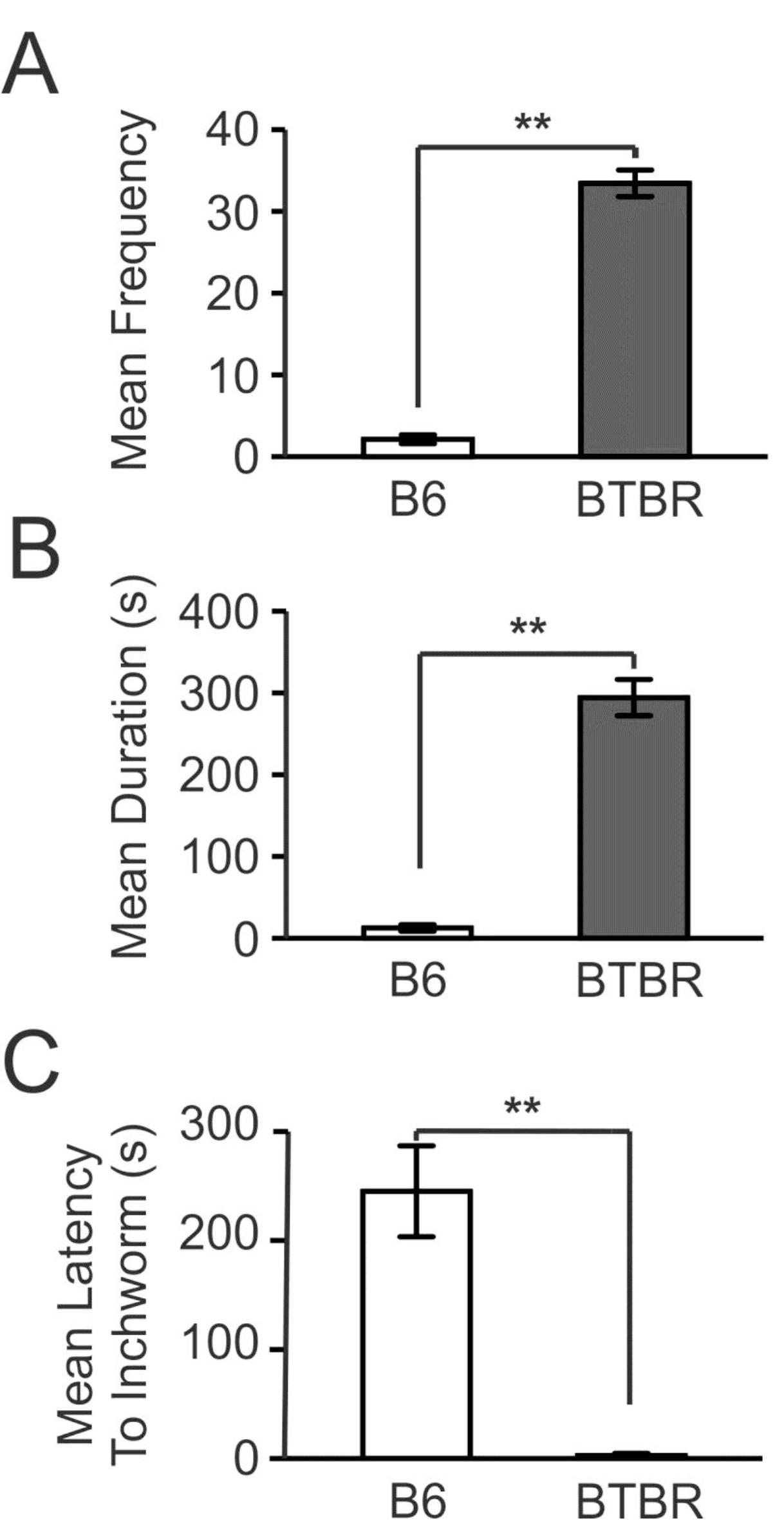
Figure 2. Average inchworming duration, frequency and latency displayed by pairs of BTBR and B6 mice. The BTBR (n = 8) and B6 (n = 8) mice were tested at P35-P40 over a two day testing period and scored for inchworming behavior. A) The BTBR strain displayed a significantly longer mean duration of inchworming than the B6 control strain (p < 0.001). B) The BTBR mice displayed a significantly higher frequency of inchworming as compared to B6 controls (p < 0.001). C) There was a significant difference in BTBR (n=8) and B6 (n=7) latency to dig with BTBR displaying a marked decrease in the time it took to display the behavior compared to B6 controls (p = 0.001).
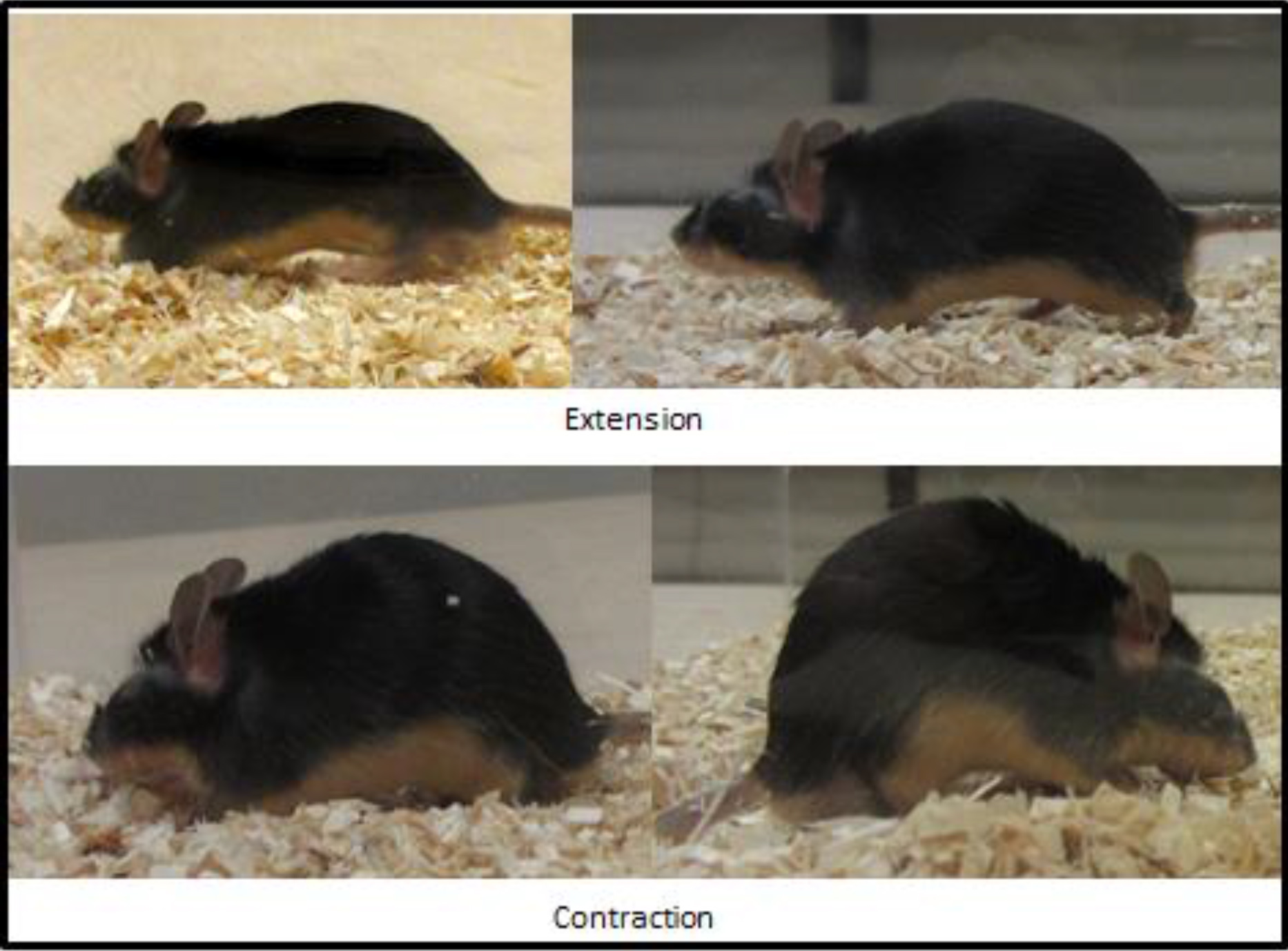
Figure 3. Illustrative representation of different mice in both aspects of the inchworming behavior. The upper panel is an example of mice in the extension phase of the inchworming behavior, whereas the lower panel is an example of mice in the contraction phase of the inchworming behavior. Inchworming is defined as the synchronous inward and synchronous outward movement of the fore and hind limbs of the mouse to displace bedding.
Discussion
Inchworming is a novel measure of repetitive behavior that can be used to study many disorders that exhibit stereotypies such as ASD. There are 4 steps that are of critical importance to generating reliable inchworming data from the modified juvenile social interaction protocol: 1) the extended isolation period is essential to the inchworming test and should not be removed from the protocol or shortened. This requirement is specific to testing of inchworming in pairs described here. 2) The video camera needs to be appropriately positioned and focused so that the mice can be fully visualized throughout the entire Plexiglas enclosure. 3) The enclosure should be thoroughly cleaned between testing pairs to ensure that the current behaviors are not influenced by scents from the prior pair of mice. 4) The bedding material for the enclosure must be sufficient (~1 inch deep) and must be sawdust; mice do not display the inchworming behavior with little/no bedding or when placed on other bedding materials.
While not described here, we have observed inchworming behavior in non-isolated, socially housed BTBR mice placed alone (rather than in pairs) in a similar bedded enclosure. Under these conditions, the BTBR mice still inchworm, but at a lower frequency and duration, thus indicating that the inchworming behavior is a stereotypic behavior possibly induced by social anxiety (data not shown). However, given that studies have failed to reliably demonstrate differences in anxiety between the BTBR and B6 control mice, anxiety is likely a interacting with other factors to induce this behavior12. However, the focus of this methodological article was the standardized modification to the juvenile social interaction protocol because it elicits the behavior rapidly and reliably, with dramatic differences between BTBR and B6 mice. In addition, data are collected from two animals in a single session, thus increasing the information gained from each testing period. In our laboratory, the inchworming behavior has only been confirmed in the age range of P35-P55. Although it is possible that mice younger and older will display this repetitive behavior, failure to identify inchworming at other ages may indicate that the behavior is specific to adolescent and young adult mice.
The data from this experiment indicate clearly that the BTBR mouse strain engages in the repetitive inchworming behavior more rapidly, more frequently and for longer durations than the B6 controls. The inchworming behavior may therefore provide researchers with a new measure of repetitive behaviors in other ASD and disease models. Although it is not yet clear what drives this repetitive digging motion, BTBR mice also display higher levels of self-grooming and social avoidance1,9. As digging is an innate genetically-regulated trait in mice, testing of the inchworming behavior can be applied easily to other strains and has the potential to be a valuable tool for assessing motor stereotypies and compulsive behaviors in other disease models.
One of the hallmark behavioral traits that characterize ASD is repetitive behavior with restricted interests9. In addition to highlighting a valuable motor stereotypy, this experiment has increased the validity of the BTBR mouse model of ASD by demonstrating an additional repetitive behavior similar to the clinical presentation of motor stereotypies in ASD patients. Inchworming is displayed in both the BTBR (ASD) mice and B6 control mice, although the behavior is comparatively rare in B6 mice. The behavior that most closely resembles inchworming is digging behaviors in rodents. Thomas et al.4 suggests that for rodents, digging serves a variety of functions including food storage, predator evasion, and protection from aversive environments. As these functions are not necessary in the laboratory setting, mice who display high levels of digging behavior may be fulfilling a compulsive behavioral need4. Furthermore, Silverman et al.9 suggests that digging behavior should be incorporated into the analysis of the juvenile social interaction test as a measure of non-social activity.
In summary, inchworming is a highly repeated behavior significantly more prevalent in BTBR mice (a model of ASD) compared to B6 mice (control strain). Although the etiology of the behavior is currently unknown, it may be a valuable resource for researchers examining compulsivity and repetition in rodent models of many diseases. The procedural techniques are simple and can be completed with ease in a 24 hr time period. Data analysis is more complex and time-consuming, but video recording the testing procedure allows the researcher to complete the analysis off-line and repetitively to ensure inter-rater consistency and reliability.
Divulgazioni
The authors have nothing to disclose.
Acknowledgements
The authors are grateful for the technical and logistical assistance and expertise provided by Rose Tobias, Younghee Ahn, and David N. Ruskin. The work described here was funded by the Alberta Children's Hospital Foundation and the Alberta Children's Hospital Research Institute.
Materials
| Material Name | Company | Catalogue Number | Comments |
| 30 cm X 30 cm Plexiglas box with lid | Can be constructed | ||
| IR Camera | Survshop (Calgary, Alberta) | Sony CCD Camera DP955V- 30′ Infrared Armor Dome | Find a camera with high-resolution and zoom capabilities |
| DVR | Supercircuits (Austin, Texas) | BLACK Enterprise-Class 4-Channel H.264 security DVR with DVD Burner | High data capacity |
| Sawdust | Same as bedding material |
Riferimenti
- McFarlane, H. G., Kusek, G. K., Yan, M., Phoenix, J. L., Bolivar, V. J., Crawley, J. N. Autism-like behavioral phenotypes in BTBR T+tf/J mice. Genes, Brain, and Behavior. 7, 152-163 (2008).
- Crawley, J. N. Designing mouse behavioral tasks relevant to autistic-like behaviors. Mental Retardation and Developmental Disabilities Research Reviews. 10 (4), 248-258 (2004).
- Deacon, R. Digging and marble burying in mice: simple methods for in vivo identification of biological impacts. Nature Protocols. 1 (1), 122-124 (2006).
- Thomas, A., Burant, A., Bui, N., Graham, D., Yuva-Paylor, L. A., Paylor, R. Marble burying reflects a repetitive and perseverative behavior more than novelty-induced anxiety. Psychopharmacology. 204, 361-373 (2009).
- Dudek, B. C., Adams, N., Boice, R., Abbott, M. E. Genetic influences on digging behaviours in mice (Mus musculus) in laboratory and seminatural settings. Journal of Comparative Psychology. 97 (3), 249-259 (1983).
- Webster, D., Williams, M. H., Owens, R., Geiger, V., Dewsbury, D. A. Digging behavior in 12 taxa of muriod rodents. Animal Learning and Behavior. 9 (2), 173-177 (1981).
- Pobbe, R. H., Pearson, B. L., Defensor, E., Bolivar, V. J., Blanchard, D. C., Blanchard, R. J. Expression of social behaviours of C57BL/6J versus BTBR inbred mouse strains in the visible burrow system. Behavioural Brain Research. 214, 443-449 (2010).
- Lewis, M. H., Tanimura, Y., Lee, L., Bodfish, J. Animal models of restricted repetitive behavior in autism. Behavioural Brain Research. 176, 66-74 (2007).
- Silverman, J. L., Yang, M., Lord, C., Crawley, J. N. Behavioural phenotyping assays for mouse models of autism. Nature Reviews. 11, 490-502 (2010).
- Deacon, R., Rawlins, J. Hippocampal lesions, species-typical behaviours and anxiety in mice. Behavioural Brain Research. 156, 241-249 (2005).
- Pearson, B. L., et al. Motor and cognitive sterotypies in the BTBR T+tf/J mouse model of autism. Genes, Brain, and Behavior. 10, 228-235 (2011).
- Pobbe, R. H., Defensor, E., Pearson, B. L., Bolivar, V. J., Blanchard, D. C., Blanchard, R. J. General and social anxiety in the BTBR T+ tf/J mouse strain. Behavioural Brain Research. 216 (1), 446-451 (2011).

