Real-time Imaging of Endothelial Cell-cell Junctions During Neutrophil Transmigration Under Physiological Flow
Summary
Leukocytes cross the endothelial monolayer using the paracellular or the transcellular route. We developed a simple assay to follow the distribution of endogenous junctional VE-cadherin and PECAM-1 during leukocyte transendothelial migration under physiological flow to discriminate between the two transmigration routes.
Abstract
During inflammation, leukocytes leave the circulation and cross the endothelium to fight invading pathogens in underlying tissues. This process is known as leukocyte transendothelial migration. Two routes for leukocytes to cross the endothelial monolayer have been described: the paracellular route, i.e., through the cell-cell junctions and the transcellular route, i.e., through the endothelial cell body. However, it has been technically difficult to discriminate between the para- and transcellular route. We developed a simple in vitro assay to study the distribution of endogenous VE-cadherin and PECAM-1 during neutrophil transendothelial migration under physiological flow conditions. Prior to neutrophil perfusion, endothelial cells were briefly treated with fluorescently-labeled antibodies against VE-cadherin and PECAM-1. These antibodies did not interfere with the function of both proteins, as was determined by electrical cell-substrate impedance sensing and FRAP measurements. Using this assay, we were able to follow the distribution of endogenous VE-cadherin and PECAM-1 during transendothelial migration under flow conditions and discriminate between the para- and transcellular migration routes of the leukocytes across the endothelium.
Introduction
Efficient and tightly controlled leukocyte transendothelial migration (TEM) is of key importance in physiological processes such as immune surveillance and acute inflammation. However, under certain patho-physiological conditions, uncontrolled and excessive TEM is observed resulting in chronic inflammatory diseases (e.g., rheumatoid arthritis, atherosclerosis, asthma). Also during tumor cell metastasis, the process of transendothelial migration is instrumental for tumor cells to leave the circulation to metastasize1-3. In order to specifically interfere with excessive leukocyte or tumor cell TEM, a detailed understanding of the regulation of this process is required.
It is believed that the TEM process occurs through different steps. Seminal studies, reviewed two decades ago by Butcher and Springer, led to the multistep model describing the process of TEM4,5. This model still holds true, although some additional steps have been included6. Alon et al. described the need for the presence of immobilized chemokines on the surface of the endothelium7. Recently, they showed that the endothelium itself generates chemokines which are presented at the endothelial apical surface8. Moreover, the same group put forward the importance of flow conditions during TEM7. Recently, several publications focused on the two different routes leukocytes can take at the final diapedesis stage of TEM. They can either go through the cell-cell junctions, i.e., the paracellular migration route, or cross through the endothelial cell body, known as the transcellular migration route9. Carman and colleagues studied these pathways in detail and concluded that leukocytes preferentially choose the paracellular migration route (90%) over the transcellular route (10%) when crossing an umbilical vein endothelial monolayer10. However, when endothelial cells from other origins were used, e.g., brain or microvasculature, more leukocytes used the transcellular route (30%)11. The Vestweber group recently showed that when the cell-cell junctions were unable to dissociate from each other by using an knock-in animal model, replacing endogenous VE-cadherin for a VE-cadherin-alpha-catenin chimera, leukocyte TEM was fully blocked12. Surprisingly, the authors noticed that TEM was blocked in several, but not all, tissues. Overall, these elegant experiments indicated that leukocytes preferred the paracellular route over the transcellular route, although the regulatory signals that trigger this decision are still unknown.
Even though the majority of the leukocytes prefer the paracellular migration route, it is still difficult to discriminate between both pathways. In addition to that, despite several studies focusing on the role of the endothelial cell-cell junctions, the dynamics of these junctions, in particular the junctional proteins VE-cadherin and PECAM-1, during leukocyte crossing is still under debate. We developed a relatively simple assay in which these junction molecules can be monitored in real-time during leukocyte diapedesis under physiological flow conditions using fluorescently-labeled antibodies. These antibodies did not interfere with or block junctional integrity or mobility of the targeted proteins. This assay allows us to follow the dynamics of junctional proteins during the process of paracellular TEM. Additionally, this assay also allows discriminating between the para- and transcellular migration routes.
Protocol
Neutrophils were isolated from healthy volunteers who have signed an informed consent. Research has been performed in compliance with the institutional and national guidelines for human welfare.
1. Plating and Maintenance of Human Umbilical Vein Endothelial Cells
- Culture Human Umbilical Vein Endothelial Cells (HUVECs) according to manufacturer’s instructions. Grow HUVECs on fibronectin (FN)-coated dishes (10 μg/ml, dissolved in demineralized water) using media (Endothelial Basal Medium (EBM-2) medium supplemented with Endothelial Growth Medium (EGM-2) singlequots). Use cell culture between 4-8 passages for experiments.
- Day 1: Coat flow-chambers with 100 μl of fibronectin (10 μg/ml in PBS) for at least 2 hr at 37 °C and 5% CO2.
- Day 2: When cells reach 80-90% confluence, trypsinize the cells after careful washing with RT phosphate buffered saline (PBS) pH 7.4, centrifuge at 800 x g and resuspend at 800,000 cells/ml using media. Plate 80,000 cells in every single channel of the FN-coated flow-chambers and gently pipette the cell suspension up and down. Culture O/N in an incubator at 37 °C and 5% CO2.
- Day 3: Refresh the media in the flow-chamber slide by gently tilting the slide at a 45° angle. (see Figure 1A).
NOTE: It is recommended to only remove the media in the reservoirs and not in the channel itself. Removal of the media in the channels may result in endothelial cell loss and death due to the dragging force of the media caused by pipetting. - Check by phase contrast microscopy if the endothelial cells have formed a monolayer (i.e., 100% confluent). If the cells are not 100% confluent, change the media two times a day until they reach 100% confluence.
- Once the cells have reached confluency, stimulate the cells with media (see step 1.1) containing inflammatory mediator (TNF-α (10 ng/ml)). Stimulating HUVECs O/N (i.e., 12 hr) with TNF-α results in an inflammatory phenotype of the endothelium, i.e., up-regulation of cell adhesion molecules such as ICAM-1 and VCAM-1 13.
2. Polymorphonuclear Leukocyte Isolation Using Percoll Gradients
- Prepare N-2-hydroxyethylpiperazine-N'-2-ethanesulfonic acid (HEPES)-buffer (hereafter called: flow-buffer) before isolating polymorphonuclear leukocytes (PMN). Flow-buffer is used to wash isolated PMNs and as buffer in the flow assay. To prepare flow-buffer: dilute 7.72 g NaCl (132 mM), 4.76 g HEPES (20 mM), 0.45 g KCl (6 mM), 0.25 g MgSO4•7H2O (1 mM), K2HPO4•3H2O (1.2 mM) in 1 L of demineralized water and adjust to pH 7.4 (this stock can be kept at 4 °C for several weeks).
- Add fresh 100 μl 1 M CaCl2 (1 mM), 2.5 ml human albumin from a 200 g/L stock concentration (0.5% v/v) and 0.1 g glucose to 100 ml (0.1% w/v) of flow-buffer. Next, filter the flow-buffer using a 0.45 μm filter. NOTE: This flow-buffer needs to be prepared fresh for every experiment.
- Prior to isolation of PMN, prepare 10% trisodium citrate (TNC) solution in PBS, pH 7.4.
- Collect 20 ml of whole blood in sodium heparine vacuettes from a healthy volunteer. Dilute whole blood 1:1 with 10% PBS/TNC in 50 ml tubes and pipette 20 ml diluted blood carefully onto 12.5 ml of Percoll (a 23% (w/w) colloidal solution in water with a density of 1.130 g/ml) in a new 50 ml tube. Carefully place the tubes in the centrifuge and spin for 20 min at 800 x g with low acceleration and no break set at RT.
NOTE: When adding the diluted blood to the Percoll, tilt the Percoll-containing tube in a 45° angle and gently pipette the diluted blood in the tube with the slowest pipette boy setting. - Remove all liquid and subsequently fill the tube with ice-cold erythrocyte lysis-buffer (4.15 g NH4Cl [0.155 M], 0.5 g KHCO3 [0.01 M] and 18.5 mg EDTA (triplex III) [0.1 mM] to 500 ml of ice-cold H2O) to lyse erythrocytes. Leave tube on ice, occasionally inverting the tube, until the suspension turns dark red, followed by centrifugation 500 x g for 5 min at 4°C with breaks enabled.
NOTE: The pellet fraction contains the PMNs (neutrophils, eosinophils, basophils) together with the erythrocytes. - Remove supernatant and wash pellet twice in ice-cold lysis-buffer at 500 x g for 5 min at 4 °C.
- Resuspend the pellet with flow-buffer at RT and determine PMNs concentration using either a hemocytometer or automated cell counter. Suspend the PMNs in flow-buffer at 1 x 106 cells/ml and keep at RT.
3. Labeling of Endothelial Junctional VE-cadherin and PECAM-1
- Add the PECAM Alexa Fluor 647 antibody (clone WM59) at a 1:100 dilution and the calcium-independent VE-cadherin-FITC antibody (clone 55-7H1) at a 1:50 dilution to HUVEC culture medium (see step 1.1), and incubate for 30 min prior to starting the flow experiment to visualize junctional dynamics of endothelial cells during neutrophil transmigration under physiological flow conditions.
NOTE: Before adding the directly-labeled antibodies to the flow-chambers, ensure that the volume in each channel does not exceed 100 μl. This helps keep the antibody expenses as low as possible.
4. PMN TEM Assay Under Flow
- Place the PMNs into a water bath for 15 min at 37 °C prior to injecting them into the flow-system.
- Connect the tubing to an empty flow-chamber and fill with warm (e.g., 37 °C) flow-buffer (see step 4.3) to prevent formation of air-bubbles when setting up the flow system.
- Connect one side of an empty flow-chamber to the syringe pump flow system by using silicone tubing containing a 20 ml syringe, and put the flow-chamber into the microscope stage.
- Connect the other side of an empty flow-chamber to the reservoir flask filled with 37 °C flow-buffer (Figure 1B) and start the syringe pump in order to fill all the tubing with flow-buffer. The pump will pull the flow-buffer from the reservoir through the flow-chamber into the syringe. NOTE: This tubing also contains an in-line Luer injection port, which allows PMNs to be injected with a needle into a running experiment without stopping the flow and creating air bubbles.
- Replace the empty flow-chamber with the flow-chamber containing TNF-α-treated HUVECs, connect the flow-buffer-containing tubing and place it into the microscope stage (Figure 1C). Pinch off the tubes before disconnecting and reconnecting them to the chambers containing the HUVECs, as not pinching off may result in the formation of air bubbles inside the tubing and/or flow-chambers.
- Adjust the flow speed to 1 dyn/cm2, in accordance with physiological flow speed in post capillary venules (1-5 dyn/cm2).
- Record Differential Interference Contrast (DIC), FITC (488 nm) and Alexa Fluor 647 (647 nm) simultaneously using a confocal laser scanning microscope.
- Inject the PMNs (step 4.1) slowly in the flow-system via the in-line Luer injection port (Figure 1D) using 1 ml syringes.
- After a few minutes, leukocytes appear, adhere, and transmigrate. Stop the experiment at any desired moment by disconnecting the tubing from the flow-chamber and pipetting fixative (3.7% formaldehyde in PBS) into the flow-chamber. Allow fixation for 10 min, followed by washing with PBS. Data is analyzed using imaging software (see materials and equipment table).
NOTE: Perform the he experiment using a confocal laser scanning microscope equipped with a climate chamber with a constant temperature at 37 °C, 5% CO2, and a 63X oil-objective.
Representative Results
We first tested if the antibodies did not interfere with the barrier function of the endothelium. We measured the resistance of endothelial monolayers by using electrical cell-substrate impedance sensing (ECIS). For details, see Van Buul et al.13 No change in resistance was observed when the anti-VE-cadherin fluorescently-labeled antibody was added to the cells (Figure 2A). An anti-VE-cadherin antibody that is well-recognized to block VE-cadherin function reduced the resistance dramatically (Figure 2A). Also, the antibodies used for imaging do not alter the dynamics of VE-cadherin, as was assessed by measuring fluorescent recovery after photo-bleaching (FRAP) of VE-cadherin-GFP (Figure 2B).
After 1-2 min, neutrophils adhered to the activated endothelial monolayer as could be visualized in the DIC channel (Figure 3A). After crawling for 5-30 sec, the vast majority of neutrophils transmigrated through the endothelial monolayer through the cell-cell junctions that were labeled with the antibodies directed against PECAM-1 and VE-cadherin. During the process of diapedesis, the distribution of VE-cadherin and PECAM-1 was followed in real-time (Figure 3B). At sites of neutrophil diapedesis, VE-cadherin was locally disrupted and PECAM-1 showed a more ring-like structure. After completion of diapedesis, the junctions close and VE-cadherin and PECAM-1 clearly relocated at the sites of diapedesis (Figure 3C). Note that parts of the anti-PECAM-1 antibody were detected on the surface of the neutrophil once the neutrophil reached the baso-lateral side of the endothelium. However, this did not prevent neutrophils from crossing the endothelium. The observed dynamics of VE-cadherin in real-time during neutrophil transendothelial migration were in agreement with the work by Shaw and colleagues who showed, using confocal microscopy and VE-cadherin-GFP-transfected endothelial cells, that VE-cadherin-GFP diffused laterally when leukocytes crossed the endothelial cell-cell junctions14. Also work by Su and coworkers underscored our observations of PECAM-1. They showed that, next to lateral VE-cadherin diffusion upon leukocyte passage, PECAM-1 was locally redistributed into a ring around the transmigrating neutrophil15.
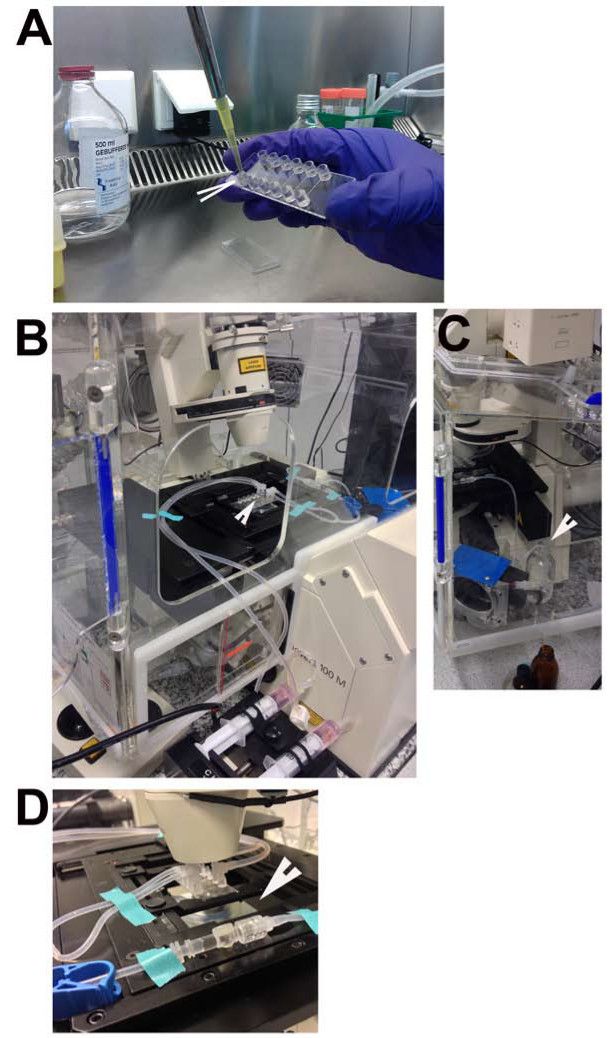
Figure 1. In vitro flow chamber. (A) Arrow indicates the reservoir from which the medium needs to be refreshed. (B) Silicone tubing that connects the flow chamber with the in-line Luer injection port used for injecting PMNs without disconnecting the tubing (arrowhead). (C) Connection of an empty flow-chamber to the reservoir flask filled with 37 °C flow-buffer (arrowhead). (D) Connection of the flow-chamber with the TNF-α-treated HUVECs to the flow-buffer-containing tubing and placed in the microscope stage (arrowhead).
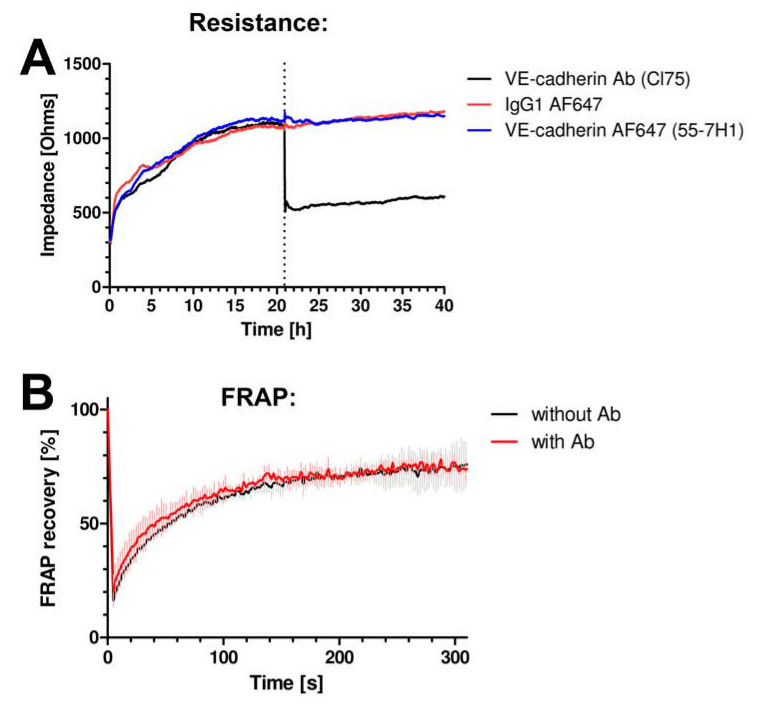
Figure 2. VE-cadherin antibodies do not interfere with junctional dynamics or function. (A) Endothelial cell monolayer impedance is measured using ECIS. Y-axis expresses impedance in Ohms and x-axis represents time in hours. VE-cadherin antibody clone 55-7H1, labeled with ALEXA647 (blue line) or isotype IgG-ALEXA647 control (red line) do not alter endothelial cell monolayer impedance, whereas the VE-cadherin blocking antibody cl75 (black line) did reduce the impedance. (B) VE-cadherin-GFP was expressed in HUVEC and FRAP analysis using confocal microscopy revealed no change in fluorescence recovery in the presence or absence of the VE-cadherin 55-7H1 antibody.

Figure 3. VE-cadherin and PECAM-1 distribution during neutrophil TEM in real-time. (A) Neutrophil (marked with white line) adhering on the endothelium and crossing the cell-cell junction without affecting the distribution of VE-cadherin and PECAM-1. (B) Neutrophil crossing the endothelial monolayer through the cell-cell junctions. A local dispersion of VE-cadherin and PECAM-1 can be observed when a neutrophil protrudes through the cell-cell junctions. White line illustrates neutrophil presence still on top of the endothelium. Yellow line shows neutrophil membrane that is already underneath endothelium. (C) Neutrophil has fully crossed the endothelial monolayer. VE-cadherin and PECAM-1 are relocated at sites of diapedesis. Yellow line illustrates borders of the transmigrated neutrophil. Please click here to view a larger version of this figure.
Discussion
For this protocol, it is critical to prevent the formation of air bubbles in the flow-chambers, since this will result in cell apoptosis and a disrupted monolayer. To avoid this, we wish to stress to pay particular interest to steps 4.2 and 4.5, where the tubes are connected to the flow-chamber. Another critical step in the protocol is the priming of the PMNs that are kept at RT by incubating them for 15-30 min at 37 °C prior to injecting them to the flow-chamber (step 4.1). This results in priming of leukocyte integrins, allowing them to adhere to adhesion molecules such as ICAM-1 or VCAM-1 at the endothelium.
This protocol is not restricted to study the transmigration of neutrophils. Also other leukocyte types such as monocytes or lymphocytes may be used. Note that step 4.1, i.e., priming the leukocytes may differ between leukocyte types. Also, other types of endothelial cells can be used. For this it remains critical to stimulate the endothelial cells with appropriate inflammatory stimuli such as TNF-α or IL-1β. If neutrophils do not respond in transmigrating, one may consider treating the neutrophils briefly (5 min) with N-formyl-L-methionyl-L-leucyl-L-phenylalanine (fMLP) peptide16. This will stimulate the neutrophils, in particular their integrins, even further, making them more prone to adhere to the endothelium.
Typically 1 dyn/cm2 flow speed is used. This flow speed is measured in post-capillary venules, sites where most leukocyte transendothelial migration occurs17. Using the described flow-chambers, it is possible to increase flow speed up to 10 dyn/cm2. However, it is not recommended to increase the shear further. It may result in unwanted leakage of the tubing and detachment of the cells from the flow-chamber.
The results described in Figure 3 indicate that these antibodies can be used to visualize and study the dynamics of endothelial cell-cell junctions during leukocyte diapedesis. In particular, in addition to the existing transmigration assays this protocol allows to discriminate paracellular from transcellular migration under physiological flow conditions in real-time. Since the antibodies stain the cell-cell junctions, one can score the number of leukocytes that cross VE-cadherin/PECAM-1-positive junctions versus nonpositive VE-cadherin/PECAM-1 sites. This way, transcellular migration can be discriminated from paracellular migration.
It is important to underscore that these antibodies do not interfere with the function of the proteins they bind to: the PECAM-1 antibody used in this study is directed against the second extracellular domain. Endothelial cells that were plated in the presence of the antibody did not show any defects in spreading or forming a monolayer, suggesting that the antibody at least did not interfere with homotypic interactions between endothelial cells. in addition to that, both antibodies do not impair the ability of neutrophils to migrate through the endothelial monolayer. Additionally, the number of neutrophils that transmigrate across the endothelial monolayer in the absence or presence of the antibodies is not altered (data not shown). Importantly, confocal laser scanning microscopy gives us the possibility to record different fluorescent channels and DIC simultaneously.
Thus, this assay allows studying the dynamics of VE-cadherin and PECAM-1 at the same time when a neutrophil crosses the endothelial cell-cell junction and will help to understand why leukocytes choose one route over the other, i.e., paracellular versus transcellular.
Divulgazioni
The authors have nothing to disclose.
Acknowledgements
The authors thank Dr. P.L. Hordijk for critically reading the manuscript. AED is supported by a Landsteiner Foundation for Blood Transfusion Research (LSBR) fellowship (grant #1028). JK is supported by the Dutch Heart Foundation (2005T3901). JDvB is a NHS Dekker fellow (grant #2005T039).
Materials
| Name of Material/ Equipment | Company | Catalog Number | Comments/Description | |
| μ-Slide VI | IBIDI | 80606 | Flow-chamber | |
| 0.45 μm filter | Whatman/GE Lifesciences | 10462100 | ||
| 1 mL syringe | BD Plastipak | 300013 | ||
| 20 mL syringe | BD Discardit II | 366296 | ||
| 21G needle | BD Microlance | 301155 | ||
| Albumin | Sanquin | 15522644 | ||
| Ammunium chloride (NH4Cl) | Merck | 1009245000 | ||
| Calcium chloride (CaCl2) | Sigma-Aldrich | 449709 | ||
| EBM-2 Basal medium + EGM-2 SingleQuot Kit Suppl. & Growth Factors | Lonza | CC-3156 + CC-4176 | media | |
| EDTA (Titriplex III) | Merck | 1370041000 | ||
| Falcon tubes | Corning Life Sciences | 352096 | ||
| Fibronectin | Sigma-Aldrich | F1141-2MG | FN | |
| Glucose | Sigma-Aldrich | G7528 | ||
| HEPES | Sigma-Aldrich | H3375 | ||
| In-line Luer Injection port | IBIDI | 10820 | ||
| Magnesium sulfate heptahydrate (MgSO4.7H2O) | Merck | 105886 | ||
| PECAM-1-ALEXA-647 | BD Pharmingen | 561654 | clone WM59 stock concentration 0.1mg/mL |
|
| Percoll | GE Healthcare Life Sciences | 17-0891-09 | ||
| Phosphate Buffered Saline | Fresenius Kabi Nederland | M090001/01NL | PBS | |
| Potassium chloride (KCl) | Sigma-Aldrich | P9541 | ||
| Potassium hydrogen carbonate (KHCO3) | Merck | 1048540500 | ||
| Potassium phosphate dibasic trihydrate (K2HPO4) | Sigma-Aldrich | P5504 | ||
| Silicone Tygon 3350 tubing | VWR | 228-4331 | tubing | |
| Sodium chloride (NaCl) | Calbiochem (Millipore) | 567441 | ||
| Syringe pump | Harvard Apparatus | model number 55-5920 | ||
| TNFα | Peprotech | 300-01A | tumor necrosis factor | |
| trisodium citrate | Merck | 1.06447.5000 | TNC | |
| Vacuettes | Greiner, Germany | 980044 | ||
| VE-Cadherin-FITC | BD Pharmingen | 560411 | clone 55-7H1 | stock concentration 0.5mg/mL |
| Zeiss LSM510 META | Carl Zeiss MicroImaging, Jena, Germany | |||
| Zen Software 2008 | Carl Zeiss MicroImaging, Jena, Germany |
Riferimenti
- Ross, R. Atherosclerosis–an inflammatory disease. N. Engl. J. Med. 340, 115-126 (1999).
- Luster, A. D., Alon, R., von Andrian, U. H. Immune cell migration in inflammation: present and future therapeutic targets. Nat. Immunol. 6, 1182-1190 (2005).
- Szekanecz, Z., Koch, A. E. Vascular involvement in rheumatic diseases: ‘ vascular rheumatology. Arthritis Res. Ther. 10, 224 (2008).
- Butcher, E. C. Leukocyte-endothelial cell recognition: three (or more) steps to specificity and diversity. Cell. 67, 1033-1036 (1991).
- Springer, T. A. Traffic signals for lymphocyte recirculation and leukocyte emigration: the multistep paradigm. Cell. 76, 301-314 (1994).
- Ley, K., Laudanna, C., Cybulsky, M. I., Nourshargh, S. Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nat. Rev. Immunol. 7, 678-689 (2007).
- Cinamon, G., Shinder, V., Shamri, R., Alon, R. Chemoattractant signals and beta 2 integrin occupancy at apical endothelial contacts combine with shear stress signals to promote transendothelial neutrophil migration. J. Immunol. 173, 7282-7291 (2004).
- Shulman, Z., Cohen, S. J., Roediger, B., et al. Transendothelial migration of lymphocytes mediated by intraendothelial vesicle stores rather than by extracellular chemokine depots. Nat. Immunol. 13, 67-76 (2012).
- Carman, C. V., Springer, T. A. Trans-cellular migration: cell-cell contacts get intimate. Curr. Opin. Cell Biol. 20, 533-540 (2008).
- Carman, C. V., Springer, T. A. A transmigratory cup in leukocyte diapedesis both through individual vascular endothelial cells and between them. J. Cell Biol. 167, 377-388 (2004).
- Millan, J., Hewlett, L., Glyn, M., et al. Lymphocyte transcellular migration occurs through recruitment of endothelial ICAM-1 to caveola- and F-actin-rich domains. Nat. Cell Biol. 8, 113-123 (2006).
- Schulte, D., Kuppers, V., Dartsch, N., et al. Stabilizing the VE-cadherin-catenin complex blocks leukocyte extravasation and vascular permeability. EMBO J. 30, 4157-4170 (2011).
- Buul, J. D., Mul, F. P., van der Schoot, C. E., Hordijk, P. L. ICAM-3 activation modulates cell-cell contacts of human bone marrow endothelial cells. J. Vasc. Res. 41, 28-37 (2004).
- Shaw, S. K., Bamba, P. S., Perkins, B. N., Luscinskas, F. W. Real-time imaging of vascular endothelial-cadherin during leukocyte transmigration across endothelium. J. Immunol. 167, 2323-2330 (2001).
- Su, W. H., Chen, H., Jen, C. J. Differential movements of VE-cadherin and PECAM-1 during transmigration of polymorphonuclear leukocytes through human umbilical vein endothelium. Blood. 100, 3597-3603 (2002).
- Paulsson, J. M., Jacobson, S. H., Lundahl, J. Neutrophil activation during transmigration in vivo and in vitro: A translational study using the skin chamber model. J. Immunol. Methods. 361, 82-88 (2010).
- Williams, M. R., Azcutia, V., Newton, G., Alcaide, P., Luscinskas, F. W. Emerging mechanisms of neutrophil recruitment across endothelium. Trends Immunol. 32, 461-469 (2011).

