A Simple Approach to Manipulate Dissolved Oxygen for Animal Behavior Observations
PREPARAZIONE ISTRUTTORI
CONCETTI
Student Protocol
Note: This experiment did not use vertebrates and therefore did not require approval by Juniata College's Institute for Animal Care and Use Committee. However for individuals adapting this method for use with vertebrates, IACUC approval should be sought.
1. Field Sample Collection
- Determine and evaluate potential field sites for the ability to collect, store, and transport stoneflies quickly to minimize time in transit with a maximum recommended time in transit of 1 hr.
- Perform kick-net sampling at the selected field site following standard kick-net procedures enough times to collect at least 35 stoneflies12.
- Collect 50 L of stream water and rocks with a maximum diameter of 2 cm from streams.
- Place aquariums in a refrigerator set to the temperature of the stream site. Distribute rocks collected at the stream site into aquariums and fill with 4 L of stream water per aquarium. Place 20-30 collected stoneflies per aquarium and place a bubbling stone attached to an aquarium bubbler into each tank and turn on bubblers to continuously add room air to the water.
- Allow the stoneflies to adjust to the new environment in the aquariums for a 48 hr period.

Figure 1. Set up for dissolved oxygen manipulation. (A) 1) Fitting for copper pipe to male hose barb 2) Location of stopper seal to examine for ensuring well sealing flask. (B) 1) 2 L side-arm flask filled with 1.9 L of water 2) Gas tube and air bubbler (blue) for use in nitrogen bubbling and room air bubbling, respectively 3) Nitrogen tank and gauged values 4) 2 L flask filled with 0.4 L of water with vacuum tube submerged 5) Dissolved oxygen meter. Please click here to view a larger version of this figure.
2. Experimental Set up
- On a bench top, connect a standard walled vacuum tube to the side arm of a 2 L side-arm flask as is shown (1 in Figure 1B).
- Fill the flask with 1.9 L of stream water from 3 L plastic containers holding collected stream water in refrigerator set to 12 °C.
- Place the flask and tubing on a tray large enough to hold an ice bath around the side-arm flask without obscuring the view of the flask interior and fill the tray with ice.
- Drill two 3 mm diameter holes in a rubber stopper to allow the passage of 1) a copper pipe to deliver the gas to the vessel and 2) the probe of a DO meter into the 2 L side-arm flask (1 in Figure 1B).
- Make a lateral incision from the edge of the stopper to one of the holes to allow seating of the wire of the DO probe into the stopper.
- Connect a coupler with a 3 mm male hose barb to a piece of 2 mm diameter copper pipe (1 in Figure 1A). Ensure that this pipe is long enough to reach to within 10 cm of the bottom of the flask while reaching through the stopper.
- Place the pipe with coupler though the second hole in the stopper until the length from the bottom of the stopper is enough to reach to within 10 cm of the bottom of the flask.
- Connect a 0.75 m length, thin walled polyethylene gas tube with a diameter of 3 mm to the coupler on the pipe.
- Slide both the DO probe and copper pipe into the flask and seal the flask with the stopper.
- Check for a secure seal between the stopper and the flask, as well as a snug fit between the pipe and the probe wire within the stopper.
- Fill a 1 L flask with 0.4 L of tap water and place adjacent to the tray with the ice bath and vacuum flask.
- Submerge the polyethylene tube coming from the large vacuum flask into the water of the 1 L flask. Secure the tube with tape such that it will remain submerged through the experiment.
- Connect the 3 mm diameter gas line from the vacuum flask to an aquarium room-air bubbler. Begin to bubble the water in the 2 L flask by plugging in the aquarium bubbler, which introduces room air and oxygen to the water.
- Monitor the DO concentration and temperature of the water with the DO meter for 5 min or until equilibrium of DO is established within the chamber such that little change in DO is occurring.
3. Testing the Stability of the Experimental Set Up
- Test each setup for DO stability prior to the addition of stoneflies.
- Add three or four rocks to the 2 L flask so that stoneflies have substrate conducive for pushups.
- Begin a trial manipulation of DO by disconnecting the gas tube from the bubbler and attaching it to the nitrogen gas line.
- Start bubbling nitrogen at 20 cubic feet per hr (CFH) for approximately 40 sec to 1 min.
- Once the DO has dropped to within 0.5 mg/L of the target concentration, reduce the flow to 15 CFH and allow the concentration to decrease to the target.
- Cease flow of nitrogen immediately once the target concentration is reached.
- Use the aquarium room-air bubbler to return the concentration to the target concentration if the DO decreases below the target.
- If the DO is unstable during the testing of a set-up then check the water volume is still at 1.9 L and no water has bubbled out, water temperature is stable and not changing, and seals on all fittings appear to be tight and sealed.
- Once three trials have been performed and the experimenter has confidence in the ability to control DO, attach the gas line to the bubbler and bubble to equilibrium again.
- Bubble to equilibrium by attaching the 3 mm diameter gas line to the aquarium bubbler and starting the addition of room air to the water until the concentration of oxygen in the water does not increase or change for 3 min.
- Once at equilibrium, stop bubbling and unseal the flask.
4. Stonefly Push-up Experiment
- Divide the total number of stoneflies by the number of observers to determine the number of trials to perform.
- Determine different DO levels between 2 and 10 mg/L to evaluate the behavioral response of stoneflies (number of pushups).
- Set up one flask per trial and add an equal number of stoneflies as there are observers to the flask (4 stoneflies within this design), place the probe and pipe back into the flask, then reseal the flask with the rubber stopper.
Note: An initial DO concentration of 10 mg/L was chosen as the first observation point since it was the DO concentration of the stream from where the stoneflies were sampled. - Once the water is at 10 mg/L by bubbling following steps 2.10-2.11, record the starting water temperature and allow the stoneflies to attach to the rock substrate in the flask.
- Assign only one observer to watch a single stonefly to ensure accurate counting of push-up behavior, which is the up and down body movement exhibited by the stonefly.
- Count and record the number of push-ups observed over the course of a 3 min observation period.
- Manipulate DO to the next experimental DO level and repeat 3 min observation period for the additional experimental levels.
Note: Within this experimental design, three different DO levels were evaluated.
5. Statistical Analysis
- To perform statistical analysis use average number of push-ups across the four stoneflies across a group for a given DO trial.
- Use the free R statistical computing software12 to perform an Analysis of Variance (ANOVA) on the number of push-ups and the DO concentrations using the order of each experimental trial (DO level) and temperature as covariates. Analyzed DO as discrete levels of a single factor.
- Use an Anderson-Darling normality test on residuals to check for normality13.
- Perform a linear regression on the data by plotting the mean number of push-ups against DO concentrations.
A Simple Approach to Manipulate Dissolved Oxygen for Animal Behavior Observations
Learning Objectives
Six trials of the described setup were performed by 24 freshmen undergraduate students in a teaching laboratory setting to quantify the number of push-ups stoneflies perform in response to different DO concentration in water. The average number of push-ups performed within a DO level and within each trial was pooled to plot push-ups against the DO level in Figure 2. An ANOVA was performed initially utilizing DO concentration, sequential order of trials, temperature, as well as the interactions between all variables. Results suggest that only DO concentration significantly influenced the number of push-ups performed by stoneflies (R2 adj. = 0.322, p = 0.004) and no other variable or interaction was a significant predictor of pushups. All data used in this analysis was confirmed for normality using an Anderson-Darling test.
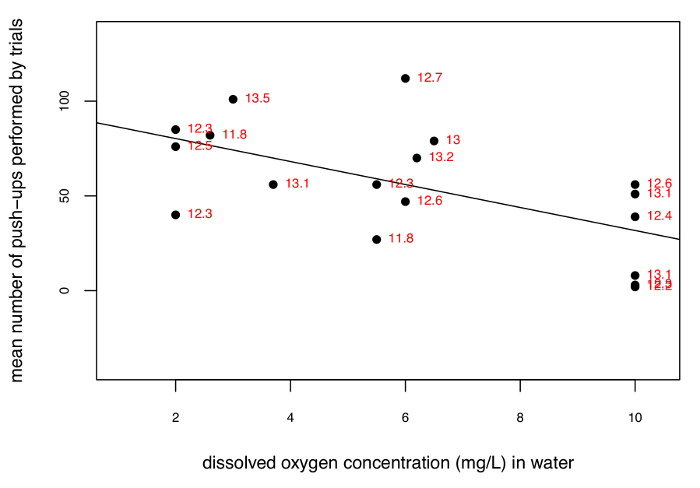
Figure 2. Mean number of push-ups performed by stoneflies grouped by trial plotted against dissolved oxygen concentration. This shows a significant negative relationship (R2 adj. = 0.322, p =0.004) between push-ups and dissolved oxygen concentration (slope of -6.063). Red numbers indicate water temperature (in °C) for a trial. The temperatures were stable across 3 min trial periods, but varied across the experiment. Please click here to view a larger version of this figure.
Supplemental Code File: R code for the statistical analyses. Please click here to download this file.
List of Materials
| Filter flask 2 L | Pyrex | 5340 | |
| Rubber Stopper size 6 | Sigma-Aldrich | Z164534 | |
| Nalgene 180 Clear Plastic Tubing | Thermo Scienfitic | 8001-1216 | |
| Whisper 60 air pump | Tetra | N/A | |
| Standard flexible Air line tubing | Penn Plax | ST25 | |
| 0.25 inch Copper tubing | Lowes Home Improvement | 23050 | |
| Male hose barb | Grainger | 5LWH1 | |
| Female Connector | Grainger | 20YZ22 | |
| Heavy Duty Dissolved Oxygen Meter | Extech | 407510 | |
| Nitrogen gas | Matheson TRIGAS | N/A | |
| Radnor AF150-580 Regulator | Airgas | RAD64003036 |
Lab Prep
The ability to manipulate dissolved oxygen (DO) in a laboratory setting has significant application to investigate a number of ecological and organismal behavior questions. The protocol described here provides a simple, reproducible, and controlled method to manipulate DO to study behavioral response in aquatic organisms resulting from hypoxic and anoxic conditions. While performing degasification of water with nitrogen is commonly used in laboratory settings, no explicit method for ecological (aquatic) application exists in the literature, and this protocol is the first to describe a protocol to degasify water to observe organismal response. This technique and protocol were developed for direct application for aquatic macroinvertebrates; however, small fish, amphibians, and other aquatic vertebrates could be easily substituted. It allows for easy manipulation of DO levels ranging from 2 mg/L to 11 mg/L with stability for up to a 5 min animal-observation period. Beyond a 5 min observation period water temperatures began to rise, and at 10 min DO levels became too unstable to maintain. The protocol is scalable to the study organism, reproducible, and reliable, allowing for rapid implementation into introductory teaching labs and high-level research applications. The expected results of this technique should relate dissolved oxygen changes to behavioral responses of organisms.
The ability to manipulate dissolved oxygen (DO) in a laboratory setting has significant application to investigate a number of ecological and organismal behavior questions. The protocol described here provides a simple, reproducible, and controlled method to manipulate DO to study behavioral response in aquatic organisms resulting from hypoxic and anoxic conditions. While performing degasification of water with nitrogen is commonly used in laboratory settings, no explicit method for ecological (aquatic) application exists in the literature, and this protocol is the first to describe a protocol to degasify water to observe organismal response. This technique and protocol were developed for direct application for aquatic macroinvertebrates; however, small fish, amphibians, and other aquatic vertebrates could be easily substituted. It allows for easy manipulation of DO levels ranging from 2 mg/L to 11 mg/L with stability for up to a 5 min animal-observation period. Beyond a 5 min observation period water temperatures began to rise, and at 10 min DO levels became too unstable to maintain. The protocol is scalable to the study organism, reproducible, and reliable, allowing for rapid implementation into introductory teaching labs and high-level research applications. The expected results of this technique should relate dissolved oxygen changes to behavioral responses of organisms.
Procedura
The ability to manipulate dissolved oxygen (DO) in a laboratory setting has significant application to investigate a number of ecological and organismal behavior questions. The protocol described here provides a simple, reproducible, and controlled method to manipulate DO to study behavioral response in aquatic organisms resulting from hypoxic and anoxic conditions. While performing degasification of water with nitrogen is commonly used in laboratory settings, no explicit method for ecological (aquatic) application exists in the literature, and this protocol is the first to describe a protocol to degasify water to observe organismal response. This technique and protocol were developed for direct application for aquatic macroinvertebrates; however, small fish, amphibians, and other aquatic vertebrates could be easily substituted. It allows for easy manipulation of DO levels ranging from 2 mg/L to 11 mg/L with stability for up to a 5 min animal-observation period. Beyond a 5 min observation period water temperatures began to rise, and at 10 min DO levels became too unstable to maintain. The protocol is scalable to the study organism, reproducible, and reliable, allowing for rapid implementation into introductory teaching labs and high-level research applications. The expected results of this technique should relate dissolved oxygen changes to behavioral responses of organisms.
