Combining Solid-state and Solution-based Techniques: Synthesis and Reactivity of Chalcogenidoplumbates(II or IV)
Summary
The synthesis of chalcogenidoplumbates(II,IV) via the in situ reduction of nominal “PbCh2” (Ch = Chalcogen) and via a solid-state reaction and subsequent solvothermal reactions is presented. Additionally, reactivities of plumbate(II) solutions are portrayed, which yield the heaviest-known CO homolog known to date: the µ-PbSe ligand.
Abstract
The phases of "PbCh2" (Ch = Se, Te) are obtained from solid-state syntheses (i.e., by the fusion of the elements under inert conditions in silica glass ampules). Reduction of such phases by elemental alkaline metals in amines affords crystalline chalcogenidoplumbate(II) salts comprised of [PbTe3]2– or [Pb2Ch3]2– anions, depending upon which sequestering agent for the cations is present: crown ethers, like 18-crown-6, or cryptands, like [2.2.2]crypt. Reactions of solutions of such anions with transition-metal compounds yield (poly-)chalcogenide anions or transition-metal chalcogenide clusters, including one with a µ-PbSe ligand (i.e., the heaviest-known CO homolog).
In contrast, the solid-state synthesis of a phase of the nominal composition "K2PbSe2" by successive reactions of the elements and by the subsequent solvothermal treatment in amines yields the first non-oxide/halide inorganic lead(IV) compound: a salt of the ortho-selenidoplumbate(IV) anion [PbSe4]4–. This was unexpected due to the redox potentials of Pb(IV) and Se(-II). Such methods can further be applied to other elemental combinations, leading to the formation of solutions with binary [HgTe2]2– or [BiSe3]3– anions, or to large-scale syntheses of K2Hg2Se3 or K3BiSe3 via the solid-state route.
All compounds are characterized by single-crystal X-ray diffraction and elemental analysis; solutions of plumbate salts can be investigated by 205Pb and 77Se or 127Te NMR techniques. Quantum chemical calculations using density functional theory methods enable energy comparisons. They further allow for insights into the electronic configuration and thus, the bonding situation. Molecular Rh-containing Chevrel-type compounds were found to exhibit delocalized mixed valence, whereas similar telluridopalladate anions are electron-precise; the cluster with the µ-PbSe ligand is energetically favored over a hypothetical CO analog, in line with the unsuccessful attempt at its synthesis. The stability of formal Pb(IV) within the [PbSe4]4– anion is mainly due to a suitable stabilization within the crystal lattice.
Introduction
Metal chalcogenides, such as SnSe or CuInSe, are versatile materials with a wide range of applications, for instance, as semiconductor, thermoelectric, or nonlinear optic materials1-6. Similar elemental compositions are found within chalcogenidometalates, where the metal is in a formally positive oxidation state and coordinated by negative (poly-)chalcogenide ligands to yield an overall anionic species. Different from the abovementioned materials, such metalates are additionally comprised of counter-ions, which are well separated from the anionic substructure. Typical cations are (solvated) alkali or alkaline earth metals, ammonium, or phosphonium ions. Most often, such salts with chalcogenidometalate anions have physical properties that are similar to their parental binary or ternary compounds, such as similar band gaps or photo- and semiconductivity properties. However, due to the broad range of possible anionic architectures within each elemental combination, ranging from isolated molecular species through strands and sheets of interconnected anions to extended three-dimensional frameworks, an even finer tuning of various properties can be achieved, ultimately aiming at the designed synthesis of compounds with the desired properties. Within the concept of dimensional reduction, it has been shown that a relative increase of counter ions per formula unit, which accompanies a reduction from 3D via 2D and 1D to 0D anionic architectures (0D representing molecular species), decreases the observed band gap7. Moreover, by the utilization of different (or mixtures of) chalcogenide ligands, it is even possible to achieve an ultra-fine adjustment of the band gap8,9.
Apart from these practical applications and visionary relevancies, chalcogenidometalates are still investigated for fundamental understanding, such as for the generation of novel anionic structure types or the discovery and interpretation of an unusual bonding, as well as for their unprecedented properties. Whereas the lighter congeners (i.e., oxidometalates, commonly referred to as oxometalates) have been extensively studied, in particular for potential catalytic applications, the heavier chalcogenidometalates are far less explored.
Our own interest has been focused on the synthesis, properties, and further reactivity of chalcogenidotetrelates (i.e., the heavier homologs of silicates)10,11. There is a broad variety of such compounds, ranging from water-stable and soluble binary anions, such as the [SnTe4]4- anion12; to organic, functionalized, and multinary cluster compounds, such as {[Ir3(cod)3(µ3-S)2](µ3-S)SnCl}2 (cod = cycloocta-1,5-diene)13. Our most recent studies deal with chalcogenidoplumbates, with lead as the central metal atom(s). In line with the inert-pair concept for heavy atoms, addressing the stabilization of the 6s orbital due to relativistic effects, lead is usually observed in the formal +II oxidation state. Exceptions like PbO2 are strong oxidizing agents, and the heavier lead(IV) chalcogenides, "PbCh2," have not been discovered to date14. The same holds for the chalcogenidoplumbate(IV) anions, of which only [PbO4]4– has been reported15 until recently (see below).
Apart from a diverse group of structurally investigated oxidoplumbates(II,IV), there have been only few examples of chalcogenidoplumbates(II), namely [PbTe3]4–, with a trigonal pyramidal anion16; and [Pb2Ch3]2–, where Ch = Se or Te, with a trigonal bipyramidal anion17. These are synthesized by a route that has also been applied for the generation of Zintl ions18. Upon preparation of multinary intermetallic phases by fusion of the elements at high temperatures, subsequent extraction by solvents in the presence of a sequestering agent affords the desired products in (single-)crystalline form. In the case of the [Pb2Ch3]2– anions, for instance, a phase of the nominal composition "KPbCh" has been extracted with 1,2-diaminoethane (en) in the presence of 4,7,13,16,21,24-Hexaoxa-1,10-diazabicyclo[8.8.8]hexacosane ([2.2.2]crypt). The cryptand is necessary both for crystallization upon increase of the effective cation radius in the {K[2.2.2]crypt}+ complex counter ion, to better match the anionic size, and for a shielding of the positive charge that suppresses an electron back-donation from the anion in solution. Such salts with encapsulated cations usually reveal high tendencies for crystallization and thus, fairly good yields when compared to the corresponding salts without sequestration agents. However, a rather cumbersome synthesis or the high prices of cryptands prevent the excessive scaling of such approaches.
In contrast, K4[PbTe3]·2en is synthesized via in situ reduction in solution, as has already been used as early as 1891 for the generation of the famous Pb94– anion19,20. For the latter, elemental alkaline metals were added to suspensions of lead in liquid ammonia at low temperatures, whereas for the telluridoplumbate, an alloy of the nominal composition "PbTe2" was reduced at room temperature, again by the addition of elemental potassium.
Our first approach towards such metalate species to be presented herein is a combination of both pathways. Here, solid-state synthesis is followed by either reduction in solution in the presence of inexpensive sequestering agents, such as 1,4,7,10,13,16-hexaoxacyclooctadecane (18-crown-6), or via reduction with alkaline metals that are chelated by the solvent itself, without the need for additional sequestering agents, similar to the synthesis of [Na4(en)7][Sn9]21. Our second approach also starts with high-temperature synthesis, but it is followed by solvothermal extraction of the resulting phases (i.e., extraction at elevated temperatures and pressures)22. In the following, we will present both synthetic approaches and some of our recent results upon application of these reaction pathways.
Protocol
Caution: Always be cautious when working with chemicals. Apply common safety precautions, including the appropriate utilization of gloves, goggles, and a lab coat at all times. In particular, be aware that all discussed compounds containing heavy elements, as well as their elemental sources, are of high toxicity. 1,2-diaminoethane is a corrosive liquid. Alkaline metals and ternary solid-state products may react pyrophorically with air and moisture.
Note: All manipulations are performed in an argon atmosphere using standard Schlenk or glovebox techniques under strict exclusion of air and external moisture. Solids or solutions containing heavy element metalate species or precursors are stored under the exclusion of light by wrapping the respective containers with aluminum foil for inhibition of a light-induced decomposition.
1. Preparation of Solvents and Solutions
- Add 1 L of freshly purchased 1,2-diaminoethane to 25 g of CaH2 and stir overnight. Reflux (Tb = 116 °C) until no H2 is generated (approximately 12 h).
- Distill at ambient pressure.
- Add 1 L of oxolane (THF) to 10 g of NaK alloy and stir overnight. Reflux (Tb = 66 °C) for at least 12 h. Distill at ambient pressure.
- Make a saturated solution of [Rh(PPh3)3Cl] by adding 150 mg of [Rh(PPh3)3Cl] to 10 mL of THF. Stir overnight at room temperature (RT) and filtrate with an inert gas filter frit of low porosity.
2. High-temperature Solid-state Reactions
- Synthesis of PbSe2
- Place 3.81 g of elemental Se in a borosilicate ampule and add 5 g of elemental Pb on top. Heat it with an oxygen/methane burner until optical homogeneity of the melt is achieved (approximately 10 min). Knock the ampule gently with a cork ring throughout the synthesis to detach sublimed Se from the ampule wall, which will then drop back into the reaction mixture.
- Allow the ampule to cool down to room temperature. Break the ampule with a pestle in a mortar and manually remove all remaining splinters of the ampule. Pestle the crude PbSe2 thoroughly.
- Synthesis of K2PbSe2
- Place 0.95 g of elemental K and 5 g of elemental Pb in a thick-walled borosilicate ampule. Slowly increase the heat with an oxygen/methane burner until optical homogeneity of the melt is achieved (approximately 20 min).
- Carefully add 1.9 g of elemental Se pellets to the molten alloy. Upon the complete addition, increase the temperature until the reaction mixture emits bright yellow/white radiation (approximately 10 min) and hold the temperature for 10 min. Decrease the reaction temperature slightly if the radiation color turns to pure, bright white (a temperature close to the melting point of the ampule).
- Allow the reaction mixture to cool down to RT. Break the ampule and manually remove all remaining splinters of the ampule and a regulus of elemental lead. Pestle the crude K2PbSe2 thoroughly.
3. In Situ Reduction
- Synthesis of a solution of [K(18-crown-6)]2[Pb2Se3]
- Place 2 g of PbSe2, 3.1 g of 18-crown-6, 250 mL of 1,2-diaminoethane, and a large stir bar in a round-bottom N2-flask on a stir plate. Stir rigorously at RT and slowly add 0.45 g of elemental K.
- Stir overnight at RT and filter the solution with an inert gas filter frit of low porosity (pore diameters: D3, 16-40 µm or D4, 10-16 µm).
4. Solvothermal Reactions
- Synthesis of K4[PbSe4]·en·NH3
- Place 0.5 g of K2PbSe2 and 2 mL of 1,2-diaminoethane in a 10-mL glass vial in a 15-mL polytetrafluorethylene vial in a standard stainless-steel autoclave. Close the autoclave tightly and it heat in an oven to 150 °C for 5 days.
- Turn off the oven and leave it for 1 d to slowly cool to RT. Transfer the reaction mixture into paratone oil and manually select crystals of K4[PbSe4]·en·NH3 under a standard light microscope at 15-40X magnification.
5. Reactive Layering
- Synthesis of [(RhPPh3)6(µ3-Se)8]·0.5en
- Place 10 mL of a solution of [K(18-crown-6)]2[Pb2Se3] in a 50-mL flask, add 10 mL of a saturated solution of [Rh(PPh3)3Cl] in THF, and stir overnight.
- Filter the reaction solution with an inert gas filter frit of low porosity and remove the solvent under dynamic vacuum slowly during 24 h. Transfer the crude reaction product into paratone oil and manually select crystals of [(RhPPh3)6(µ3-Se)8]·0.5en under a standard light microscope at 15-40X magnification.
- Synthesis of {[K(18-crown-6)]-[K(en)2]K[Rh3(CN)2(PPh3)4(µ3-Se)2(µ-PbSe)]}2·1.3en
- Place 10 mL of a solution of [K(18-crown-6)]2[Pb2Se3] in a Schlenk tube and carefully layer it with 10 mL of a saturated solution of [Rh(PPh3)3Cl] in THF. Cover the Schlenk tube completely in aluminum foil and leave it undisturbed for 4 weeks.
- Transfer the resulting solid into paratone oil and select single crystals quickly under a light microscope.
6. Analysis of the Solutions and Compounds
- Place 50 mg of "K2PbSe2" onto an acrylic glass sample carrier (the compound reacts with elemental Si) and cover it with tape. Place it under ambient conditions in a powder X-ray diffractometer (PXRD) and record the diffraction data within 1 h23.
- Place 0.6 mL of a solution of [K(18-crown-6)]2[Pb2Se3] in a nuclear magnetic resonance (NMR) tube and thoroughly seal the latter with protective tape. Transfer it quickly into the NMR probe and record 77Se and 205Pb NMR with at least 2,000 and 5,000 pulses, respectively25.
- Select a single crystal under a light microscope and mount it on the goniometer head of the diffractometer. Measure it with high redundancy to enable the adequate absorption corrections23-33.
- Perform a simultaneous optimization of the electronic and geometric structure of [Rh3(CN)2(PPh3)4(µ3Se)2(µPbSe)]3–. Apply the conductor-like screening model (COSMO) with a 10% increase of the default radii to account for charge compensation28.
- Calculate the vibrational frequencies to ensure the energetic minimum28.
- Perform Mulliken and/or natural bond orbital (NBO) analyses based on the density functional theory (DFT) wave function to obtain the atomic charges28.
- Calculate in-orbital contributions to the "relaxed" and the original structures of a) the complete cluster, b) the CO/PbSe-free cluster, and c) the CO/PbSe ligand, and compare the results28.
Representative Results
The existence of an ortho-selenidoplumbate anion [PbSe4]4–23 (see Figure 1, top right) has been confirmed by single crystal diffraction experiments, elemental analysis, and quantum chemical calculations. The crystal structure refinement confirms the almost-perfect tetrahedral coordination geometry, as would be expected for a lead(IV) ion, whereas DFT calculations rationalize the energetically stabilized a1 representation, which contributes to the overall stability of the anion (see Figure 1, bottom right). The isolation of the anion as its potassium salt was possibly caused by the unexpected stability of the anion itself, but it was mainly due to the incorporation within a reasonable crystal structure. This is rationalized by similar measures of the anion as compared to its homolog, the well-known [SnTe4]4–. K4[PbSe4]·en·NH3 represents the first inorganic lead(IV) compound without highly electronegative ligands, such as oxygen or fluorine atoms.
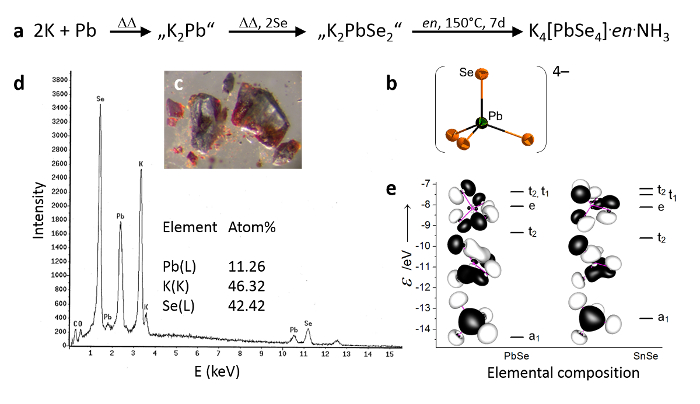
Figure 1: K4[PbSe4].en.NH3. Reaction pathway for the synthesis of K4[PbSe4].en.NH3 (a). Representation of the [PbSe4]4– anion, as determined by means of single crystal X-ray diffraction with thermal ellipsoids at 50% probability (b). Macroscopic appearance (c). Results of the elemental analysis via energy dispersive X-ray spectroscopy (d). Results of the quantum chemical calculations with amplitudes drawn at 0.033 a.u. (e). Parts of the figure were reproduced with permission from Wiley-VCH. Please click here to view a larger version of this figure.
By means of the same synthesis protocol as applied for K4[PbSe4]·2en·NH3, yet using a different elemental combination and stoichiometry, further metalate materials can be obtained. For example, K2Hg2Se324 is a semi- and photoconductor material with a polyanionic substructure that is based on three-dimensionally connected selenidomercurate tubes. The compound can be obtained on large scales and in high yield. It is a promising archetype for thermoelectric applications, even though the very elemental combination exhibits a too-large band gap, as demonstrated by means of DFT calculations with periodic boundary conditions and by ultra-violet (UV)-visible spectroscopy. This causes a rather low electronic conductivity, as rationalized experimentally. However, the band gap can be decreased by synthesizing the heavier homolog, K2Hg2Te3 (Figure 2), which indeed gives rise to an increase in the photoconductivity by several orders of magnitude.
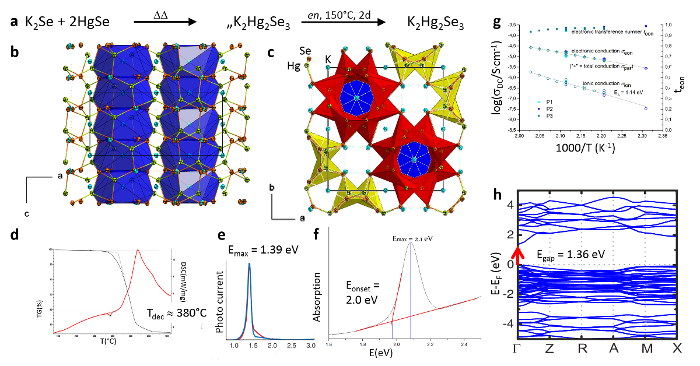
Figure 2: K2Hg2Se3. Reaction pathway for the synthesis of K2Hg2Se3 (a). Representation of the structures with channels along c (b, c) according to single-crystal X-ray diffraction, thermal (d), and optoelectronic analysis (e, f). Impedance spectroscopy results (g) and calculated band structure (h). Parts of the figure were reproduced with permission from the American Chemical Society. Please click here to view a larger version of this figure.
High yield and purity solutions of metalate anions do not only facilitate their isolation and full characterization25, but they can also be utilized for further reactivity studies, yielding, for example, molecular Chevrel-type compounds, such as [(RhPPh3)6(µ-Ch)8] (Ch = Se, Te) or anionic [Pd6(µ-Te)8]4– (Figure 3)26,27. Interestingly, the phosphine-saturated (thus, overall neutral) species include mixed valence Rh2+/Rh3+ ions, as rationalized by means of quantum chemical calculations. As the charge is highly delocalized over the cluster core, the structure determined by single crystal diffraction does not allow for an assignment of the different formal oxidation states. The anionic telluridopalladate cluster, in contrast, is electron-precise. Pd(II) ions adopt a distorted square-planar coordination environment and are thus interesting for further reactions towards Lewis-basic compounds, like in catalytic processes.
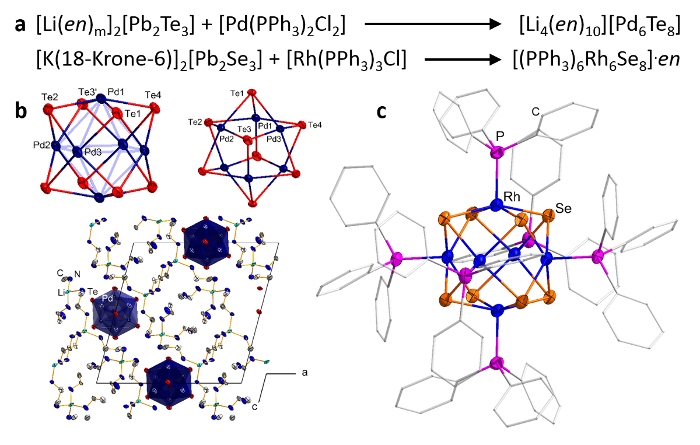
Figure 3: Molecular CHEVREL-type compounds. Reaction pathway for the synthesis of [Li4(en)10][Pd4Te8] and [(PPh3)6Rh6Se8]·en (a). Structural representation as determined by means of single-crystal X-ray diffraction (b, c). Parts of the figure were reproduced with permission from the American Chemical Society. Please click here to view a larger version of this figure.
Very similar reaction conditions, but a different work-up procedure, afford compounds with [Rh3Se2] units, adopting a trigonal bipyramidal shape, with Se at the apical positions and Rh in the basal plane28. These units represent the core of different anionic cluster complexes that can be isolated selectively by the addition of a certain counter-ion sequestering agent (Figure 4). [Rh3(PPh3)6(µ3-Se)2]–, with two PPh3 ligands coordinating each of the Rh(I) atoms, is crystallized as its salt upon the addition of [2.2.2]crypt. The use of 18-crown-6 instead yields a salt of the [Rh3(CN)2(PPh3)4(µ3-Se)2(µ-PbSe)]3– anion, in which only one of the Rh(I) atoms bears two phosphine ligands, whereas the two others are coordinated by one CN– ligand each. Additionally, and most remarkably, a µ-PbSe ligand bridges between the latter two metal atoms. The PbSe fragment is the second-heaviest carbon monoxide homolog and the heaviest one observed to date. Quantum chemical calculations helped to show that a corresponding complex with CO instead of PbSe in the bridging position would be disfavored, as the size and bonding properties of PbSe better match the requirements of the cluster core. In line with this, experiments in a CO atmosphere failed to yield a corresponding µ-CO-bridged species.
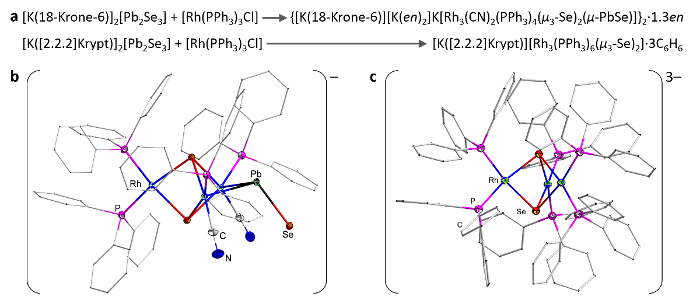
Figure 4: µ-PbSe: a very heavy CO analog. Reaction pathways (a) and structural representations, as determined by single-crystal X-ray diffraction for the [Rh3(CN)2(PPh3)4(µ3-Se)2(µ-PbSe)]3- (b) and [Rh3(PPh3)6(µ3-Se)2]– (c) anions. Parts of the figure were reproduced with permission from Wiley-VCH. Please click here to view a larger version of this figure.
Discussion
The combination of classical high-temperature, solid-state reactions with solution-based methods allows for the generation and isolation of novel compounds that cannot be synthesized by only one of these pathways. Even though, in most cases, a clear identification and full characterization of the intermediate species is difficult or essentially impossible, the general idea is straightforward and can be applied to a variety of elemental combinations. Furthermore, the actual synthetic conditions for the generation of one specific compound are rather flexible, and the presence of further ionic species and/or differing relative proportions of the involved elements affect the yield, but not the formation itself. The synthesis of K4[PbSe4]·2en·NH3,23 for instance, has to be performed by starting out from a phase of the nominal composition "K2PbSe2" for achievement of highest yields, while the same compound is obtained in lower yields upon the use of other phases, like "KPbSe," "K4PbSe4," or "K2PbSe4." Furthermore, the utilization of technical Pb, which contains up to 30% of Sb, affords the same product, to our surprise, in even better yields than with the phases mentioned before. This suggests a reaction mechanism involving a Sb3+/Sb5+ redox step — present, for example, in a phase with the nominal composition "K4PbxSb1-xSe4" — as a sacrificial oxidant for the generation of "Pb4+." The same applies for the generation of mercurates, thallates, and bismuthates: the solvothermal reaction of "K2PbSe2" with HgSO4 yields K2Hg2Se3, as does a solvothermal extraction of "KxHgySez" (where x ≥ y and z ≤ 2y; K2Hg2Se3 is the primary reaction product). Again, in most cases of such solvothermal "extractions," K2Hg2Se3 can be obtained with elemental proportions not too far off the nominal product of the solid-state fusion reaction. Depending on the respective amounts, K2Hg3Se4, K2Sex (x = 1.3), HgSe, and elemental Hg are obtained as side-products in corresponding yields.
Apparently, it is necessary to provide preformed multinary phases, as can be deduced from the generation of K4[HgSe3]·H2O29, which was synthesized from K2Se and HgSO4·nH2O. Attempts to synthesize this compound from a phase of the nominal composition "K4HgSe3," with varying percentages of water within the solvent, failed. Only the abovementioned mercurates, K2Hg2Se3 and K2Hg3Se4, were obtained instead. Vice versa, K2Hg2Se3 could so far not be obtained from solvothermal reactions starting out from K2Se and HgSO4 or HgSe.
In contrast to the aforementioned flexibility of the synthesis concerning elemental ratios, the change of solvent or the addition of trace amounts of different solvents had a large impact on the reaction product. Whereas N,N-dimethylformamide slowly decomposes into CO and HNMe2, to slowly increase the basicity of the mixture, or forms formate anions, which help with crystallization, primary amines either tend to form ammonia in situ or coordinate the metal ions in various ways, such as in [Ba(trien)2]2+ (trien = 2,2'-diaminodiethylamine) or [(pren)3Eu(Te3)2Eu(pren)3] (pren = 1,3-diaminopropane)30. Traces of water may act as a crystal solvent, affect the acidity and basicity of the solution, and/or act as templates by H-bonding.
A different approach towards these metalates is the pathway of in situ reduction. Formally chalcogen-rich chalcogenides or elemental chalcogens in the presence of metal chalcogenides are treated with elemental alkali metals in amines. As known for solutions of alkali metals in liquid ammonia, amine solutions of alkali metals possess a high reduction potential. Thus, the chalcogen is reduced, forming soluble chalcogenides that can further react with metal chalcogenides to yield chalcogenidometalates. However, the formal oxidation state of the metal within the metal chalcogenide is usually not affected. Thus, a large variety of metalate species can be obtained via this synthetic approach25. Additionally, the application of primary amines, such as 1,2-diaminoethane, yields stable alkali metal solutions that can be stored at RT (with the exception of cesium and, to a lesser extent, rubidium, which cause the instantaneous reduction of the amine). Furthermore, unlike reactions in liquid ammonia, the reactions in amines do not have to be performed at low temperatures. As would be expected, the reduction of tellurium usually proceeds much faster than that of selenium. Additionally, the solubility of telluride species in amines is generally enhanced by an order of magnitude. However, the resulting telluridometalate compounds are usually extremely sensitive to air, moisture, and — depending on the central metal ion — also to light.
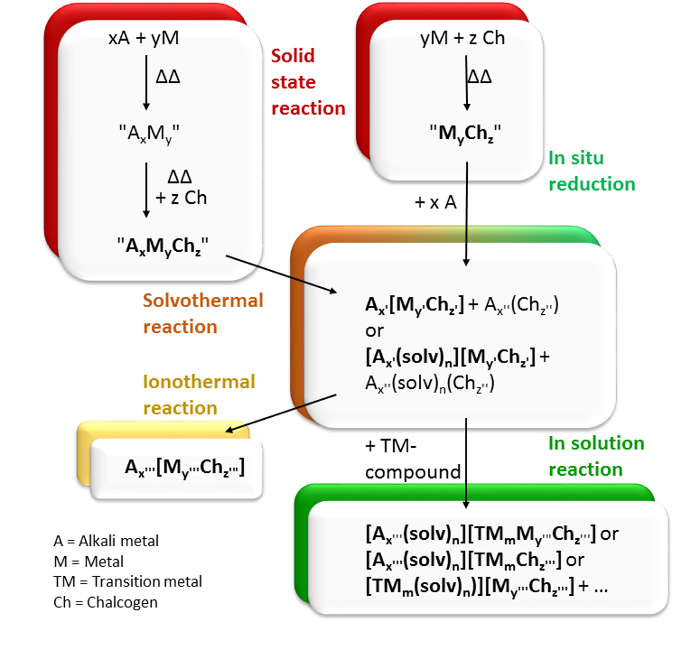
Figure 5: Summary of synthetic approaches. Reaction pathways starting out from the solid-state reactions to generate metalate species by solvothermal reactions or in situ reduction and the subsequent derivatization of the metalate species by means of ionothermal reactions or solution-based techniques. Please click here to view a larger version of this figure.
Each of the individual reaction approaches depicted in Figure 5 has intrinsic limitations. Solid-state reactions usually yield thermodynamic products, and since hardly any phase diagrams for the ternary compounds were investigated, the stoichiometries have to be investigated via an educated trial-and-error approach. The in situ reactions do not allow for unusual high oxidation states of the central metal (such as Pb+IV), and the solvent is restricted to primary amines. Transformation reactions in ionic liquids need to start from pure educts, and the exact nature of the reaction product cannot yet be predicted. This also applies to the reported reactions in solution, which additionally suffer from low yields, thus making subsequent reaction studies and physical investigations cumbersome.
However, apart from all metalate compounds that could be identified and isolated so far (see the summary of synthetic approaches in Figure 5), a vast variety of polychalcogenides have been detected and isolated31,32. These investigations have been neglected so far, even though they possess intriguing properties and can also be synthesized by a direct approach via the in situ method in high yields and purities. In contrast, for the solvothermal reaction pathway, no general pattern is observed for the resulting type of metalate. Neither have the exact influences of solvent and temperature been elucidated thus far. A predictive model, however, seems to be the ultimate goal for the synthesis of this versatile class of materials.
Divulgazioni
The authors have nothing to disclose.
Acknowledgements
This work was supported by the Deutsche Forschungsgemeinschaft (DFG) within the framework of SPP 1708. GT thanks the Leopoldina Nationale Akademie der Wissenschaften for a postdoctoral scholarship.
Materials
| Ethan-1,2-diamine | Sigma-Aldrich | E26266-2.5L | |
| Calcium hydride | Sigma-Aldrich | 213268-100G | |
| Tetrahydrofuran | Sigma-Aldrich | 401757-1L | |
| Sodium | Sigma-Aldrich | 71172-1KG | |
| Potassium | Sigma-Aldrich | 244864-50G | |
| Tris-triphenylphosphine rhodium chloride | Sigma-Aldrich | 199982-5G | |
| Lead | Acros | 222625000 | |
| Selenium | Sigma-Aldrich | 209643-50G | |
| 18-crown-6 | Acros | 181561000 |
Riferimenti
- Zhao, L. D., et al. Ultralow thermal conductivity and high thermoelectric figure of merit in SnSe crystals. Nature. 508 (7496), 373-377 (2014).
- Chung, I., Kanatzidis, M. G. Metal Chalcogenides: A Rich Source of Nonlinear Optical Materials. Chem. Mater. 26 (1), 849-869 (2014).
- Lhuillier, E., et al. Two-Dimensional Colloidal Metal Chalcogenides Semiconductors: Synthesis, Spectroscopy, and Applications. Acc. Chem. Res. 48 (1), 22-30 (2015).
- Heine, T. Transition metal chalcogenides: ultrathin inorganic materials with tunable electronic properties. Acc. Chem. Res. 48 (1), 65-72 (2015).
- Gao, M. R., Xu, Y. F., Jiang, J., Yu, S. H. Nanostructured metal chalcogenides: synthesis, modification, and applications in energy conversion and storage devices. Chem. Soc. Rev. 42, 2986-3017 (2013).
- Jackson, C., et al. Multiexciton Solar Cells of CuInSe2 Nanocrystals. J. Phys. Chem. Lett. 5 (2), 304-309 (2014).
- Androulakis, J., et al. Dimensional Reduction: A Design Tool for New Radiation Detection Materials. Adv. Mater. 23 (36), 4163-4167 (2011).
- Sheldrick, W. S. Polychalcogenide Anions: Structural Diversity and Ligand Versatility. Z. Anorg. Allg. Chem. 638 (15), 2401-2424 (2012).
- Dehnen, S., Melullis, M. A coordination chemistry approach towards ternary M/14/16 anions. Coord. Chem. Rev. 251 (9-10), 1259-1280 (2007).
- Santner, S., Heine, J., Dehnen, S. Synthesis of Crystalline Chalcogenides in Ionic Liquids. Angew. Chem. Int. Ed. 55 (3), 876-893 (2015).
- Heine, J., Dehnen, S. From Simple Chalcogenidotetrelate Precursors to Complex Structures and Functional Compounds. Z. Anorg. Allg. Chem. 638 (15), 2425-2440 (2012).
- Ruzin, E., Zent, E., Matern, E., Massa, W., Dehnen, S. Syntheses, Structures, and Comprehensive NMR Spectroscopic Investigations of Hetero-Chalcogenidometallates: The Right Mix toward Multinary Complexes. Chem. Eur. J. 15 (21), 5230-5244 (2009).
- Leusmann, E., Geringer, E., Weinert, B., Dehnen, S. Ir3(cod)3(µ3-S)2](µ3-S)SnCl} 2 – a Ternary Ir-Sn-S cluster with the Iridium Atoms in Three Different Chemical Environments. Dalton Trans. 45, 15298-15302 (2016).
- Pyykkö, P. Relativistic Effects in Chemistry: More Common Than You Thought. Ann. Rev. Phys. Chem. 63, 45-64 (2012).
- Brazel, B., Hoppe, R. Zur Kenntnis von K4PbO4 und Rb4PbO4. Z. Anorg. Allg. Chem. 505 (10), 99-104 (1983).
- Jones, C. D. W., DiSalvo, F. J., Haushalter, R. C. Synthesis and X-ray Crystal Structure of K4PbTe3·2(en). Inorg. Chem. 37 (4), 821-823 (1998).
- Björgvinsson, M., Sawyer, J. F., Schrobilgen, G. J. Dilead(II) Chalcogenide anions Pb2Ch32- (Ch = Se, Te): A 207Pb, 125Te, and 77Se Solution NMR Study. X-ray crystal structure of (2,2,2-crypt-K+)2Pb2Se32-. Inorg. Chem. 26 (5), 741-749 (1987).
- Scharfe, S., Kraus, F., Stegmaier, S., Schier, A., Fässler, T. F. Zintl Ions, Cage Compounds, and Intermetalloid Clusters of Group 14 and Group 15 Elements. Angew. Chem. Int. Ed. 50 (16), 3630-3670 (2011).
- Joannis, C. R. Action du sodammonium et du potassamonium sur quelques métaux. C. R. Hebd. Seances Acad. Sci. 113, 795-798 (1891).
- Joannis, C. R. Sur quelques alliages bien dèfinis de sodium. C. R. Hebd. Seances Acad. Sci. 114, 585-587 (1892).
- Diehl, L., Khodadeh, K., Kummer, D., Strähle, J. Anorganische Polyederverbindungen, III. Zintl’s „Polyanionige Salze": Darstellung und Eigenschaften der kristallinen Verbindungen [Na4·7 en]Sn9, [Na4·5 en]Ge9 und [Na3·4 en]Sb7 und ihrer Lösungen. Die Kristallstruktur von [Na4·7 en] Sn9. Chem. Ber. 109 (10), 3404-3418 (1976).
- Demazeau, G. Solvothermal Processes: Definition, Key Factors Governing the Involved Chemical Reactions and New Trends. Z. Naturforsch. 65b, 999-1006 (2010).
- Thiele, G., Krüger, T., Dehnen, S. K4[PbSe4]⋅en⋅NH3: A Non-Oxide, Non-Halide Inorganic Lead(IV) Compound. Angew. Chem. Int. Ed. 53 (18), 4699-4703 (2014).
- Thiele, G., et al. K2Hg2Se3: Large-Scale Synthesis of a Photoconductor Material Prototype with a Columnar Polyanionic Substructure. Chem. Mater. 27 (11), 4114-4118 (2015).
- Thiele, G., Vondung, L., Dehnen, S. About the Syntheses of Chalcogenidometalates by in-situ Reduction with Elemental Alkali Metals. Z. Anorg. Allg. Chem. 641 (2), 247-252 (2015).
- Thiele, G., You, Z., Dehnen, S. Molecular CHEVREL-like Clusters [(RhPPh3)6(µ3-Se)8] and [Pd6(µ3-Te)8]4-. Inorg. Chem. 54 (6), 2491-2493 (2015).
- Thiele, G., Balmer, M., Dehnen, S. Synthesis, Structure and Electronic Situation of [Rh6Te8(PPh3)6]·4C6H6. Zeitschrift für Naturforschung B. 71 (5), 391-394 (2016).
- Thiele, G., Franzke, Y., Weigend, F., Dehnen, S. {µ-PbSe}: A Heavy CO Homologue as an Unexpected Ligand. Angew. Chem. Int. Ed. 54 (38), 11283-11288 (2015).
- Thiele, G., et al. Smallest molecular chalcogenidometalate anions of the heaviest metals: syntheses, structures, and their interconversion. Dalton Trans. , (2016).
- Thiele, G., et al. Solvothermal and ionothermal synthses and structures of amine- and/or (poly-)chalcogenide coordinated metal complexes. Z. Kristallogr. 229 (7), 489-495 (2014).
- Thiele, G., Vondung, L., Donsbach, C., Pulz, S., Dehnen, S. Organic Cation and Complex Cation-Stabilized (Poly-)Selenides, [Cation]x(Sey)z: Diversity in Structures and Properties. Z. Anorg. Allg. Chem. 640 (14), 2684-2700 (2014).
- Thiele, G., Lichtenberger, N., Tonner, R., Dehnen, S. Syntheses, Structures and Electronic Properties of a New Series of Tellurides of the Type [Sequestrated Cation]2[Tex] (x = 1-4). Z. Anorg. Allg. Chem. 639 (15), 2809-2815 (2013).

