Stencil Micropatterning of Human Pluripotent Stem Cells for Probing Spatial Organization of Differentiation Fates
Summary
Human pluripotent stem cells (hPSCs) have the intrinsic ability to differentiate and self-organize into distinct tissue patterns; although this requires the presentation of spatial environmental gradients. We present stencil micropatterning as a simple and robust method to generate biochemical and mechanical gradients for controlling hPSC differentiation patterns.
Abstract
Human pluripotent stem cells (hPSCs), including embryonic stem cells and induced pluripotent stem cells, have the intrinsic ability to differentiate into all three germ layers. This makes them an attractive cell source for regenerative medicine and experimental modeling of normal and diseased organogenesis. However, the differentiation of hPSCs in vitro is heterogeneous and spatially disordered. Cell micropatterning technologies potentially offer the means to spatially control stem cell microenvironments and organize the resultant differentiation fates. Micropatterning hPSCs needs to take into account the stringent requirements for hPSC survival and maintenance. Here, we describe stencil micropatterning as a method that is highly compatible with hPSCs. hPSC micropatterns are specified by the geometries of the cell stencil through-holes, which physically confine the locations where hPSCs can access and attach to the underlying extracellular matrix-coated substrate. Due to this mode of operation, there is greater flexibility to use substrates that can adequately support hPSCs as compared to other cell micropatterning methods. We also highlight critical steps for the successful generation of hPSC micropatterns. As an example, we demonstrate that stencil micropatterning of hPSCs can be used to modulate spatial polarization of cell-cell and cell-matrix adhesions, which in turn determines mesoendoderm differentiation patterns. This simple and robust method to micropattern hPSCs widens the prospects of establishing experimental models to investigate tissue organization and patterning during early embryonic development.
Introduction
Human pluripotent stem cells (hPSCs), including embryonic stem cells (hESCs) and induced pluripotent stem cells (hiPSCs), are widely exploited in regenerative medicine as well as experimental modeling of normal and diseased organogenesis because of their differentiation potential into cell lineages of all three germ layers1,2. The differentiation fates of hPSCs are highly sensitive to local environmental factors that can modulate autocrine or paracrine signaling1 as well as mechanotransduction processes mediated by physical cues3-5. Cell micropatterning encompasses a set of techniques that have been developed to spatially organize the geometry and location of a cell population as a mean to control the local cellular microenvironment, such as cell-cell interactions6 and cell-matrix interactions3. In the context of hPSCs, cell micropatterning has been employed to gain significant insights into how niche-dependent autocrine signaling modulates hESC pluripotency-differentiation decisions7 and organization into early embryonic differentiation patterns6. 2D and 3D micropatterned hPSCs have been used to control the colony size of multicellular patterns, which in turn influenced differentiation decisions into the three germ layers8,9. We have employed multicellular hPSC micropatterns to modulate the extent of cell-cell and cell-matrix interactions within a hPSC colony to probe how integrin-E-cadherin crosstalk can give rise to cell fate heterogeneity10. The demonstrations from the above reports open new avenues towards the application of multicellular micropatterns of hPSCs as experimental models for drug toxicity screening for developmental diseases11, to study the effect of growth factors and hormones during tissue or organ development, and to unravel the formation of tissue patterns.
A myriad of cell micropatterning techniques have been developed as reviewed by Falconnet et. al.12 but only a handful, such as micro-contact printing7,8,13, microwell culture14,15, photopatterning6 and microstencils16 have been successfully implemented with hPSCs. The challenge with micropatterning hPSCs lies in their vulnerability and a stringent requirement of specific extracellular matrices (ECM) and growth conditions for cell attachment and survival. For 2D hPSC patterns, micro-contact printing is one of the most common methods to generate hPSC micropatterns on tissue culture and glass substrates13. The method can be used to pattern common ECM used in hPSC culture, including laminin and basement membrane matrices, such as Matrigel. However, it typically requires a two-step coating process aided by Poly-D-Lysine, and needs specific inert atmospheric and humidity conditions to make stable ECM micropatterns for hPSCs to attach on6,13. The foremost consideration of each micropatterning method is whether the surface modification regime can generate hPSC-adhesive ECM patterns at the desired geometrical resolution while minimizing unspecific cell attachment to the surrounding areas.
Here, we report the use of stencil micropatterning as a simple method to generate hPSC micropatterns without additional surface modification steps prior to the generation of adhesive ECM patterns for hPSCs to attach on. The cell stencil comprises of a thin membrane, e.g., polydimethylsiloxane (PDMS) sheet, with micron to millimeter size through-holes sealed onto a cell culture substrate to physically contain ECM coatings and subsequently seeded hPSCs. As stencil patterning works by physically restraining the location where hPSC can access and attach directly to the underlying ECM coated substrate, this method is compatible with various substrates that can support hPSC cultures. The only requirement is that the choice of stencil material can form a reversible seal with the substrate. These substrates include conventional tissue culture polystyrene (TCPS)17, ligand conjugated substrates18, as well as elastomeric substrates with tunable stiffness (e.g., PDMS)19. This method also allows coating of different ECM, such as vitronectin (or VTN protein), laminin and basement membrane matrices (e.g., Matrigel and Geltrax) to allow for proper attachment and differentiation of hPSCs. Therefore, we can transfer optimized ECM-substrate configurations for a specific hPSC line to stencil micropatterning for optimal cell-matrix adhesion, survival and differentiation. Recently, a similar method has also been reported to direct hepatic differentiation by micropatterning hESCs using poly(methyl methacrylate) (PMMA) micro-stencil arrays16.
Cell stencils can be fabricated from different materials, including metals20,21, poly(p-xylylene) polymers22,23, PMMA16 and most commonly, PDMS24-28. Silicon and poly(p-xylylene) polymers stencils require direct etching of the through-holes with specialized equipment20-23, which limits their accessibility to biological users. PDMS stencils can be fabricated by different methods depending on the feature size required, which typically ranges from 3 µm to 2,000 µm11,26-29. If small features are desired, thin stenciling sheets can be produced by press molding PDMS pre-polymer on a microfabricated silicon template containing reliefs of the micropatterns28. For features > 1,000 µm, a CO2 laser cutter provides an easy and low cost method to directly cut the patterns on a pre-casted PDMS sheet during stencil fabrication. The recyclability of PDMS stencils also makes them cost-effective to conduct a series of experiments with sufficient consistency.
Here, we present the detailed methodology for the fabrication of a PDMS stencil with 1,000 µm features by laser cutting and the generation of hESC micropatterns. These hESC micropatterns were used to modulate the extent of integrin and E-cadherin mediated adhesions within a cohesive hESC colony so as to investigate how spatial polarization of cell adhesion resulted in cell fate heterogeneity10.
Protocol
NOTE: This protocol describes the fabrication of PDMS stencil with 1,000 µm patterns by laser-cutting and micropatterning of the hESC line, H9 using the PDMS stencil.
1. Design and Fabrication of PDMS Stencil for Micropatterning
- Design the stenciling sheet with through-holes of the desired geometry and size (e.g., 1,000 µm circles) and the stencil gasket using computer-aided design software10.
- Laser-cut the stenciling sheet and gasket on 120-150 µm and 2 mm thick polydimethylsiloxane (PDMS) sheets respectively using a CO2 laser-cutter10.
- Bond the PDMS gasket with the PDMS stencil sheet using liquid uncured PDMS and bake at 60 °C for 3-4 hr to obtain the PDMS stencil for micropatterning.
- Sterilize the PDMS stencil by autoclaving at 120 °C for 30 min and drying in an oven before use.
2. hESCs Maintenance
- Culture the hESC lines in a feeder-free maintenance medium on 1× hESC-qualified basement membrane matrix coated cell culture plates at 37 °C and 5% CO2.
- Passage the hESCs when they are approximately 70% confluent by 3 min incubation at 37 °C with dispase treatment and mechanically dissociating the hESC colonies into 100-200 µm clumps. Plate the hESCs clumps at a density of 30-50 clumps per 9.6 cm2 of growth area on basement membrane matrix-coated tissue culture polystyrene at 1:6 splitting ratio.
3. Stencil Micropatterning of Human Embryonic Stem Cells
- Preparation prior to cell patterning
- Seal a PDMS stencil onto a 60 mm Petri dish by dispensing 700 µl 70% analytical grade ethanol in ultrapure water into the Petri dish and placing the stencil on top.
- Place the Petri dish inside biosafety cabinet overnight to let the ethanol dry.
- Check no bubble exists underneath the stencil to ensure that the stencil forms a good seal with the Petri dish. This prevents leakages of ECM coating solution. Seal the Petri dish and store under sterile conditions until it is ready for cell seeding.
- Prepare aliquots of hESC-qualified basement membrane matrix according to the Certificate of Analysis. Ensure that each aliquot yields 1× hESC-qualified basement membrane matrix solution when diluted in 25 ml of Dulbecco's Modified Eagle Medium: Nutrient Mixture F-12 (DMEM/F12) according to manufacturer's product sheet.
- Prepare the ECM coating solution by adding one aliquot of hESC-qualified basement membrane matrix into 16.7 ml of DMEM/F12 to make 1.5× hESC-qualified basement membrane matrix solution. Keep all the ECM coating solution on ice to prevent gelation.
- Supplement hESC maintenance medium with 10 µM ROCK inhibitor (Y27632).
- ECM coating on the stenciled substrate
- Treat the Petri dish with 100 W O2 plasma for 90 sec. To ensure sterility, only open the Petri dish cover inside the plasma chamber prior to plasma treatment, and quickly cover back the Petri dish after completion of treatment. This facilitates surface wetting and prevents air bubble formation in the micropattern through-holes during the addition of ECM coating solution.
- Add 450 µl of 1.5× hESC-qualified basement membrane matrix solution to cover the whole stencil. Seal the Petri dish with self-sealable film, such as Parafilm, to prevent the solution from drying out, and incubate for 5 hr at 37 °C before use.
- Seeding hESCs onto the stenciled substrate
- Examine hESC colonies in 6-well plate to identify the differentiated cell areas showing loss of typical hESC morphology (e.g., loss of rounded, tightly packed epithelial morphology, high nucleus/cytoplasm ratio with prominent nucleoli). Remove differentiated regions using a vacuum aspirator.
- Wash twice with 2 ml DMEM/F12 per well.
- Add 1 ml of digestive enzymes, such as Accutase, per well of 6-well plate and incubate at 37 °C for 8 min. Tap the plate gently to detach all colonies from substrate.
- Rinse each well with at least 4 ml of DMEM/F12 per 1 ml of digestive enzymes and collect the cell suspension into 15 ml conical tube.
- Centrifuge the cell suspension at 200 × g for 3 min at room temperature.
- Aspirate to remove the supernatant and add 400 µl of hESC maintenance medium supplemented with ROCK inhibitor (ROCKi) to re-suspend the cells. Pipette the cell suspension up and down 3 times gently to break clumps into single cells.
- Mix the single cell suspension well and dilute 10 µl of cell samples into 190 µl of DMEM/F12 (1:20 dilution). Use a hemocytometer to determine the cell density in the stock cell suspension.
- Calculate the required cell seeding density for a given stencil.
NOTE: For example, we have experimentally determined the cell seeding density to obtain a confluent monolayer of single cells is approximately 4,444 cells/mm2. Thus, a stencil with an area of 450 mm2 and seeding volume of 400 µl will require a cell suspension to be at a density of 2 million cells / 400 µl. - Dilute the stock cell suspension to the required seeding density (see step 3.3.8 NOTE) with hESC culture medium supplemented with ROCKi.
- Add a designated volume of cell suspension containing the required number of cells into each stencil and leave the Petri dish undisturbed in hood for 5 min at room temperature to allow cells to settle.
- Transfer the Petri dish into incubator and incubate for 1 hr to allow for cell attachment. Take care to keep the Petri dish level during the transfer process so that cells remain as a monolayer in the stencil.
- Stencil removal and passivation of unpatterned substrate
- Examine the Petri dish under a microscope to check if cells are properly attached onto the underlying substrate.
- Aspirate away cell suspension from the stencil.
- Add 2 ml/dish of 0.5% cell culture compatible non-ionic surfactant in DMEM/F12 to the area surrounding the stencil.
- Use a pair of autoclaved forceps to gently peel off the stencil. Swirl the non-ionic surfactant solution around as the stencil is peeled off to prevent the cells from drying out. Visually observe the micropatterned-cells in the Petri dish.
- Incubate the micropatterned-cells in 0.5% non-ionic surfactant-DMEM/F12 solution for 10 min at 37 °C.
- Aspirate away the non-ionic surfactant-DMEM/F12 solution. Wash 3 times with 2 ml/dish of DMEM/F12.
- Add 2 ml/dish of hESC maintenance medium supplemented with ROCKi and incubate at 37 °C overnight.
- After overnight incubation, aspirate hESC maintenance medium supplemented with ROCKi, wash once with DMEM/F12 and induce mesoendoderm differentiation by adding 2 ml/dish of differentiation medium supplemented with 100 ng/ml Activin, 25 ng/ml BMP4 and 10 ng/ml FGF2.
- Evaluate the micropatterns using phase contrast imaging and compute the average Brachyury (T) intensity profiles for each pattern as described previously8.
Representative Results
In this paper, we describe the fabrication of a cell stencil by using a laser cutter to generate 1,000 µm features. The stencil was composed of 2 parts: a thin stenciling sheet (approximately 100-200 µm thick) containing the micropattern through-holes, and a PDMS gasket to contain the ECM coating solution or cell suspension. Here, 127 µm and 2 mm thick commercially available PDMS sheets were used as the stenciling sheet and gasket respectively. Other methods for preparing PDMS sheets of controllable thickness, such as spin-coating, can also be used to fabricate the parts. The two components were bonded together by using liquid uncured PDMS prepolymer-crosslinker mixture as an adhesive or plasma bonding, and baked at 60 °C for 3-4 hr (Fig. 1).
The material for the stenciling sheet was selected based on the choice of the cell culture substrate. When using conventional tissue culture polystyrene (TCPS) as the culture substrate, PDMS stencils can be used since the compliance of the PDMS stenciling sheet will form a good seal with the rigid TCPS (E ~ 3 GPa) (Fig. 2A). In experimental designs where one wishes to investigate spatial heterogeneity of stem cell fate on softer cell culture substrates, such as elastomeric PDMS substrates with a tunable stiffness range of 5 kPa to 2 MPa30, stiffer stenciling materials can be employed. As an example, we demonstrated the use of a polyethylene terephthalate (PET) stencil (E ~ 2 GPa) to generate 1 mm micropatterned hPSC colonies on softer PDMS substrate having a stiffness of ~ 5 kPa (Fig. 2B). The PET stencil was fabricated by gluing a PDMS gasket onto a stenciling sheet made of commercially available PET films (Fig. 2B, inset). We note that the required cell attachment time during the micropatterning process was longer (~3 hr) on the softer PDMS substrate of stiffness ~5 kPa as compared to conventional TCPS substrates.
We utilized stencil micropatterned hESC colonies to demonstrate that spatial heterogeneity in cell-adhesion mediated mechanical forces could pattern early mesoendoderm differentiation. We have previously shown that H9 hESCs at the colony periphery experienced higher integrin adhesions than cells at the colony interior, which resulted in their preferential differentiation into Brachyury (T)-positive mesoendoderm cells when induced with Activin, BMP4 and FGF231 (Fig. 3A). When the cells were patterned into different geometries (circle, square, rectangle and semi-circular arc) at a constant colony area, geometric anisotropy at the corners of squares, rectangles, and convex curvatures led to a local concentration of integrin-mediated traction forces and resulted in increased mesoendoderm differentiation32,33. Indeed, by mapping T expression intensity at different localities within a single colony, we found that the extent of mesoendoderm induction was higher in sharp corners of square and rectangular colonies (Fig. 3B) and at convex curvatures of a semi-circular arc (Fig. 3C), which corresponded to reported high integrin-mediated stress regions in an anisotropic geometry32,33. Therefore, micropatterning can be used to modulate the spatial distribution of cell adhesion mediated mechanical forces within an intact hPSC colony as a means to control the ensuing differentiation process.
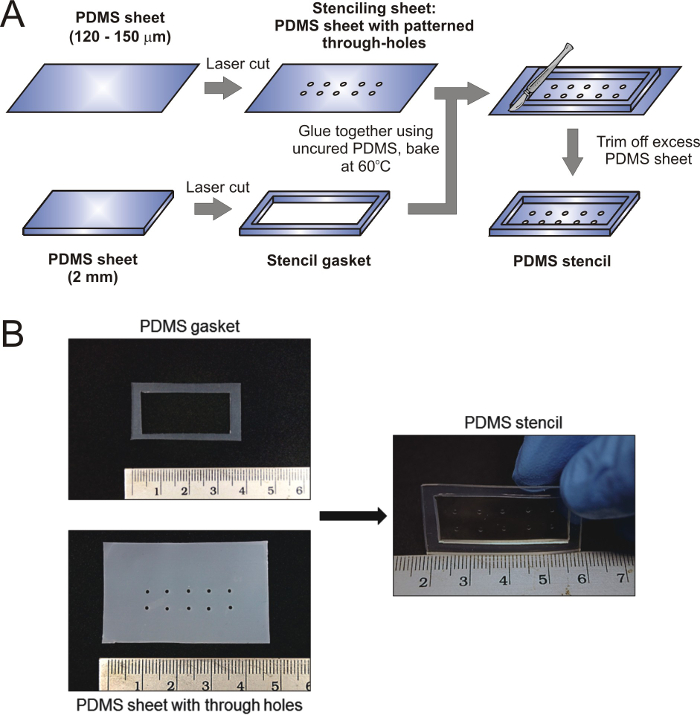
Figure 1. Generation of PDMS stencil for micropatterning. (A) Schematic representing the critical steps in stencil fabrication. A 127 µm thick PDMS sheet was laser-cut to produce the designed patterns, and another 2 mm thick PDMS sheet was laser-cut to produce the gasket. These two components were bonded together with uncured PDMS to assemble the stencil. (B) Images showing assembly of the components to form a PDMS stencil with 1 mm circular through holes. Please click here to view a larger version of this figure.
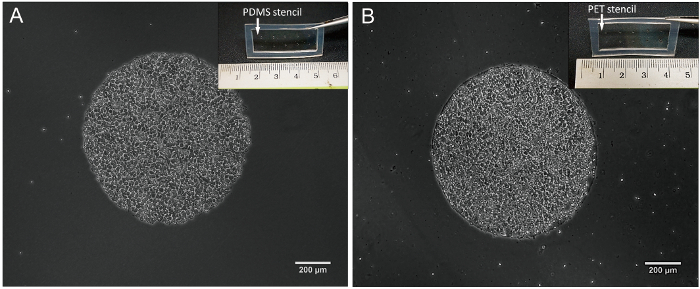
Figure 2. Micropatterned hESC colonies (1,000 µm circles) on different culture substrates. Microscopic images of hESC micropattern colony immediately after the removal of (A) PDMS stencils (inset) used to generate hESC micropatterns on stiff substrates, e.g., TCPS of stiffness ~ 2 GPa, and (B) PET stencils (inset) used to generate micropatterns on softer substrates, e.g., PDMS of stiffness ~5 kPa. Please click here to view a larger version of this figure.
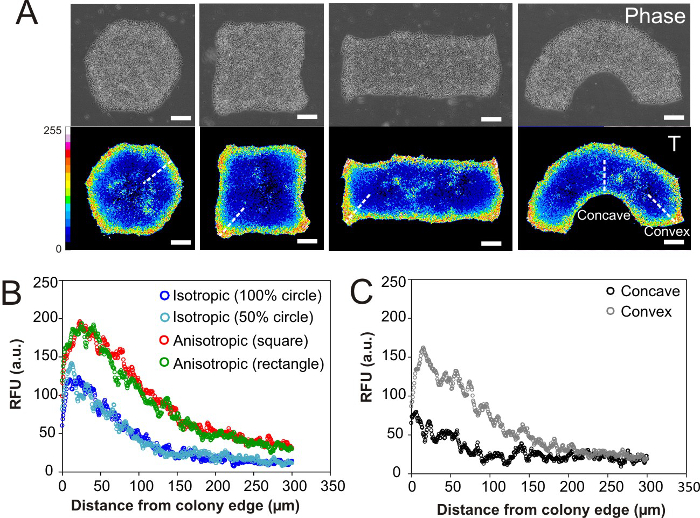
Figure 3. Effect of hESC micropatterns of different geometrical shapes on their mesoendoderm differentiation8. (A) hESC micropatterns of different geometrical shapes but same colony area were generated using PDMS stencils. Phase images (top panel) and intensity maps of T expression (bottom panel) after 24 hr of mesoendoderm differentiation. (B) Average T intensity profiles (along white dotted lines in (A)) in isometric circular colonies or anisometric square and rectangular colonies. All colonies had the same area except the 50% circle, which had half of the colony area. (C) Average T intensity profiles from the concave or convex edges into the colony interior in a semi-circular arc, as indicated by white dotted lines in (A). Each intensity profile in (B-C) is an average of 16 intensity profiles obtained from 4 colonies. Images are re-printed with permission from Toh et. al., 8. Scale bar = 200 µm and RFU is relative fluorescence unit. Please click here to view a larger version of this figure.
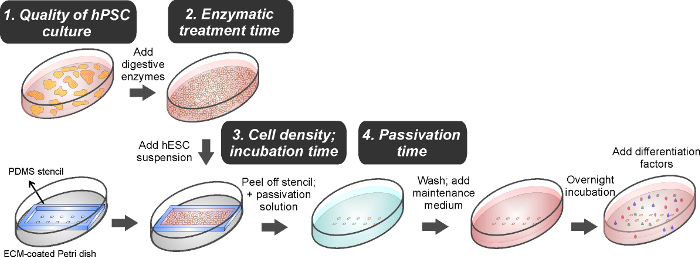
Figure 4. Schematic representation of the workflow to micropattern hESC colonies and induce differentiation. Key steps for the successful generation of hESC micropatterns are highlighted in grey boxes. Please click here to view a larger version of this figure.
Discussion
Fabrication of micropatterning stencils
Stencil micropatterning provides an ideal method to generate hPSC micropatterns for investigating niche-mediated differentiation patterning. The key advantage of stencil patterning over other micropatterning techniques, such as microcontact printing and photopatterning, is that it does not require surface modification and can be implemented on conventional TCPS substrates. Therefore, optimized culture media and ECM coatings for different hPSC lines can be readily transferred to stencil micropatterning.
There are a number of key steps during the fabrication of the cell stencil that contribute to the generation of good micropatterns. The fidelity of reproducing the designed geometries in the hPSC micropatterns depends on the quality and consistency of through-hole fabrication in the stenciling sheet. For studies involving multicellular micropatterns of > 1,000 µm, the through-holes can be directly fabricated by laser cutting on a pre-casted PDMS sheet. The resolution of laser cutting is dependent on the spot size of the laser beam and the precision of the mechanical stage controlling the path of the laser beam. For conventional CO2 laser cutters, we found that it can produce circular or curved features with good fidelity but not geometries with sharp corners (e.g., square). In applications where small or sharp features are desired, the through-holes in the stenciling sheet can be fabricated by molding PDMS on a microfabricated silicon template containing reliefs of the micropatterns28. The challenge of the molding method is to produce micro-size through-holes in thin PDMS sheets with no residual PDMS over the silicon template reliefs. Several strategies have been developed to address this problem as described by Li et. al.28
During the assembly of the stenciling sheet and gasket to produce the cell stencil, one should ensure that the stenciling sheet is flat and free of dust during the bonding process in order to prevent leakages from the stencil. Buckling of the stenciling sheet during the bonding process may compromise the sealing of the stencil to the culture substrate, causing ECM and cell solutions to leak from the micropattern through-holes. The stencil should be sterilized by autoclaving (120 °C, 30 min) and be thoroughly dried in an oven to ensure that it can form a good seal with the culture substrate.
Another important factor is the selection of the stenciling sheet material. The PDMS stencil is ideal for micropatterning hPSCs onto rigid culture substrates, such as TCPS with a stiffness of ~ 2 GPa. However, if one wishes to micropattern hPSCs onto softer substrates, such as on PDMS substrates commonly used to tune cell substrate stiffness34, the compliance of a PDMS stenciling sheet will make it difficult to peel off the stencil after the cell seeding process, therefore destroying the hPSC micropatterns. In this case, the material choice for the stenciling sheet of the stencil must be selected such that it can form a seal with the culture substrate but can be peeled off with ease.
Generating hPSC micropatterns
The protocol for micropatterning H9 hESCs onto TCPS substrates using a PDMS stencil presented here has been optimized to achieve a confluent colony with good cell viability and patterning fidelity. It is essential that the hPSCs form proper cell-cell and cell-matrix interactions within the spatial confinement of a particular geometry defined by the micropatterns so that one can investigate how geometry-dependent factors influence differentiation fates. We have identified a few critical steps to successfully generate hPSC micropatterns (Fig. 4) and they are discussed as follows.
First, the quality of the hPSC culture matters. It is important to harvest the hPSC colonies at 70-80% confluence, with majority of colonies showing undifferentiated hPSC morphology while removing the differentiated areas. Over-confluent hPSC cultures usually cause spontaneous differentiation, and if the differentiated areas are not removed prior to cell seeding, the differentiated cells will disrupt normal differentiation pattern formation.
Second, when harvesting hPSC colonies to single cell suspension, avoiding over-treatment with digestive enzymes is critical because this often results in cell death after overnight incubation even if the cells can attach initially. We found that 8 minutes of enzymatic treatment is optimal for the H9 hESC line cultured in our lab; although we recommend that the cell detachment treatment time should be optimized separately for different cell lines.
Third, the cell seeding density plays a critical role in generating a confluent monolayer of cells with defined cell-cell and cell-matrix adhesions. Cell seeding density is calculated based on the average cell size, stencil surface area and cell seeding volume, and then optimized experimentally. A low cell seeding density often results in poor patterning fidelity. Given their epithelial nature, hPSCs have a strong tendency to aggregate and grow as colonies. Therefore, when there are insufficient cells within the micropattern, the attached cells will aggregate resulting in "bald spots" within the micropattern. Conversely, an overly high cell seeding density will cause over-crowding of cells in the micropatterns. This results in multiple cell layers being formed, especially at the periphery of the micropattern. At these regions, cell adhesion mediated mechanical forces are no longer defined solely in the x-y plane by the micropatterns. Hence, the resultant differentiation fates are more random and cannot be correlated to the mechanical factors modulated by the cell micropattern.
Lastly, there is a need to passivate the substrate surrounding the hPSC micropatterns with non-cell adhesive molecules in order to constrain proliferating or differentiating cells to within the micropattern. This is essential to maintain the fidelity of the hPSC micropattern, and in turn, the biochemical and mechanical environmental gradient for differentiation patterns to develop. Cell culture compatible non-ionic surfactants, such as Pluronic F-127 can be used as a passivating agent. When passivating with surfactants, the treatment time and concentration should be optimized due to surfactant's cytotoxicity at long exposure. As we discussed above, the presented protocol provides a detailed guideline to micropattern hPSCs using a PDMS stencil, but optimization of this protocol, especially for the above critical steps, is encouraged to successfully generate hPSC micropatterns.
One limitation of stencil micropatterning is that it is more suitable for generating larger micropatterns ranging from 100-1,500 µm. To generate very small features intended for single cell patterning, the fabrication of stenciling sheets with < 50 µm through-holes is very challenging as mentioned previously. Hence, we expect that the use of stencil micropatterned hPSCs is well suited to investigate multicellular tissue pattern formation during different developmental processes (e.g., neuroepithelium folding or endoderm patterning).
By using micropatterning to geometrically confine a hPSC population, we can establish cell-adhesion mediated mechanical10 and paracrine signaling gradients6 to control and understand the spatial organization of the resultant differentiation fates. These micropatterned hPSC models with spatially organized differentiation can in turn be translated into human-specific disease or teratogen screening models11 for congenital birth defects.
Divulgazioni
The authors have nothing to disclose.
Acknowledgements
This work is supported by NUS Start up grant (R-397-000-192-133) and ETPL Gap Fund (R-397-000-198-592). G.S. is a NUS Research scholar. Authors would like to thank Dr. Jiangwa Xing for her technical support on cell micropatterning.
Materials
| 2 mm thick PDMS sheet | Specialty Silicone Products Inc., USA | SSPM823-.005 | Used to form reservoir for stencil |
| 120-150 μm thick PDMS sheet | Specialty Silicone Products Inc., USA | SSPM823-.040 | Used to form stencil |
| 60 mm petri dish | Nunc Nunclon Delta | 150326 | Substrate for micropatterning |
| Accutase | Accutase, Merck Millipore, Singapore | SCR005 | Enzyme to break H9 Cells into single cells |
| Activin | R&D Systems, Singapore | 338-AC-010 | Growth factor for H9 differentiation |
| BMP4 | R&D Systems, Singapore | 338-BP-010 | Growth factor for H9 differentiation |
| Plasma system | Femto Science, Korea | CUTE-MP | For plasma oxidation of stencil |
| Dispase | StemCell™ Technologies, Singapore | 7923 | Enzyme used to weaken the cell-ECM adhesion during passaging |
| DMEM/F12 | GIBCO, USA | 11330032 | Basal medium for H9 cells |
| FGF2 | R&D Systems, Singapore | 233–FB–025 | Growth factor for H9 differentiation |
| H9 Cell line | WiCell Research Institute, Inc., USA | WA09 | Human embryonic stem cells |
| hESC-qualified basement membrane matrix | Matrigel, BD Biosciences, Singapore | 354277 | Extra-cellular matrix coating to support growth of H9 cells |
| Inverted microscope | Leica Microsystems, Singapore | DMi1 | For capturing bright-field images |
| Laser cutter | Epilog Helix 24 Laser System | Used to generate through holes in PDMS sheet | |
| mTeSR™1 medium | StemCell™ Technologies, Singapore | 5850 | Maintainence medium for H9 cells |
| PDMS | SYLGARD® 184, Dow Corning Co., USA | 3097358-1004 | Used for sticking the PDMS stencil and reservior |
| ROCKi Y27632 | Calbiochem, Merck Millipore, Singapore | 688000 | Maintains H9 cells as single cells |
| STEMdiff™ APEL™ medium | StemCell™ Technologies, Singapore | 5210 | Differentiation medium for H9 cells |
| Polyethylene terephthalate film | SureMark Singapore | SQ-6633 | Used to form stencil |
| Cell culture compatible non-ionic surfactant | Pluronic acid F-127, Sigma, Singapore | P2443 | Passivating reagent to repel cell adhesion in non-micropatterned substrates |
Riferimenti
- Graf, T., Stadtfeld, M. Heterogeneity of embryonic and adult stem cells. Cell Stem Cell. 3 (5), 480-483 (2008).
- Nishikawa, S., Goldstein, R. A., Nierras, C. R. The promise of human induced pluripotent stem cells for research and therapy. Nat Rev Mol Cell Biol. 9 (9), 725-729 (2008).
- Guilak, F., Cohen, D. M., Estes, B. T., Gimble, J. M., Liedtke, W., Chen, C. S. Control of stem cell fate by physical interactions with the extracellular matrix. Cell Stem Cell. 5 (1), 17-26 (2009).
- Dalby, M. J., Gadegaard, N., Oreffo, R. O. Harnessing nanotopography and integrin-matrix interactions to influence stem cell fate. Nat Mater. 13 (6), 558-569 (2014).
- Joddar, B., Ito, Y. Artificial niche substrates for embryonic and induced pluripotent stem cell cultures. J Biotechnol. 168 (2), 218-228 (2013).
- Warmflash, A., Sorre, B., Etoc, F., Siggia, E. D., Brivanlou, A. H. A method to recapitulate early embryonic spatial patterning in human embryonic stem cells. Nat Methods. 11 (8), 847-854 (2014).
- Peerani, R., et al. Niche-mediated control of human embryonic stem cell self-renewal and differentiation. EMBO J. 26 (22), 4744-4755 (2007).
- Lee, L. H., Peerani, R., Ungrin, M., Joshi, C., Kumacheva, E., Zandstra, P. Micropatterning of human embryonic stem cells dissects the mesoderm and endoderm lineages. Stem Cell Res. 2 (2), 155-162 (2009).
- Hwang, Y. S., Chung, B. G., Ortmann, D., Hattori, N., Moeller, H. C., Khademhosseini, A. Microwell-mediated control of embryoid body size regulates embryonic stem cell fate via differential expression of WNT5a and WNT11. Proc Natl Acad Sci U S A. 106 (40), 16978-16983 (2009).
- Toh, Y. C., Xing, J., Yu, H. Modulation of integrin and E-cadherin-mediated adhesions to spatially control heterogeneity in human pluripotent stem cell differentiation. Biomaterials. 50, 87-97 (2015).
- Xing, J., Toh, Y. C., Xu, S., Yu, H. A method for human teratogen detection by geometrically confined cell differentiation and migration. Sci Rep. 5, 10038 (2015).
- Falconnet, D., Csucs, G., Grandin, H. M., Textor, M. Surface engineering approaches to micropattern surfaces for cell-based assays. Biomaterials. 27 (16), 3044-3063 (2006).
- Bauwens, C. L., et al. Control of human embryonic stem cell colony and aggregate size heterogeneity influences differentiation trajectories. Stem Cells. 26 (9), 2300-2310 (2008).
- Khademhosseini, A., et al. Co-culture of human embryonic stem cells with murine embryonic fibroblasts on microwell-patterned substrates. Biomaterials. 27 (36), 5968-5977 (2006).
- Mohr, J. C., de Pablo, J. J., Palecek, S. P. 3-D microwell culture of human embryonic stem cells. Biomaterials. 27 (36), 6032-6042 (2006).
- Yao, R., et al. Hepatic differentiation of human embryonic stem cells as microscaled multilayered colonies leading to enhanced homogeneity and maturation. Small. 10 (21), 4311-4323 (2014).
- Mei, Y., et al. Combinatorial development of biomaterials for clonal growth of human pluripotent stem cells. Nat Mater. 9 (9), 768-778 (2010).
- Melkoumian, Z., et al. Synthetic peptide-acrylate surfaces for long-term self-renewal and cardiomyocyte differentiation of human embryonic stem cells. Nat Biotechnol. 28 (6), 606-610 (2010).
- Evans, N. D., et al. Substrate stiffness affects early differentiation events in embryonic stem cells. Eur Cell Mater. 18, 1-13 (2009).
- Carter, S. B. Haptotactic islands: a method of confining single cells to study individual cell reactions and clone formation. Exp Cell Res. 48 (1), 189-193 (1967).
- Jimbo, Y., Robinson, H. P., Kawana, A. Simultaneous measurement of intracellular calcium and electrical activity from patterned neural networks in culture. IEEE Trans Biomed Eng. 40 (8), 804-810 (1993).
- Wright, D., et al. Reusable, reversibly sealable parylene membranes for cell and protein patterning. J Biomed Mater Res. A. 85 (2), 530-538 (2008).
- Jinno, S., et al. Microfabricated multilayer parylene-C stencils for the generation of patterned dynamic co-cultures. J Biomed Mater Res A. 86 (1), 278-288 (2008).
- Jackman, R. J., Duffy, D. C., Cherniavskaya, O., Whitesides, G. M. Using elastomeric membranes as dry resists and for dry lift-off. Langmuir. 15 (8), 2973-2984 (1999).
- Folch, A., Jo, B. H., Hurtado, O., Beebe, D. J., Toner, M. Microfabricated elastomeric stencils for micropatterning cell cultures. J Biomed Mater Res. 52 (2), 346-353 (2000).
- Park, J., et al. Microfabrication-based modulation of embryonic stem cell differentiation. Lab Chip. 7 (8), 1018-1028 (2007).
- Choi, J. H., Lee, H., Jin, H. K., Bae, J. S., Kim, G. M. Micropatterning of neural stem cells and Purkinje neurons using a polydimethylsiloxane (PDMS) stencil. Lab Chip. 12 (23), 5045-5050 (2012).
- Li, W., et al. NeuroArray: a universal interface for patterning and interrogating neural circuitry with single cell resolution. Sci Rep. 4, 4784 (2014).
- Guvanasen, G. S., Mancini, M. L., Calhoun, W. A., Rajaraman, S., DeWeerth, S. P. Polydimethylsiloxane Microstencils Molded on 3-D-Printed Templates. J Microelectromech S. 23 (5), 1045-1053 (2014).
- Palchesko, R. N., Zhang, L., Sun, Y., Feinberg, A. W. Development of polydimethylsiloxane substrates with tunable elastic modulus to study cell mechanobiology in muscle and nerve. PLoS One. 7 (12), 51499 (2012).
- Chowdhury, F., et al. Material properties of the cell dictate stress-induced spreading and differentiation in embryonic stem cells. Nat Mater. 9 (1), 82-88 (2010).
- Gjorevski, N., Boghaert, E., Nelson, C. M. Regulation of Epithelial-Mesenchymal Transition by Transmission of Mechanical Stress through Epithelial Tissues. Cancer Microenviron. 5 (1), 29-38 (2012).
- Thery, M. Micropatterning as a tool to decipher cell morphogenesis and functions. J Cell Sci. 123, 4201-4213 (2010).
- Eroshenko, N., Ramachandran, R., Yadavalli, V. K., Rao, R. R. Effect of substrate stiffness on early human embryonic stem cell differentiation. J Biol Eng. 7 (1), 7 (2013).

