Protective Efficacy and Pulmonary Immune Response Following Subcutaneous and Intranasal BCG Administration in Mice
Summary
We herein detail the methodology followed to compare protective efficacy and lung immune response induced by intranasal and subcutaneous immunization with BCG in mouse model. Our results show the benefits of pulmonary vaccination and suggest a role for IL17-mediated response in vaccine-induced protection.
Abstract
Despite global coverage of intradermal BCG vaccination, tuberculosis remains one of the most prevalent infectious diseases in the world. Preclinical data have encouraged pulmonary tuberculosis vaccines as a promising strategy to prevent pulmonary disease, which is responsible for transmission. In this work, we describe the methodology used to demonstrate in the mouse model the benefits of intranasal BCG vaccination when compared to subcutaneous. Our data revealed greater protective efficacy following intranasal BCG administration. In addition, our results indicate that pulmonary vaccination triggers a higher immune response in lungs, including Th1 and Th17 responses, as well as an increase of immunoglobulin A (IgA) concentration in respiratory airways. Our data show correlation between protective efficacy and the presence of IL17-producing cells in lungs post-Mycobacterium tuberculosis challenge, suggesting a role for this cytokine in the protective response conferred by pulmonary vaccination. Finally, we detail the global workflow we have developed to study respiratory vaccination in the mouse model, which could be extrapolated to other tuberculosis vaccines, apart from BCG, targeting the mucosal response or other pulmonary routes of administration such as the intratracheal or aerosol.
Introduction
Tuberculosis (TB) is one of the leading infectious diseases causing more associated deaths than HIV in the world and combined with rising increase of multidrug resistant strains makes TB an alarming global health problem1. New diagnostic tools, more effective and less toxic drugs, and new safe and effective TB vaccines are an urgent need, especially in the developing world.
Live attenuated Bacille Calmette-Guerin (BCG) is currently the only licenced vaccine against TB, which has been administered intradermally at birth since the 1970s worldwide. BCG is considered effective in preventing severe forms of the disease (meningitis and miliary TB) in children, but has shown inconsistent efficacy against pulmonary TB responsible of disease transmission 2.
Pulmonary vaccination, which mimics natural route of TB infection, represents an attractive approach for priming local host immune responses. In this regard, various preclinical works in different relevant TB animal models have demonstrated greater vaccine efficacy following pulmonary immunization as compared to the subcutaneous or intradermal route 3-6. Nevertheless, the protective mechanisms triggered by pulmonary vaccination are not well understood. In the last years, several works have pointed towards IL17-mediated response as an important factor of TB-specific mucosal immune response, as in mouse models deficient for IL17 mucosal vaccine-induced protective efficacy is impaired 7,8.
Recently we demonstrated for the first time that intranasal BCG administration protected DBA/2 mice, a mouse strain characterized by the lack of protection after subcutaneous BCG immunization 9. These results suggested that respiratory TB vaccination could be more effective in reducing rate of TB in endemic countries, where intradermal BCG is considered ineffective against pulmonary TB.
Protocol
All mice were kept under controlled conditions and observed for any sign of disease. Experimental work was conducted in agreement with European and national directives for protection of experimental animals and with approval from the competent local ethics committees.
1. Preparation of Quantified Glycerol Stocks of BCG Danish and Mycobacterium tuberculosis H37Rv
NOTE: All the protocols described were performed under BSL3 conditions.
- Thaw frozen glycerol stocks of BCG Danish or H37Rv strains and inoculate 100 μl in 10 ml of 7H9 medium10 supplemented with Tween-80 0.05% (v/v) and ADC (Albumin, Dextrose, Catalase) 10% (v/v).
- Incubate under static conditions for one week at 37 °C until the culture is in log-phase growth.
- Scale up bacterial culture by transferring the 10-ml culture to 200 ml of fresh medium. Incubate for 20 additional days under static conditions.
- Transfer to 50-ml centrifuge tubes and centrifuge for 15 min at 2500 x g.
- Discard supernatants and resuspend bacterial pellet in the residual volume. Add to each tube sterile 3-mm-diameter glass beads (10 – 15 per tube).
- Vortex vigorously during 1 min in order to dissociate bacterial clumps. Resuspend each pellet in 10 ml of PBS with Tween-80 0.05% (v/v).
- Leave tubes for 10 min allowing the greatest bacterial aggregates and glass beads to settle down.
- Recover 9.5 ml of supernatant from each of the 4 tubes containing small clumps and single bacteria and transfer volumes to a single fresh 50-ml centrifuge tube (final recovered volume 38 ml). Centrifuge for 10 min at 400 x g.
- Recover 35 ml of supernatant (containing mainly single bacteria) in a single fresh 50-ml centrifuge tube and add 15 ml of PBS with glycerol 50% (v/v), obtaining a final glycerol concentration of 15% (v/v).
- Mix gently and distribute in 1-ml aliquots in appropriate tubes for freezing. Store at -80 °C (one lot provides approximately 50 aliquots glycerol stock).
- To quantify glycerol stocks, thaw a glycerol the day after freezing and perform seven ten-fold serial dilutions in separate 1.5-ml tubes containing 900 μl of PBS.
- Plate 100 μl of each dilution in 7H10 solid-agar medium10 supplemented with ADC 10% (v/v) prepared in 55-mm diameter Petri dishes.
- Carefully add 3 mm-diameter glass beads (approx. 10 per plate) and gently shake plate to equally distribute volume over agar plate. Discard beads and seal agar plate with parafilm.
- Incubate for 21 days at 37 °C and determine bacterial concentration by counting colony-forming units (CFU) in the dilutions where single colonies can be accurately distinguished.
2. Mouse Vaccination
- Thaw a pre-quantified BCG glycerol and dilute it in PBS to prepare two suspensions. Calculate final volume considering the dose per animal is 100 μl for subcutaneous administration (107 CFU per ml for subcutaneous vaccination) and 40 μl for intranasal administration (2.5 x 107 CFU for intranasal vaccination).
- Use a specialized anesthesia workstation for rodents to anesthetize mice with a mixture of isoflurane and oxygen. Use isoflurane 5% to induce anesthesia and 2% to maintain the mice in the anesthesia chamber. (Other accepted anesthesia method can be used).
- For subcutaneous vaccine administration fill a 1-ml syringe with a 26 G needle with 1 ml of bacterial suspension (107 CFU/ml). Remove air bubbles.
- Place an anesthetized mouse on a flat surface in prone position inside a laminar flow hood and inoculate subcutaneously 100 μl (106 CFU/dose) of vaccine in a flank of the mouse back. Return the mouse to the cage and monitor it to ensure that it properly recovers from anesthesia.
- Change the syringe needle between each mouse administration. Removed air bubbles as described above.
- For intranasal administration, place an anesthetized mouse in supine position inside the hood.
- With a micropipette take 20 μl of the suspension with 2.5 x 107 CFU/ml. Place the inoculum drop-by-drop between the two nostrils until the whole volume has been deposited, leaving time between drops to allow the mouse breathe the volume in. Note: mice can suffer from respiratory distress during this procedure so they should be left to assimilate each drop prior to administrate the next one to prevent it as much as possible.
- Re-fill the micropipette with another 20 μl and repeat the operation. If the mouse starts to wake up from the anesthesia after the first inoculation, place it in the anesthesia chamber prior to the second one. Return the mouse to the cage and ensure that it properly recovers from anesthesia.
3. Mouse Intranasal Challenge with H37Rv Strain
- Thaw a pre-quantified H37Rv glycerol and dilute it in PBS to prepare a bacterial suspension of 2,500 CFU per ml. Calculate final volume considering the dose per animal is 40 μl.
- H37Rv is inoculated intranasally as described in 2.5 – 2.7. Final challenge dose per mouse is 102 CFU/40 µl.
4. Analysis of Induced Immune Response in Lungs
- Lung cellular suspension
NOTE: Assessment of vaccine-induced adaptive immune response in lungs requires the generation of single cell suspension. For this purpose, we use a tissue dissociator such as GentleMACS .- Sacrifice the mouse by cervical dislocation (or other accepted humane methodology) and ensure mouse is dead touching the eyeball to confirm absent of blink reflex.
- Inside a laminar flow hood, extract the lungs using sterile scissors and forceps and place them on a clean surface. Clean lungs removing trachea and connective tissue, and transfer the lungs to a 1.5-ml tube with PBS (maintain on ice until processing).
- To generate a cellular suspension, place lungs in a proper tube for organ dissociation (C-dissociator tube) containing 5 ml of 10 mM HEPES-NaOH pH 7.4, 150 mM NaCl, 5 mM KCl, 1 mM MgCl2 and 1.8 mM CaCl2 buffer.
- Add 100 μl of Collagenase D 100 mg/ml (2 mg/ml final concentration), and 40 μl of DNAse I 20,000 IU/ml (160 IU/ml final concentration.
NOTE: Stock solutions of Collagenase D or DNaseI are prepared dissolving lyophilized enzymes in PBS or glycerol 50% (v/v), 20 mM Tris-HCl pH 7.5 y MgCl2 1 mM, respectively. - Place the tube in the tissue dissociator and use the predefined program m_lung_01 for lung fragmentation. Incubate the tube at 37 °C in a water bath for 30 min.
- Place the tube in the dissociator and use the predefined program m_lung_02 for complete lung homogenization into single cellular suspension.
- Pass the cellular suspension through a 70-μm nylon cell strainer fitted to a 50-ml centrifuge tube. Wash the strainer with 10 ml of RPMI 1640 medium.
- Centrifuge cells for 5 minutes at 400 x g and discard supernatant.
- Add 1 ml of erythrocytes lysing buffer, mix by vortexing and incubate for 1 min at room temperature.
- Add 10 ml of RPMI 1640 medium and centrifuge cells for 5 minutes at 400 x g.
- Resuspend cells in 0.5 ml of complete RPMI 1640 medium supplemented with 10% heat-inactivated Foetal Calf Serum (FCS), 2 mM L-Glutamin, 100 μg/ml Streptomycin, 100 IU/ml Penicillin and 50 μM 2-mercaptoethanol.
NOTE: FCS is inactivated by incubation at 56°C for 30 minutes. - Count cells and add complete RPMI 1640 medium to reach final cellular density of 107 cells/ml.
NOTE: For cell count, we use the automated cell counter. - Distribute 100 μl of cellular suspension in U-bottom 96-well sterile plate (106 cells/well).
- Add 50 μl/well of complete RPMI 1640 medium with 15 μg/ml of commercial H37Rv Purified Protein Derivative (PPD) (5 μg/ml final concentration).
- Supernatant collection for cytokine determination by ELISA
- Incubate plate from 4.1.14 for 48 hr at 37 °C and 5% CO2.
- Centrifuge plate for 5 min at 400 x g and recover 100 μl of supernatant.
- Store at -80 °C.
- Dilute supernatants 1/10 and 1/2 in assay buffer for IFNγ and IL17A determination, respectively. Determine cytokine concentrations with commercial ELISA kits, according to manufacturer instructions (ELISA Development kits).
- Intracellular staining for determination of cytokine-producing CD4+ cells by flow cytometry
- Incubate plate from 4.1.14 for 18 hr at 37 °C and 5% CO2.
- Add 1.5 μl of Brefeldin A 1 mg/ml (stock dissolved in DMSO) to each well (10 μg/ml final concentration) and incubate for six additional hours.
- Centrifuge the plate from 4.3.2 for 1 min at 3200 x g and discard supernatant.
- Resuspend cells in 50 μl of anti-mouse CD4-FITC diluted in RPMI 1640 with 10% FCS (1/500) and incubate for 10 min at 4 °C.
- Centrifuge the plate from 4.3.4 for 1 min at 3,200 x g, discard supernatant and add 100 μl/well of fixation solution. Incubate at 4 °C for 20 min.
- Wash cells twice with 150 μl/wash of permeabilization solution.
NOTE: 10x concentrated stock is diluted in deionized water to prepare 1x work solution. - Resuspend cells in 50 μl of anti mouse IFNγ-APC and anti-mouse IL17A-APC.Cy7 diluted in permeabilization solution (1/250 both antibodies).
- Incubate for 1 hr at RT.
- Wash cells twice with 150 μl/wash of permeabilization solution.
- Resuspend cells in 200 μl of PBS for flow cytometry acquisition.
5. Analysis of Immunoglobulins in Bronchoalveolar Lavage (BAL)
- Obtainment of BAL samples
- Sacrifice the mouse by cervical dislocation (or other accepted humane methodology) and ensure mouse is dead touching the eyeball to confirm absent of blink reflex.
- Expose the trachea using proper precision sterile forceps and scissors and perform a small cut in the trachea, making sure not to excise it completely. Take care not to cause bleeding that could contaminate BAL sample.
- Introduce into the trachea a cannula connected to a sterile 1-ml syringe with 800 μl of ice-cold PBS.
- Inoculate slowly the PBS into the lungs and then recover BAL volume pulling back the syringe's piston very slowly in order to avoid lung collapse.
NOTE: Recovery of 500 to 600 μl is satisfactory. - Transfer BAL volume into a 1.5-ml tube and confirm BAL remains uncoloured, indicating that is not contaminated with blood (maintain on ice until processing).
- Centrifuge BAL samples for 5 min at 400 x g, and transfer supernatant to a new tube.
- Store supernatants at -80 °C.
- Determination of IgA in BAL supernatants
NOTE: All reagents used are at RT.- Coat high-protein binding polystyrene flat-bottom 96-well plates with 100 μl of PPD diluted in PBS (10 μg/ml) for M. tuberculosis-specific IgA determination, or with 100 μl of BAL supernatant diluted 10 times in PBS for total IgA determination.
- Incubate overnight at 4 °C.
- Discard supernatant and tap the plate on paper towel to dry. Wash with 200 μl/well of PBS.
- Block with 200 μl/well of a bovine serum albumin (BSA) 1% (w/v) in PBS-Tween-80 0.05% (v/v) (Blocking buffer).
- Incubate 1 hr at RT.
- Wash once with 200 μl/well of PBS-Tween-80 0.05% (v/v) (Washing buffer).
- Add 100 μl of undiluted BAL supernatant in the PPD-coated wells. Incubate two hours at room temperature.
NOTE: If the total IgA determination is done in parallel with the PPD-specific IgA, leave total IgA wells with 100 μl washing buffer during this incubation. - Wash three times with 200 μl/well of washing buffer.0170004
- Add 100 μl of horseradish peroxidase (HRP)-conjugated anti-mouse IgA diluted in blocking buffer (1/10,000).
- Incubate 1 hr RT.
- Wash five times with 200 μl/well of washing buffer.
- Add 100 μl/well of 3,3′,5,5′-Tetramethylbenzidine (TMB). Incubate 20 min at room temperature in the dark.
- Add 100 μl/well of H2SO4 0.5N. Read the plate in a spectrophotometer at a wavelength of 450 nm.
6. Bacterial load determination in lungs
- Lung homogenization and plating
- Four weeks after challenge, sacrifice the mouse by cervical dislocation (or other accepted humane methodology) and ensure mouse is dead touching the eyeball to confirm absent of blink reflex.
- Place the mouse in supine position fixed on a flat and disinfected surface inside a laminar flow hood.
- Make an incision and remove the skin from chest region to leave visible the thoracic area.
- Cut out the ribs and harvest the lungs and heart using sterile scissors and forceps. Place organs on a clean surface.
- Clean out lungs by removing the heart, trachea and connective tissue and transfer the lungs to a 1.5-ml tube with 1 ml of deionized water (maintain on ice until processing).
- Use sterile forceps to transfer the lungs of each mouse into a proper tube for organ homogenization (M – dissociator tube), containing 1 ml of deionized water.
- Place the tube in the tissue dissociator and use the predefined program RNA_1 to homogenise the lungs.
- Make five ten-fold serial dilutions in 1.5-ml tubes, starting with 100 μl of lung homogenate diluted in 900 μl.
- Plate dilutions as described in 1.11-1.14.
- Differentiation between BCG and H37Rv by PCR
NOTE: In the plates from 6.1.6 corresponding to the group vaccinated with BCG by the intranasal route, we analyzed a representative number of colonies by a discerning PCR between BCG and H37Rv to ensure that the CFU considered to assess vaccine-conferred protective efficacy corresponded uniquely to H37Rv. This PCR targets amplification of RD9, a genomic region different in BCG and M. tuberculosis, giving a fragment of 2618 bp for H37Rv, and 465 bp for BCG 11.- Prepare a master mix stock for all samples with volumes per sample as indicated, and then distribute in PCR tubes (9 μl/sample).
NOTE: Buffer 5x: 2 μl. Forward primer 25 μM (GTGTAGGTCAGCCCCATCC): 0.32 μl, Reverse primer 25 μM (GCCCAACAGCTCGACATC): 0.32 μl, Taq polymerase 5 units/μl: 0.04 μl, Deionized H2O: 6.32 μl. - Touch a colony with a sterile toothpick and immerse the toothpick with the sample in a PCR tube containing the master mix.
- Run the PCR with the following program: 10 min 95 °C (this step is to trigger bacterial disruption), 10 min 95 °C (this step is to trigger bacterial disruption), 35 cycles ( 30 seconds at 95 °C, 1 min at 58 °C and 4 min at 72 °C).
- Load the PCR samples in a 1% agarose gel with ethidium bromide and run samples to visualize the fragments.
- Prepare a master mix stock for all samples with volumes per sample as indicated, and then distribute in PCR tubes (9 μl/sample).
Representative Results
This work describes the comparison of two routes of administration of BCG: subcutaneous and intranasal. Subcutaneous route is comparable to the intradermal, which is the current clinical route for BCG worldwide. Intranasal route of vaccination aims to mimic the natural route of infection of M. tuberculosis, with the objective to induce immune response directly in the lungs, the primary target organ of this pathogen.
Figure 1 describes the workflow followed. Eight to ten week-old female DBA/2 mice are vaccinated with 106 CFU of BCG Danish by the subcutaneous or intranasal route of administration. Eight weeks later, a group of mice is sacrificed to analyze lung immune response induced by vaccination. BAL samples are first obtained and then we harvest lungs. In order to study vaccine-conferred protective efficacy, we inoculate a different group of mice with a low-dose intranasal challenge of M. tuberculosis H37Rv strain. One month later, we sacrifice animals and harvest lungs. In this case, for each animal we use the left lung to determine bacterial load and the right lung to assess vaccine-induced immune response post-challenge. The objective is to generate bacterial load and immune response data in lungs for each animal in order to study potential correlates of protection.
As shown in Figure 2, lungs were dissociated to obtain either an organ homogenate or a cellular suspension. Homogenized lungs were plated on solid agar medium to determine bacterial load four weeks post-challenge. Cellular suspension to study vaccine-induced immune response was obtained following lung enzymatic digestion with collagenase D and DNaseI.
Our results clearly indicate that, when compared to the subcutaneous route, the intranasal route of vaccination confers a much greater protective efficacy in lungs four weeks after challenge (Figure 3A). In addition, we confirmed that bacterial load in lungs from the intranasal BCG-vaccine group correspond to H37Rv and not to BCG vaccine. To this end, we analyzed a representative number of colonies from this group by specific PCR for the RD9 genome region, which amplifies different length fragments in BCG and M. tuberculosis (Figure 3B). Figure clearly showed that all the colonies analyzed provided a fragment of 0.4 kbp, which corresponded to H37Rv.
Our data revealed correlation between protective efficacy conferred by intranasal BCG vaccination and vaccine-induced immune response in lungs prior to challenge. Intranasal BCG clearly triggered higher IL17 and IFNγ production in lungs, measured by ELISA (Figure 4A). These data were confirmed by intracellular staining (ICS) and flow cytometry (data not shown). In addition, we also found a higher concentration of both total and PPD-specific IgA concentration in BAL samples (Figure 4B), indicating that pulmonary BCG vaccination induces production of IgA and translocation to respiratory airways.
Finally, we studied BCG-induced immune response in lungs after challenge (Figure 5). Our data revealed differences between IL17 and IFNγ; IL17A-producing CD4+ cells were only detected in the intranasal BCG group, whereas IFNγ-producing cells were found in all groups infected with H37Rv regardless of vaccination. Representation of data of each animal corresponding to IL17-producing cells and bacterial load showed a significant correlation between presence of IL17 and lung bacterial load reduction, which was not observed in the case of IFNγ.
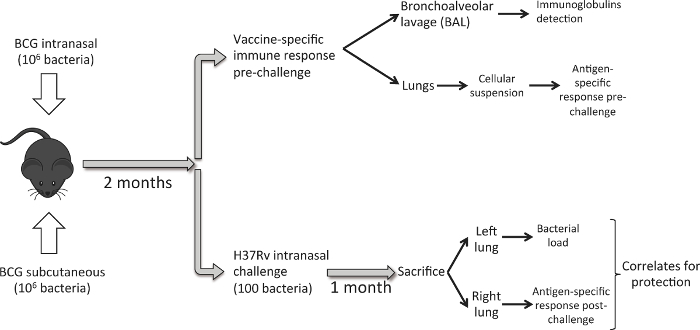
Figure 1. Workflow to Compare Intranasal and Subcutaneous Route of BCG Vaccination. Eight weeks post BCG immunization, a set of mice (6 per experimental group) is used to harvest lungs and perform a BAL, and analyze pulmonary immune response induced by vaccination. Another set of mice (6/group), is inoculated with a low-dose intranasal challenge of M. tuberculosis H37Rv strain (100 CFU). One month later, animals are sacrificed and bacterial load is analyzed in the left lung and PPD-specific immune response in the right lung. Please click here to view a larger version of this figure.
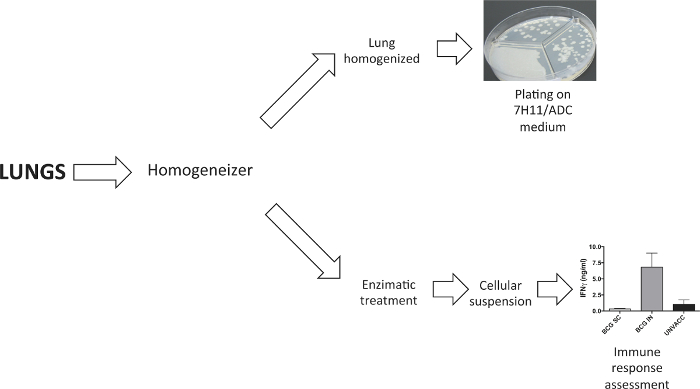
Figure 2. Processing of Lung Samples. A tissue dissociator was used to process lungs. In the experiments to determine bacterial load, lungs were homogenised prior to plate them in solid agar medium. In experiments that require a lung cellular suspension, this was generated following enzymatic digestion with collagenase D and DNaseI. Please click here to view a larger version of this figure.
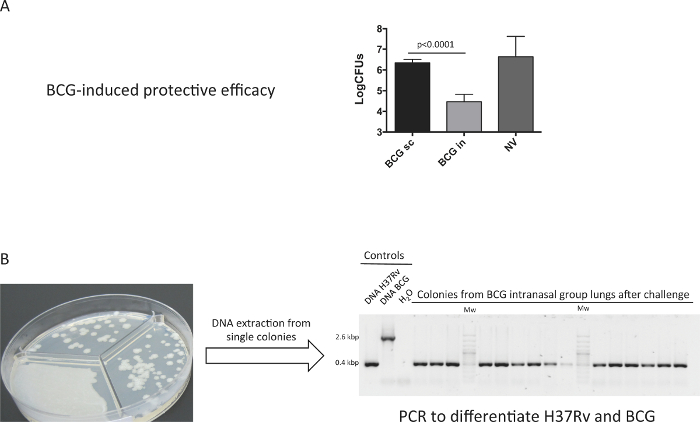
Figure 3. Protective Efficacy Conferred by BCG Immunization. Groups of 6 DBA/2 mice were vaccinated by the subcutaneous (BCG sc), intranasal (BCG in) route, or non-vaccinated (Unvacc) with BCG Danish vaccine 106 CFU. At two months post-vaccination, mice were inoculated intranasally with a low dose (100 CFU) H37Rv challenge, and one-month later bacterial burden in lungs was determined. A representative experiment of two independent is shown. (A) Data in the graphs are represented as mean+SD. One-way ANOVA test with Bonferroni post analysis was performed to calculate statistical significance. (B) A representative number of single colonies from the BCG intranasal group were analyzed by PCR specific for RD9 region (different in BCG and H37Rv genomes) to discern BCG and H37Rv colonies. (Previously published 9). Please click here to view a larger version of this figure.
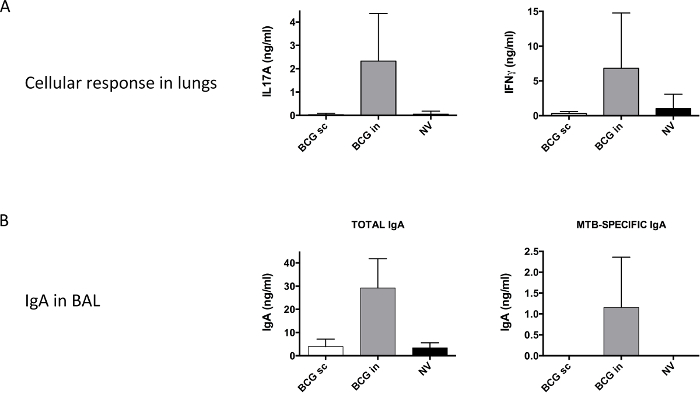
Figure 4. Vaccine-specific Pulmonary Immune Response Analyzed Prior to Challenge with H37Rv. Groups of 6 DBA/2 mice were vaccinated by the subcutaneous (BCG sc) or intranasal (BCG in) route, or non-vaccinated (Unvacc) with BCG Danish vaccine 106 CFU. (A) At two months post-vaccination, a cellular suspension from harvested lungs was obtained. Cells were stimulated with PPD as described in methods section and IL17A (left panel) and IFNγ (right panel) production were analyzed by ELISA. (B) Total IgA, and M. tuberculosis (MTB)-specific IgA were analyzed from BAL samples by ELISA. Pooled data from two independent experiments are shown. Data in the graphs are represented as mean + SD. (Previously published 9). Please click here to view a larger version of this figure.
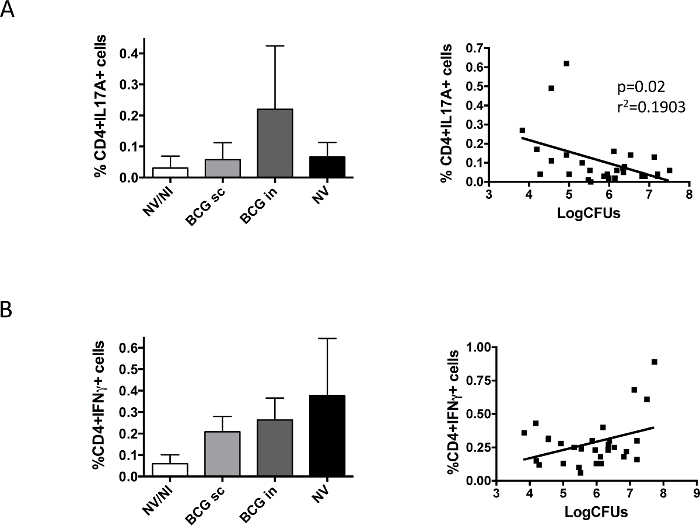
Figure 5. Vaccine-specific Pulmonary Immune Response Analyzed prior to Challenge with H37Rv. Groups of 6 DBA/2 mice were vaccinated by the subcutaneous (BCG sc) or intranasal (BCG in) route, or non-vaccinated (NV) with BCG Danish vaccine 106 CFU. A control group of non-vaccinated, non-infected mice was also included (NV/NI). At two months post-vaccination, mice were challenged intranasally with a low H37Rv dose (100 CFU), and one-month later animals were euthanized. Left and right lungs from the same animal were used to determine bacterial load and IL17A- (A) or IFNγ- (B) producing CD4+ cells, respectively. Data in left panels correspond to percentage of cytokine-producing cells measured by flow cytometry, and are represented as mean + SD. Right panels represent data from bacterial load and cytokine- producing CD4+ cells obtained for each mouse. Linear regression was calculated and the p-value obtained in each case is shown in the case of IL17A. Pooled data from two independent experiments are shown in the figure. (Previously published 9). Please click here to view a larger version of this figure.
Discussion
Although current vaccine against tuberculosis, BCG, is the most widely administered vaccine in history, tuberculosis remains one of the leading causes of death and morbidity from infectious diseases worldwide. This paradox is explained by the lack of protection of this vaccine against pulmonary tuberculosis, the responsible form of transmission. New vaccination approaches effective against pulmonary forms of the disease are urgently needed, as they would have the greatest impact on disease transmission globally.
Our data clearly show that a change in the route of administration of BCG to mimic the natural route of infection could be a successful strategy to prevent pulmonary tuberculosis. Our results are in accordance with other authors showing equivalent data in different animal models, including guinea pigs and non-human primates (NHP) 6,9,12.
Remarkably, our unpublished data indicated that the volume of administration by the intranasal route is a critical step of the protocol. These results revealed that following intranasal delivery of 100 H37Rv bacteria resuspended in 10 μl (instead of 40 μl), we only recovered lung CFUs from around 20% of the animals (data not shown.
Importantly, one of the possible limitations of intranasal administration as a vaccine delivery route in clinic could be its proximity with the central nervous system13,14. In this regard, aerosol immunization might be safer as delivery route for pulmonary tuberculosis vaccines in humans.
This protocol describes a standardized methodology that can be adapted to other studies, as comparison of pulmonary vaccines, or different routes of pulmonary immunization, including aerosol.
Our data suggest that the analysis in parallel of protective efficacy and immune response, using lung samples from the same animal, could be a useful tool to identify biomarkers of protection. In this regard, our results reveal that lung IL17, but not IFNγ, seems to correlate with a better vaccine protective efficacy. These data highlight a possible role of IL17 in the protective response induced by intranasal BCG, which is in accordance with data reported by other authors using mucosal subunit TB vaccines 7,15. Our data also indicate that presence of IgA (both total and MTB-specific) in BAL samples correlates with protection conferred by intranasal BCG. Importantly, we described previously, in agreement with other works, that IL17 contributes to traslocation of IgA to respiratory airways and gut lumen.9,16,17
Divulgazioni
The authors have nothing to disclose.
Acknowledgements
This work was supported by “Spanish Ministry of Economy and Competitiveness” [grant number BIO2014-5258P], “European Commission” by the H2020 programs [grant numbers TBVAC2020 643381].
Materials
| Middlebrook 7H9 broth | BD | 271310 | |
| Middlebrook ADC Enrichment | BD | 211887 | |
| Tween 80 | Scharlau | TW00800250 | |
| 3-mm diameter Glass Beads | Scharlau | 038-138003 | |
| Middlebrook 7H10 Agar | BD | 262710 | |
| 1-ml syringe 26GA 0.45×10 mm | BD | 301358 | |
| GentleMACS dissociator | Miltenyi Biotec | 130-093-235 | |
| C tubes | Miltenyi Biotec | 130-093-237 | |
| M tubes | Miltenyi Biotec | 130-093-236 | |
| Collagenase D | Roche | 11088882001 | |
| DNaseI | Applichem | A3778,0100 | |
| Falcon 70µm Cell Strainer | Corning | 352350 | |
| RPMI 1640 | Sigma | R0883 | |
| Red Blood Cell Lysing Buffer | Sigma | R7757 | |
| GlutaMAX Supplement | Gibco | 35050-061 | 100X concentrated |
| Penicillin-Streptomycin Solution | Sigma | P4333 | 100X concentrated |
| Fetal Calf Serum | Biological Industries | 04-001-1A | |
| 2-Mercaptoethanol | Sigma | M3148-25ML | |
| Scepter 2.0 Handheld Automated Cell Counter | Millipore | PHCC20040 | |
| Scepter Cell Counter Sensors, 40 µm | Millipore | PHCC40050 | |
| Mycobacterium Tuberculosis – Tuberculin PPD | Statens Serum Institut (SSI) | 2390 | |
| Mouse IFN-γ ELISA development kit | Mabtech | 3321-1H | |
| Mouse IL17A ELISA development kit | Mabtech | 3521-1H | |
| Brefeldin A | Sigma | B7651 | |
| FITC Rat Anti-Mouse CD4 | BD | 553047 | |
| BD Cytofix/Cytoperm Kit | BD | 555028 | |
| APC-Cy7 Rat Anti-mouse IL-17A | BD | 560821 | |
| APC Mouse Anti-mouse IFNg | BD | 554413 | |
| LACHRYMAL OLIVE LUER LOCK 0.60 x 30 mm. 23G x 1 1/4” | UNIMED | 27.134 | Used as trachea cannula for BAL |
| high-protein binding polystyrene flat-bottom 96-well plates MAXISORP | NUNC | 430341 | |
| Albumin, from bovine serum | Sigma | A4503 | |
| Goat Anti-Mouse IgA (α-chain specific)−Peroxidase antibody | Sigma | A4789 | |
| 3,3′,5,5′-Tetramethylbenzidine (TMB) | Sigma | T0440 | |
| MyTaq DNA Polymerase | Bioline | BIO-21107 | The kit Includes Buffer 5x |
Riferimenti
- Zumla, A., et al. The WHO 2014 global tuberculosis report–further to go. Lancet Glob Health. 3 (1), e10-e12 (2015).
- Mangtani, P., et al. Protection by BCG vaccine against tuberculosis: a systematic review of randomized controlled trials. Clin Infect Dis. 58 (4), 470-480 (2014).
- Aguilo, N., et al. Pulmonary Mycobacterium bovis BCG vaccination confers dose-dependent superior protection compared to that of subcutaneous vaccination. Clin Vaccine Immunol. 21 (4), 594-597 (2014).
- Chen, L., Wang, J., Zganiacz, A., Xing, Z. Single intranasal mucosal Mycobacterium bovis BCG vaccination confers improved protection compared to subcutaneous vaccination against pulmonary tuberculosis. Infect Immun. 72 (1), 238-246 (2004).
- Giri, P. K., Verma, I., Khuller, G. K. Protective efficacy of intranasal vaccination with Mycobacterium bovis BCG against airway Mycobacterium tuberculosis challenge in mice. J Infect. 53 (5), 350-356 (2006).
- Lagranderie, M., et al. BCG-induced protection in guinea pigs vaccinated and challenged via the respiratory route. Tuber Lung Dis. 74 (1), 38-46 (1993).
- Gopal, R., et al. Interleukin-17-dependent CXCL13 mediates mucosal vaccine-induced immunity against tuberculosis. Mucosal Immunol. 6 (5), 972-984 (2013).
- Khader, S. A., et al. IL-23 and IL-17 in the establishment of protective pulmonary CD4+ T cell responses after vaccination and during Mycobacterium tuberculosis challenge. Nat Immunol. 8 (4), 369-377 (2007).
- Aguilo, N., et al. Pulmonary but Not Subcutaneous Delivery of BCG Vaccine Confers Protection to Tuberculosis-Susceptible Mice by an Interleukin 17-Dependent Mechanism. J Infect Dis. , (2015).
- Middlebrook, G., Cohn, M. L. Bacteriology of tuberculosis: laboratory methods. Am J Public Health Nations Health. 48 (7), 844-853 (1958).
- Brosch, R., et al. A new evolutionary scenario for the Mycobacterium tuberculosis complex. Proc Natl Acad Sci U S A. 99 (6), 3684-3689 (2002).
- Kaushal, D., et al. Mucosal vaccination with attenuated Mycobacterium tuberculosis induces strong central memory responses and protects against tuberculosis. Nat Commun. 6, 8533 (2015).
- Lochhead, J. J., Thorne, R. G. Intranasal delivery of biologics to the central nervous system. Adv Drug Deliv Rev. 64 (7), 614-628 (2012).
- Lochhead, J. J., Wolak, D. J., Pizzo, M. E., Thorne, R. G. Rapid transport within cerebral perivascular spaces underlies widespread tracer distribution in the brain after intranasal administration. J Cereb Blood Flow Metab. 35 (3), 371-381 (2015).
- Griffiths, K. L., et al. Cholera toxin enhances vaccine-induced protection against Mycobacterium tuberculosis challenge in mice. PLoS One. 8 (10), e78312 (2013).
- Hirota, K., et al. Plasticity of Th17 cells in Peyer’s patches is responsible for the induction of T cell-dependent IgA responses. Nat Immunol. 14 (4), 372-379 (2013).
- Jaffar, Z., Ferrini, M. E., Herritt, L. A., Roberts, K. Cutting edge: lung mucosal Th17-mediated responses induce polymeric Ig receptor expression by the airway epithelium and elevate secretory IgA levels. J Immunol. 182 (8), 4507-4511 (2009).

