通过热烫完整植株或提取物热处理烟草宿主细胞蛋白去除方法的比较
Summary
之前的任何其他纯化步骤三个换热沉淀方法都能够有效地除去90%以上的烟草宿主细胞蛋白质(医疗专业人员)的提取物。植物医疗专业人员不可逆聚合在温度高于60℃。
Abstract
植物不仅对人类提供食物,饲料和原料,但也已经被开发作为一种经济的生产系统,用于生物制药的蛋白质,如抗体,疫苗候选和酶。这些必须从植物生物质进行纯化,但色谱法步骤是由高浓度的植物提取液中宿主细胞蛋白(医疗专业人员)的阻碍。然而,大多数医疗专业人员不可逆聚合在温度高于60℃,促进靶蛋白的随后的纯化。这里,被呈现给实现烟草医疗专业人员的热沉淀在任一完整叶片或提取物的三种方法。完整叶片的热烫可以容易地纳入现有过程,但可能对随后的过滤步骤产生负面影响。相反的是一种用于在搅拌容器叶片提取物的热析出,从而可以提高下游操作的性能虽然有在工艺设备设计的重大变化,如真均质几何。最后,一个热交换器设置是公特征的传热条件方面和易于规模,但清洗可能是困难和可能存在于过滤器的能力造成负面影响。设计的-实验的方法可以被用来识别影响HCP去除和产物回收最相关的工艺参数。这有利于在其他平台上的表达每一种方法和最合适的方法鉴定为给定的纯化策略的应用。
Introduction
现代医疗保健系统越来越依赖于生物制药蛋白质1。在植物中生产这些蛋白质相比于常规表达系统2-4由于低病原体负担和更大的可扩展性是有利的。然而,植物性药物下游处理(DSP)是很有挑战性的,因为颠覆性的提取程序导致高粒子负担,与浊度超过5000浊度单位(NTUs)和宿主细胞蛋白(HCP)含量往往超过95 %[M / M] 5,6。
精细澄清过程需要以除去分散颗粒7-9,但色谱仪器是如果存在用于高效医疗专业人员去除10,11较早步骤初始产物回收过程中结合和洗脱模式运行成本更低。这可以通过沉淀使用floccul靶蛋白可以实现任蚂蚁12或低pH 13,14,以及通过使医疗专业人员聚集。核酮糖-1,5-二磷酸羧化酶/加氧酶(Rubisco),最丰富的HCP在绿色植物如烟草( 烟草 ),所述的选择性汇聚可以通过添加聚乙二醇15来促进,但是这是昂贵且具有大不相容-scale制造。热处理已显示变性和沉淀烟草医疗专业人员的超过95%,而蛋白质疟疾疫苗候选物如Vax8留在溶液中16-18稳定。
三种不同的方法被用来实现烟草医疗专业人员的热诱导析出:(ⅰ)热烫, 即,完整叶片在热液体的浸入,(ⅱ)一个温度受控的搅拌容器,和(iii)的热交换器( 图1)16。对于完整叶片,漂烫实现医疗专业人员的快速,高效的降水,也容易扩大规模,并与现有的大型的制造工艺,其中包括一个初始步骤洗植物生物质19兼容。与此相反,温度控制的容器已经在一些工艺可用,并且可以用于植物的热处理提取20,但其可扩展性和能量转移率是有限的,因为罐的表面-体积比逐渐减少,并变得不适合于生产规模。的热交换器是一个在技术上明确替代加热搅拌容器,但需要加热和冷却介质, 例如,蒸汽和冷水的供应充足,以及其适合于热交换器的几何形状的严格控制的体积流动速率和媒体属性, 例如 ,比热容量。本文显示了所有三种方法可如何用于在普通烟草医疗专业人员和植物医疗专业人员的热诱导沉淀。建立和EAC的操作在实验室设置h方法可用于评估其对于大规模的工艺的适用性。主要的挑战是确定适当尺度缩小的模型和运行条件类似于过程大规模生产过程中使用的设备和条件的每个操作。此处呈现的数据是指与表达的疟疾疫苗候选Vax8和荧光蛋白DsRed的16转基因烟草植物进行的实验,但该方法也已成功地应用到N.植物本生瞬时表达等生物制药蛋白21。
一个设计-的-实验(DOE)接近22可促进过程发展,絮凝剂23也可以是在这种情况下有益的,如前所述8。热烫,加热的容器和热交换器之间的主要区别是,热烫施加到完整叶片在过程的早期,而OT她的被施加到植物提取物( 图1)。
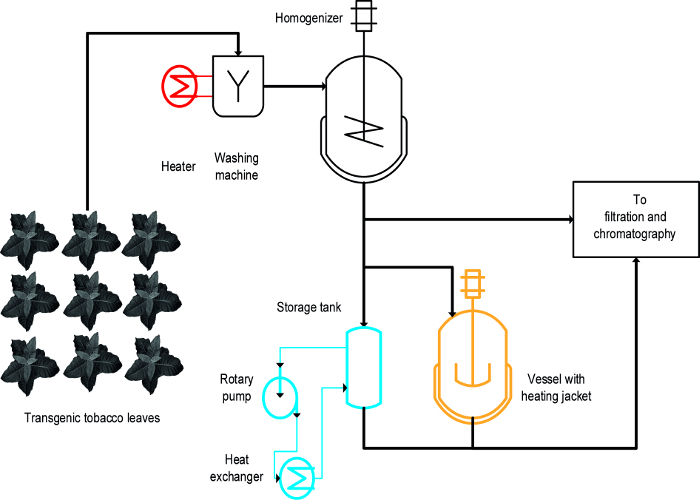
图 1: 工艺流程图图解三种不同方法进行烟草HCP热沉淀实施植物材料洗净,澄清和纯化前均化。用于漂白步骤(红色)的设备可以很容易地添加到现有的机械。相比之下,使用搅拌容器(橙色)和特别是换热器(蓝色)需要一个或几个额外的设备和管道。 请点击此处查看该图的放大版本。
Protocol
Representative Results
Discussion
以上所述的三种方法为热沉淀能有效地去除前的任何层析纯化步骤16,17烟草医疗专业人员。它们补充,旨在增加初始产品的纯度, 例如,吐水29,rhizosecretion 30或离心萃取31,32,所有这些都限于分泌蛋白的其他策略。但是,基于热的方法只能在一个有意义的方式使用,如果待纯化的目的蛋白可承受〜60℃的最低析出温度超过1分钟。因此,在任何的三种方法的?…
Divulgazioni
The authors have nothing to disclose.
Acknowledgements
We would like to acknowledge Dr. Thomas Rademacher, Alexander Boes and Veronique Beiß for providing the transgenic tobacco seeds, and Ibrahim Al Amedi for cultivating the tobacco plants. The authors wish to thank Dr. Richard M. Twyman for editorial assistance as well as Güven Edgü for providing the MSP1-19 reference. This work was funded in part by the European Research Council Advanced Grant ”Future-Pharma”, proposal number 269110, the Fraunhofer-Zukunftsstiftung (Fraunhofer Future Foundation) and Fraunhofer-Gesellschaft Internal Programs under Grant No. Attract 125-600164.
Materials
| 2100P Portable Turbidimeter | Hach | 4650000 | Turbidimeter |
| Amine Coupling Kit | GE Healthcare | BR100050 | SPR chip coupling kit |
| Autoclaving basket | Nalgene | 6917-0230 | Basket for leaf blanching |
| Biacore T200 | GE Healthcare | 28-9750-01 | SPR device |
| Bio Cell Analyser BCA 003 R&D with 3D ORM | Sequip | n.a. | Particle size analyzer |
| Blender | Waring | 800EG | Blender |
| BP-410 | Furh | 2632410001 | Bag filter |
| Centrifuge 5415D | Eppendorf | 5424 000.410 | Centrifuge |
| Centrifuge tube 15 mL | Labomedic | 2017106 | Reaction tube |
| Centrifuge tube 50 mL self-standing | Labomedic | 1110504 | Reaction tube |
| CM5 chip | GE Healthcare | BR100012 | Chip for SPR measurements |
| Cuvette 10x10x45 | Sarsted | 67.754 | Cuvette for Zetasizer Nano ZS |
| Design-Expert(R) 8 | Stat-Ease, Inc. | n.a. | DoE software |
| Disodium phosphate | Carl Roth GmbH | 4984.3 | Media component |
| Ferty 2 Mega | Kammlott | 5.220072 | Fertilizer |
| Forma -86C ULT freezer | ThermoFisher | 88400 | Freezer |
| Greenhouse | n.a. | n.a. | For plant cultivation |
| Grodan Rockwool Cubes 10x10cm | Grodan | 102446 | Rockwool block |
| Twentey-loop heat exchanger (4.8 m length) | n.a. (custom design) | n.a. | Heat exchanger |
| HEPES | Carl Roth GmbH | 9105.3 | Media component |
| K200P 60D | Pall | 5302303 | Depth filter layer |
| KS50P 60D | Pall | B12486 | Depth filter layer |
| Lauda E300 | Lauda Dr Wobser GmbH | Z90010 | Water bath thermostat |
| L/S 24 | Masterflex | SN-06508-24 | Tubing |
| mAb 5.2 | American Type Culture Collection | HB-9148 | Vax8 specific antibody |
| Masterflex L/S | Masterflex | HV-77921-75 | Peristaltic pump |
| Miracloth | Labomedic | 475855-1R | Filter cloth |
| MultiLine Multi 3410 IDS | WTW | WTW_2020 | pH meter / conductivity meter |
| Osram cool white 36 W | Osram | 4930440 | Light source |
| Phytotron | Ilka Zell | n.a. | For plant cultivation |
| Sodium disulfit | Carl Roth GmbH | 8554.1 | Media component |
| Sodium chloride | Carl Roth GmbH | P029.2 | Media component |
| Stainless-steel vessel; 0.7-kg 2.0-L; height 180 mm; diameter 120 mm | n.a. (custom design) | n.a. | Container for heat precipitation |
| Synergy HT | BioTek | SIAFRT | Fluorescence and spectrometric plate reader |
| VelaPad 60 | Pall | VP60G03KNH4 | Filter housing |
| Zetasizer Nano ZS | Malvern | ZEN3600 | DLS particle size distribution measurement |
| Zetasizer Software v7.11 | Malvern | n.a. | Software to operate the Zetasizer Nano ZS device |
Riferimenti
- PhRMA. . 2013 Report: Medicines in Development – Biologics. , (2013).
- Buyel, J. F. Process development strategies in plant molecular farming. Curr. Pharm. Biotechnol. 16, 966-982 (2015).
- Stoger, E., Fischer, R., Moloney, M., Ma, J. K. C. Plant molecular pharming for the treatment of chronic and infectious diseases. Annu. Rev. Plant Biol. 65, 743-768 (2014).
- Melnik, S., Stoger, E. Green factories for biopharmaceuticals. Curr. Med. Chem. 20, 1038-1046 (2013).
- Buyel, J. F., Twyman, R. M., Fischer, R. Extraction and downstream processing of plant-derived recombinant proteins. Biotechnol. Adv. 33, 902-913 (2015).
- Wilken, L. R., Nikolov, Z. L. Recovery and purification of plant-made recombinant proteins. Biotechnol. Adv. 30, 419-433 (2012).
- Buyel, J. F., Fischer, R. Scale-down models to optimize a filter train for the downstream purification of recombinant pharmaceutical proteins produced in tobacco leaves. Biotechnol. J. 9, 415-425 (2014).
- Buyel, J. F., Fischer, R. Flocculation increases the efficacy of depth filtration during the downstream processing of recombinant pharmaceutical proteins produced in tobacco. Plant Biotechnol. J. 12, 240-252 (2014).
- Buyel, J. F., Opdensteinen, P., Fischer, R. Cellulose-based filter aids increase the capacity of depth filters during the downstream processing of plant-derived biopharmaceutical proteins. Biotechnol. J. 10, 584-591 (2014).
- Buyel, J. F., Fischer, R. Generic chromatography-based purification strategies accelerate the development of downstream processes for biopharmaceutical proteins produced in plants. Biotechnol. J. 9, 566-577 (2014).
- Buyel, J. F., Woo, J. A., Cramer, S. M., Fischer, R. The use of quantitative structure-activity relationship models to develop optimized processes for the removal of tobacco host cell proteins during biopharmaceutical production. J. Chromatogr. A. 1322, 18-28 (2013).
- Holler, C., Vaughan, D., Zhang, C. M. Polyethyleneimine precipitation versus anion exchange chromatography in fractionating recombinant beta-glucuronidase from transgenic tobacco extract. J. Chromatogr. A. 1142, 98-105 (2007).
- Buyel, J. F., Fischer, R. Downstream processing of biopharmaceutical proteins produced in plants: the pros and cons of flocculants. Bioengineered. 5, 138-142 (2014).
- Hassan, S., van Dolleweerd, C. J., Ioakeimidis, F., Keshavarz-Moore, E., Ma, J. K. Considerations for extraction of monoclonal antibodies targeted to different subcellular compartments in transgenic tobacco plants. Plant Biotechnol. J. 6, 733-748 (2008).
- Arfi, Z. A., Drossard, J., Hellwig, S., Fischer, R., Buyel, J. F. Polyclonal antibodies can effectively detect tobacco host cell proteins after RuBisCO depletion and endotoxin removal. Biotechnol. J. , (2015).
- Buyel, J. F., Gruchow, H. M., Boes, A., Fischer, R. Rational design of a host cell protein heat precipitation step simplifies the subsequent purification of recombinant proteins from tobacco. Biochem. Eng. J. 88, 162-170 (2014).
- Buyel, J. F., Fischer, R. A juice extractor can simplify the downstream processing of plant-derived biopharmaceutical proteins compared to blade-based homogenizers. Process Biochem. 50, 859-866 (2014).
- Beiss, V., et al. Heat-precipitation allows the efficient purification of a functional plant-derived malaria transmission-blocking vaccine candidate fusion protein. Biotechnol. Bioeng. 112, 1297-1305 (2015).
- Ma, J. K., et al. Regulatory approval and a first-in-human phase I clinical trial of a monoclonal antibody produced in transgenic tobacco plants. Plant Biotechnol. J. 13, 1106-1120 (2015).
- Mahajan, P. V., Caleb, O. J., Singh, Z., Watkins, C. B., Geyer, M. Postharvest treatments of fresh produce. Philos T R Soc A. 372, (2014).
- Menzel, S., et al. Optimized blanching reduces the host cell protein content and substantially enhances the recovery and stability of two plant derived malaria vaccine candidates. Front. Plant Sci. , (2015).
- Buyel, J. F., Fischer, R. Characterization of complex systems using the design of experiments approach: transient protein expression in tobacco as a case study. J. Vis. Exp. , e51216 (2014).
- Buyel, J. F. Procedure to evaluate the efficiency of flocculants for the removal of dispersed particles from plant extracts. J. Vis. Exp. , e53940 (2016).
- Simonian, M. H., Smith, J. A. Spectrophotometric and colorimetric determination of protein concentration. Curr. Protoc. Mol. Biol. 76, (2006).
- Buyel, J. F., Kaever, T., Buyel, J. J., Fischer, R. Predictive models for the accumulation of a fluorescent marker protein in tobacco leaves according to the promoter/5’UTR combination. Biotechnol. Bioeng. 110, 471-482 (2013).
- Piliarik, M., Vaisocherova, H., Homola, J. Surface plasmon resonance biosensing. Methods Mol. Biol. 503, 65-88 (2009).
- Kim, T. D., Ryu, H. J., Cho, H. I., Yang, C. H., Kim, J. Thermal behavior of proteins: Heat-resistant proteins and their heat-induced secondary structural changes. Biochemistry-Us. 39, 14839-14846 (2000).
- Kwon, S., Jung, Y., Lim, D. Proteomic analysis of heat-stable proteins in Escherichia coli. Bmb Rep. 41, 108-111 (2008).
- Komarnytsky, S., Borisjuk, N. V., Borisjuk, L. G., Alam, M. Z., Raskin, I. Production of recombinant proteins in tobacco guttation fluid. Plant Physiol. 124, 927-933 (2000).
- Drake, P. M. W., et al. Development of rhizosecretion as a production system for recombinant proteins from hydroponic cultivated tobacco. FASEB J. 23, 3581-3589 (2009).
- Turpen, T. H. Tobacco mosaic virus and the virescence of biotechnology. Philos. Trans. R. Soc. Lond., Ser. B: Biol. Sci. 354, 665-673 (1999).
- Kingsbury, N. J., McDonald, K. A. Quantitative Evaluation of E1 Endoglucanase Recovery from Tobacco Leaves Using the Vacuum Infiltration-Centrifugation Method. Biomed. Res. Int. , (2014).
- Buyel, J. F. Numeric simulation can be used to predict heat transfer during the blanching of leaves and intact. Biochem. Eng. J. , (2015).
- Mandal, M. K., Fischer, R., Schillberg, S., Schiermeyer, A. Inhibition of protease activity by antisense RNA improves recombinant protein production in Nicotiana tabacum cv. Bright Yellow 2 (BY-2) suspension cells. Biotechnol. J. 9, 1065-1073 (2014).
- Welty, J. R., Wicks, C. E., Wilson, R. E. . Fundamentals of momentum, heat, and mass transfer. , (1976).
- Lowe, D., et al. Aggregation, stability, and formulation of human antibody therapeutics. Advances in protein chemistry and structural biology. 84, 41-61 (2011).
- Gong, R., et al. Engineered human antibody constant domains with increased stability. J. Biol. Chem. 284, 14203-14210 (2009).
- Rouet, R., Lowe, D., Christ, D. Stability engineering of the human antibody repertoire. FEBS Lett. 588, 269-277 (2014).
- Stabel, J. R., Lambertz, A. Efficacy of pasteurization conditions for the inactivation of Mycobacterium avium subsp paratuberculosis in milk. J. Food Prot. 67, 2719-2726 (2004).
- Wichers, H., Mills, C., Wichers, H., Hoffmann-Sommergruber, K. Ch. 12. Managing Allergens in Food. , 336 (2006).
- Davis, P. J., Williams, S. C. Protein modification by thermal processing. Allergy. 53, 102-105 (1998).
- Dubois, M. F., Hovanessian, A. G., Bensaude, O. Heat-shock-induced denaturation of proteins. Characterization of the insolubilization of the interferon-induced p68 kinase. J. Biol. Chem. 266, 9707-9711 (1991).

