Automated Radiochemical Synthesis of [18F]3F4AP: A Novel PET Tracer for Imaging Demyelinating Diseases
Summary
We demonstrate the semi-automated radiochemical synthesis of [18F]3F4AP and quality control procedures.
Abstract
3-[18F]fluoro-4-aminopyridine, [18F]3F4AP, is a radiofluorinated analog of the FDA-approved drug for multiple sclerosis 4-aminopyridine (4AP). This compound is currently under investigation as a PET tracer for demyelination. We recently described a novel chemical reaction to produce metafluorinated pyridines consisting of direct fluorination of a pyridine N-oxide and the utilization of this reaction for the radiochemical synthesis of [18F]3F4AP. In this article, we demonstrate how to produce this tracer using an automated synthesizer and an in-house made flow hydrogenation reactor. We also show the standard quality control procedures performed before releasing the radiotracer for preclinical animal imaging studies. This semi-automated procedure may serve as the basis for future production of [18F]3F4AP for clinical studies.
Introduction
The ability to trace a small-molecule drug non-invasively within the human body has great potential towards precision medicine. Among molecular imaging techniques, positron emission tomography (PET) has many favorable characteristics: the high sensitivity of PET detectors allows detection and quantification of very small amounts of radioactive material and the characteristics of the scanners allow accurate spatial mapping of the drug localization1,2,3. For example, PET allows detection and localization of tumors and metastasis based on the level of uptake of a radioactive glucose analog, [18F]FDG4. PET can also provide localization and quantification of specific brain receptors and their occupancy which can be valuable for diagnosing and understanding neurological and psychiatric disorders5. In order to develop a small molecule PET tracer, the compound of interest must be labeled with a positron-emitting isotope, typically 11C or 18F. Between these two radioisotopes, 18F has a longer half-life (109 min vs. 20.3 for 11C), which allows multi-dose and offsite production. Nevertheless, adding 18F to a molecule can be challenging. 18F labeling requires fast reactions compatible with automation relieving the chemist of direct handling of the activity and receiving high-absorbed radiation doses.
We recently described the use of pyridine N-oxides as precursors for the fluorination of pyridines and the use of this chemistry in the radiochemical synthesis of [18F]3F4AP6, a radiofluorinated analog of the FDA-approved drug for multiple sclerosis, 4-aminopyridine (4AP)7,8,9. This novel radiotracer is currently under investigation as a PET tracer for demyelination10,11,12. In this video article, we demonstrate the semi-automated synthesis of this compound using an IBA Synthera Synthesis Unit (henceforth referred to as "the synthesizer") and an in-house made flow hydrogenation device. The synthesis is based on the reaction shown in Figure 1. Preparation for the procedure takes approximately 1 h, radiolabeling and purification 1.5 h and quality control procedures 0.5 h.
Protocol
CAUTION: All procedures involving the use of radioactive materials must be approved by the local Office of Radiation Safety. When working with radioactive materials wear a lab coat and personal radiation badges. Use two layers of gloves at all times and check hands with a Geiger counter after each step that involves handling radioactivity. If the gloves are contaminated with radioactivity discard and replace outer gloves. Use appropriate shielding, minimize time in contact with the radiation source and maximize distance.
1. One Week before Experiment: Preparation of Materials
- Download [18F]3F4AP sequence: Synthera users can log into the Users Database (http://www.iba-radiopharmasolutions.com/products/chemistry) and download the sequence file for 3F4AP. Users of other synthesizers may need to write their own script based on the sequence of steps. Browse through the annotated sequence to become familiar with the steps involved in the synthesis.
- Ensure that there is enough gas for the synthesis. The synthesizer requires compressed gas, either helium or nitrogen. It also requires >75 psi compressed air. Ensure that the pressures are within the recommended by the manufacturer.
- Prepare HPLC mobile phase: prepare 1 L of 50 mM sodium phosphate and 10 mM triethyl amine. Using a pH meter adjust the pH to 8.0 ±0.1 by adding dropwise saturated sodium hydroxide while stirring. Filter the solution through a 0.22 µm bottletop filter and add 5% volume of ethanol.
- Dry glassware in the oven overnight.
2. Day of Experiment: Before Arrival of Fluorine-18
- Using 1 mL syringes, fill reagent vials with the appropriate reagents. For vials 2 and 3 use oven-dried vials and anhydrous solvents kept under argon. Seal the vials with crimp seals using a crimper.
- Fill Vial 1 (11 mm diameter / 2 mL volume vial) with 400 µL of TBA-HCO3 + 800 µL of acetonitrile (MeCN).
- Fill Vial 2 (13 mm / 4 mL vial) with 50 µL of precursor solution 1.0 mg/mL + 450 µL of MeCN.
- Fill Vial 3 (11 mm / 2 mL vial) with 500 µL of MeCN.
- Fill Vial 4 (13 mm / 4 mL vial) with 4 mL of 0.2% oxalic acid in methanol (MeOH).
- Condition the QMA (strong anion exchange) and Alumina-N solid phase extraction cartridges. Using a 10 mL syringe, pass 5 mL of 8.4% NaHCO3 drop-wise through the QMA followed by 5 mL of ultrapure deionized Type I water (18.2 ΜΩ•cm at 25 ºC). Pass 5 mL of ultrapure water drop-wise through Alumina-N cartridge followed by 5 mL of MeOH + 0.2% oxalic acid.
- Turn on the HPLC and condition the C-18 column with 4 mL per min of mobile phase for 30 min.
- Load a new catalyst cartridge onto the hydrogenator cartridge holder and start a flow of 0.5 mL/min of 100% MeOH. Set the hydrogen regulator to 50 psi and condition the cartridge for 15 min (Figure 2).
- Assemble the Integrated Fluid Processor (IFP) by introducing vials 1 through 4 in their positions, attaching the cartridges and collection vial as shown in Figure 3. Attach a collection vial with a vent needle to the output line of the hydrogenator.
- Start the synthesizer's software. Enter login and password. Perform pre-run checks on the synthesizer according to manufacturer instructions.
- Click on "Sequences" and then "Open" to load the 3F4AP sequence.
- Load the IFP by clicking the "Load" button on the screen. Type a file name for the run and start the sequence by clicking "Start". (The automated synthesizer will automatically pause before the 18F loading step.)
- Watch as the synthesizer goes through the routine self-check steps (part one of the sequence). Look at the screen to ensure that there are no warnings or alarms. Pay attention to sounds as the synthesizer flushes lines and preheats reaction vessel in preparation for the run. The temperature indicator should rise and stay at 65 ºC. Wait for the signal (auditory beep) indicating that the synthesizer is ready for the 18F transfer.
3. Day of Experiment: 18F Labeling
- Remotely transfer desired amount of cyclotron-produced 18F from the cyclotron target to 18F vial. Verify the amount of radioactivity and record it with the time of delivery.
NOTE: If not using a direct line for 18F– transfer use a prefilled syringe with a needle attached to transfer the activity to the vial through the septum. The amount of starting radioactivity depends on the limits set by the Office of Radiation Safety and the desired amount of final tracer. Typical amount ranges between 50 and 500 mCi. - Resume sequence on the synthesizer by pressing "Resume". This will initiate the transfer of the 18F into the QMA.
- Monitor the progression of the synthesis throughout the automated sequence on the computer screen.
- Watch the transfer the 18F from the vial onto the QMA for 90 s. After trapping 18F– on QMA it elutes with TBA-HCO3 solution (vial 1). (Part two of the sequence)
- Monitor the pressure and temperature traces on the synthesizer while the TBA18F is dried under reduced pressure (5 kPa) and heating (100 ºC), followed by additional drying and cool down steps. (Part 3 of the sequence)
- Watch the transfer of anhydrous MeCN (vial 3) and precursor solution (vial 2) to the reactor and how it reacts for 1 min at room temperature. The solution should be colorless or very faint yellow. (Part 4 of the sequence)
- Watch the transfer of oxalic acid solution (vial 4) to the reactor. Watch as the solution is pressure transferred from the reactor through alumina-N cartridge to the final product vial. (Part 5 of the sequence)
- At the end of the sequence, print out the report, eject the IFP, shut off gas tanks, and close the software.
- While first establishing the procedure, measure the radioactivity in the alumina-N cartridge and the collection vial by separately introducing the cartridge and vial in the dose calibrator. Record the activity and time of measurement. Place the used cartridge in a leaded waste container. Place the collection vial in a shielded container for transport to the next step.
- Using a 1 mL syringe with a 2" needle attached, manually transfer approximately 100 μL of sample of the intermediate product solution into a standard HPLC vial for in-process quality control. Inject 10 μL of this sample into the HPLC to evaluate the purity and identity of the intermediate compound.
NOTE: HPLC conditions: XDB 5 µm, 9.4 x 250 mm C18 column. Flow 4 mL/min. Mobile phase (50 mM Na2HPO4, 10 mM TEA, 5% EtOH). Isocratic 15 min.
4. Day of Experiment: Hydrogenation
CAUTION: Injection of the product into the hydrogenator has to be done using proper shielding precautions. Hydrogen gas must be properly handled and vented.
NOTE: the hydrogenation reactor can be connected in place of the HPLC column on the synthesizer and controlled using the synthesizer software.
- Set up the hydrogenator flow at 0.5 mL/min by starting the synthesizer HPLC sequence. Manually set up hydrogen pressure to 50 psi.
- After finishing the labeling and quenching steps the synthesizer will transfer the intermediate product solution into hydrogenator/HPLC loop.
- When the radioactive peak appears on the HPLC software toggle the collection valve to collect the product. Measure radioactivity of crude product using a dose calibrator.
NOTE: The crude product should be injected into an automated HPLC system inside a hot cell. After purification, the final product is then collected and dispensed into an aseptic ISO class 5 laminar air flow hot cell as per USP and FDA regulations.
5. Day of Experiment: Purification and Preparation of the Dose
- Inject crude product into the HPLC and use the automated fraction collector to collect the fractions corresponding to the final product peak. Each tube contains 0.66 mL of solution.
NOTE: HPLC conditions: XDB 5 µm, 9.4 x 250 mm C18 column. Flow 4 mL/min. Mobile phase (50 mM Na2HPO4, 10 mM TEA, 5% EtOH). Isocratic 15 min. Collect 4-15 min. - Measure the radioactivity of each fraction using a dose calibrator and record it. Combine the fractions with highest amounts of radioactivity (typically tubes 14-18).
- Draw the product solution with a 10 mL syringe and pass the sample through 0.22 µm filter into a sterile vial. Record the amount of radioactivity, end of synthesis time and solution volume on the vial label. This is the final dose for injection. Put aside ~0.8 mL of the solution for quality control tests.
6. Day of Experiment: Quality Control (QC) Tests
- Before dose release:
Inspect the dose through lead-shielded glass. The solution must be clear, colorless and free of particulate matter. - Radiochemical identity:
- For RadioTLC: spot a drop of the sample on a TLC plate side-by-side with the reference standard. Run TLC plate on a TLC chamber using 95% MeOH: 5% acetic acid. Visualize the reference standard under UV illumination and mark its position with pencil.
- Tape the TLC plate to the stage of the radioTLC scanner and record time of the peak. Rf values of the reference standard and radioactive peak must match within 5%.
- For RadioHPLC: run 10 µL of the dose with and without the reference standard on the HPLC. Retention time of the reference standard and the radioactive peak must match. A single coelution peak must be seen on the spiked sample.
- For radiochemical purity: measure the area under curve for the radioHPLC and radioTLC target peaks. The area of the target peak must be >95% of the area for all radioactive peaks combined.
- For the specific radioactivity: calculate the specific radioactivity as the amount of radioactivity in the peak (measured on step 5.2) over the mass amount determined from the area under the curve of the UV HPLC trace using a pre-established calibration curve. The specific radioactivity must be higher than 50 mCi/µmol.
- For the residual solvent analysis: measure the amount of residual solvents (MeCN, MeOH) in the dose using gas chromatography. Solvents levels must be < 0.04% for acetonitrile and <3,000 ppm for methanol. The amount of EtOH must be less than 10% w/v.
- For the sterile filter integrity test (bubble point): connect the filter used in step 5.3 to a nitrogen supply equipped with a pressure regulator and submerge the needle in water. Gradually open the gas valve while watching the pressure gauge. The filter should withstand pressures up to 50 psi without bursting as evidenced by the lack of a stream of bubbles from the needle. Increase the pressure beyond 50 psi until a stream of bubbles comes out of the needle. Record this pressure, it is the burst pressure and it must be >50 psi.
- For the radionuclide half-life: measure the radioactivity of the product at two time points ≥10 min apart in a dose calibrator. Calculate the half-life using the equation below. The half-life must match that of 18F to within 5 minutes (109 ±5 min):
t½calculated = 0.693 t ÷ ln (A1/A2)
where t is the interval between measurements and A 1, A 2 the activity measured at each time point. - For the radionuclidic identity and purity: obtain the γ-ray spectrum of a sample of the product using a gamma counter. The spectrum should exhibit one single photo-peak at an energy of 511 keV. There should be no other photo-peaks in the spectrum.
- For the endotoxin analysis: measure the endotoxin levels using a LAL chromogenic endotoxin quantitation test. Endotoxin levels must be <1.75 EU/mL for a 1:10 diluted product with a final product volume of 10 mL.
- Document the results from each QC test. Release the dose for animal studies only if all the tests passed.
- Post-dose release:
For the sterility test: add a sample of the dose to both fluid thioglycolate and trypticase soy broth. No growth must be seen on the media after 14 days.
7. Day of Experiment: Calculations (Table 1)
- For the non-decay corrected radiochemical yield (n.d.c. RCY): calculate the n.d.c. RCY as the amount of radioactivity in the final product over the starting radioactivity.
- For the radiolabeling efficiency: calculate the labeling yield as the ratio of radioactivity in the collection vial over radioactivity in the alumina-N cartridge (unincorporated [18F]F–) and the collection vial.
- For the hydrogenation yield: calculate the hydrogenation yield as the amount of radioactivity in the desired peak over the radioactivity injected into the HPLC.
- For the filtering losses: calculate filtering loses as the radioactivity remaining in the filter and syringe over radioactivity before filtering.
Representative Results
The radiochemical synthesis of [18F]3F4AP comprises two steps (Figure 1). The first step is carried out in a fully automated fashion using the synthesis unit (Figure 3). This cassette-based system uses four reagent vials and one reactor vial and has computer-controlled valves that allow transfer and mixing of reagents as well as heating, pressurizing and evacuating the reactor. In addition, it supports standard solid-phase extraction cartridges for separation of reagents. The computer interface allows users to write and modify scripts in order to run their own syntheses. In the case of [18F]3F4AP, the synthesis procedure is comprised of five basic parts. In the first part, the synthesizer performs self-check steps, preheats the reactor and waits for operator's signal that the 18F is ready. During the second part, the [18F]fluoride is transferred from the 18F vial into the anion exchange cartridge and eluted from the cartridge into the reactor using a solution tetrabutyl ammonium bicarbonate. The third part, the synthesizer azeotropically dries the [18F]fluoride under vacuum to make it reactive towards nucleophilic displacement. In the fourth part, the precursor is automatically added to the reactor where it reacts with the 18F– to generate the labeled compound. Finally, the reaction is quenched by the addition of 0.2% oxalic acid in methanol, which prevents base-promoted decomposition of the product, and the final solution is pressure-transferred to the collection vial after passing through an alumina-N cartridge that traps any unreacted fluoride.
After the labeling step is completed a small sample can be taken for quality control. Running a sample on the HPLC provides confirmation that the labeling step worked and an estimation of the radiochemical purity (Figure 4). Also, from the UV trace on the HPLC the mass amount of product can be calculated using a pre-established calibration curve.
While the in-process quality control HPLC is running, the second reaction step, reduction of the N-oxide and nitro groups, is performed. In order to do this, the labeled product is automatedly injected into an in-house hydrogenation device based on the method published by Yoswathananont et al.13 (Figure 2). This device consists of an HPLC pump and a compressed hydrogen tank connected to the flow hydrogenation device through lines equipped with check valves to prevent back-streaming. The product is pushed by the HPLC pump and mixed with hydrogen in a T-shaped mixer. This mixture is then passed through a small cartridge containing 10% Pd/C catalyst on a solid support. After passing through the catalyst the reduced product is then collected in small fractions.
Following hydrogenation, the crude product is transported and manually injected into the HPLC for purification of the final product (Figure 5). The mobile phase of the HPLC has been selected to be compatible with animal injection. The peaks corresponding to the product are then collected and filtered-sterilized to obtain the final dose.
Prior to releasing the dose for PET imaging studies, quality control tests are performed. These tests are performed to ensure that the tracer is the chemical entity that it is supposed to be and that it is safe for injection. Some of these tests may not be required for injection into animals but it is generally recommended to follow the human use guidelines. Doing so ensures quality of the product, which increases confidence in the results and greatly facilitates future transition to manufacturing the product for human injection.
Table 1 contains the typical synthesis parameters including initial amount radioactivity, initial amount of precursor, yield for each step, specific activity, filtering loses, etc. These parameters are useful troubleshooting occasional failures and future optimization of the procedure.
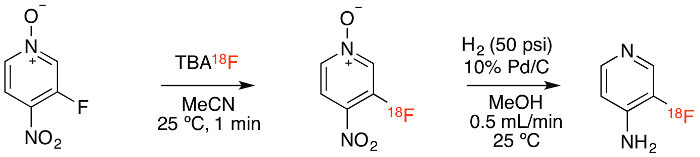
Figure 1. Reaction scheme. Radiochemical synthesis consists of labeling by 19F/18F exchange followed by palladium-catalyzed hydrogenation. Please click here to view a larger version of this figure.
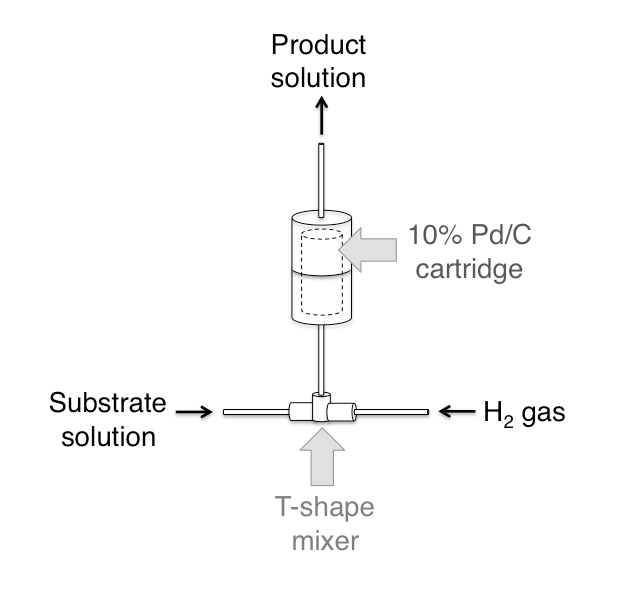
Figure 2. Hydrogenation system. Schematic of the device. This device is based on the publication by Yoswathananont et al. (ref 13).
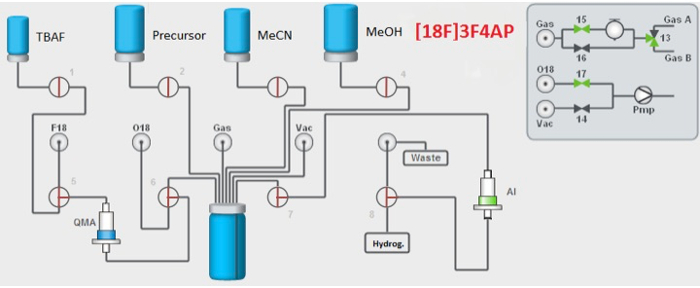
Figure 3. Scheme of synthesizer integrated fluidic processor (IFP) and reagents. IFP contains four reagent vials, a QMA cartridge and one reactor vial. Please click here to view a larger version of this figure.
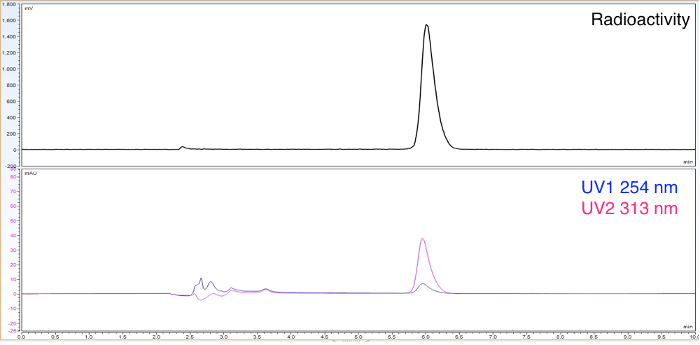
Figure 4. UV and radioHPLC tracers for intermediate product. 3-fluoro-4-nitropyridine N-oxide has a characteristic absorption at 313 nm. Please click here to view a larger version of this figure.
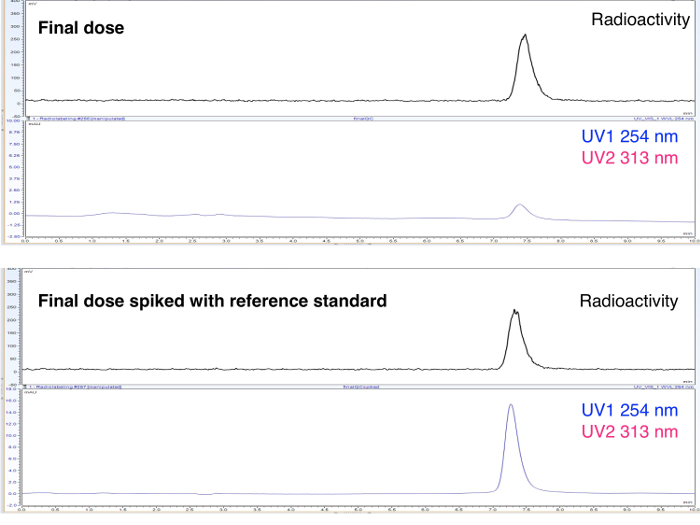
Figure 5. UV and radioHPLC tracers for final product. 3-fluoro-4-aminoopyridine absorbs at a 254 nm. Please click here to view a larger version of this figure.
| Concept | Mean (n = 4) | S.D. | Comments |
| Initial 18F activity (mCi) | 148.0 | 44.9 | Start of synthesis |
| Precursor amount (μg) | 50 | Use 50 μL of 1.0 mg/mL stock | |
| Activity left in QMA (mCi) | 3.0 | 1.7 | Measured at the end of labeling step |
| Radiolabeling yield | 29.7% | 6.3% | Act_collection_vial ÷ (Act_collection_vial + Act_AluN) |
| Radiochemical purity (HPLC-1) | > 98% | From HPLC-1 QC | |
| Spec. act. intermediate (mCi/μmol) | 122.9 | 29.7 | From HPLC-1 using calibration curve |
| Hydrogenation recovery (d.c.) | 74% | 9.0% | Corrected for decay |
| HPLC radiochemical purity (HPLC-2) | 90.7% | 2.9% | Calculated from HPLC-2 |
| Drying efficiency | > 98% | Corrected for decay | |
| Filtering recovery | 93.5% | 1.7% | Corrected for decay |
| Dose volume (mL) | 3.3 | Collect fractions with highest radioactivity | |
| Spec. act. final product (mCi/µmol) | 75.5 | 30.0 | From HPLC-3 using calibration curve |
| Synthesis efficiency | 8.5% | 3.6% | Non-decay corrected |
| Synthesis time (min) | 104 | 11.2 |
Table 1. Radiochemical synthesis parameters.
| Common problems | Potential reasons and solutions |
| [18F]fluoride is not efficiently eluted from the QMA | · TBA-HCO3 was not prepared correctly. Ensure the concentration is adequate. |
| · There are leaks on the TBA-HCO3 vial. Make sure the crimp seal is tight and the septum is not pierced prior to installing it on the IFP. | |
| · TBA-HCO3 is not in good condition. Order a fresh batch. | |
| Labeling yield is low | · There is moisture in the precursor solution. Dry precursor and solvents. |
| · Temperature is too low. | |
| Reaction solution is yellow | · The product is decomposing due to base. Use less TBA-HCO3. |
| · There is too much precursor. Use less precursor. | |
| · There is too little solvent for the amount of 18F–. Use more solvent. | |
| Additional peaks on radioHPLC | · Nitro group is being substituted: reduce the reaction temperature or shorten reaction time. |
| Hydrogenation reaction does not work | · Catalyst is not good. Use a new cartridge. |
| · Flow is too fast and does not allow sufficient contact between catalyst and substrate. Decrease flow. | |
| · Hydrogen pressure is too low. Increase H2 pressure. | |
| Hydrogen pressure increases dramatically during procedure | · Cartridge integrity is compromised and solid support is clogging the lines. Stop the flow and shut off the gas. Let radioactivity decay. Remove catalyst cartridge and flush the system. Put a new cartridge. |
| Hydrogenation yield is low | · Too many impurities competing for the catalyst (MeCN, oxalic acid). Decrease amount of impurities or increase mass of precursor (Warning: increasing precursor amount will reduce specific activity). |
| Recovery of radioactivity from hydrogenation step is low | · There is a leak in the system. Check for leaks and backflush into the hydrogen line. |
| · Compound is defluorinating in the reactor. Evaluate different reaction conditions (pressure, temperature, flow, etc.). | |
| Too much radioactivity is lost during filtration | · Wet the filter prior to use. |
| · Use filter with a lower dead volume. | |
| The final product peak on the HPLC looks broad | · Too much volume injected. Inject lower amount. Use column with larger diameter. |
| · The column is not well conditioned. Condition the column for at least 30 column volumes. | |
| · pH of the mobile phase is low. Make sure that the pH ≥ 8. | |
| · Column is not in good condition. Replace column. Use column compatible with basic pH. |
Table 2. Troubleshooting guide.
Discussion
The preparation of PET tracers requires efficient labeling with minimal user intervention to minimize radiation exposure14. Here, we described the first semi-automated procedure for the radiochemical synthesis of [18F]3F4AP, a PET tracer currently under investigation for imaging demyelination. This semi-automated method produces the radiotracer with high purity and sufficient specific activity for animal studies. Prior methods for the synthesis of this compound relied on manual synthesis6, which significantly limits the amount of radioactive tracer that can be produced. Having an automated method for the synthesis also provides more reproducible yields and makes it easier to transfer the procedure to other laboratories with similar equipment. Future efforts to fully automate the procedure will be instrumental to the production of the tracer in high amounts for studies in large animals or humans.
This procedure uses nucleophilic exchange of 19F for 18F to incorporate the radioisotope into the molecule of interest. The advantages of this reaction are that it is fast and produces almost exclusively the desired product without the need to perform a potentially lengthy purification step to remove excess of precursor. One limitation of using fluoride-exchange labeling reactions such as the one used here is that due to initial mass of cold compound the final specific activity defined as amount of radioactivity in mCi over amount of compound in µmol may be limited. Under our standard conditions, starting with 100-200 mCi of 18F– and 50 µg of precursor, the typical specific activity at the end of synthesis is up to 100-200 mCi/µmol, which appears to be sufficient for preclinical PET imaging studies. Nevertheless, the specific activity may improve by increasing the starting amount for 18F– while keeping the mass amount low. There have been several reports of producing radioligands by fluoride-exchange with high specific activity (1-3 Ci/µmol) by starting with high activity and low precursor amounts15,16.
As with all radiochemical syntheses of PET tracers, it is critical to work quickly in order to minimize radioactive decay. It is also important to minimize the time handling the radioactive materials, use proper shielding and maximize the distance between the radioactive material and the user to minimize radiation exposure. These aspects are particularly important during the second half of the protocol (purification and quality control) in which the user has to manually inject the solution into the HPLC, collect the fractions and filter the final product.
As with all radiochemical syntheses of PET tracers, it is critical to work quickly in order to minimize radioactive decay. It is also important to minimize the time handling the radioactive materials, use proper shielding and maximize the distance between the radioactive material and the user to minimize radiation exposure. These aspects are particularly important during the second half of the protocol (hydrogenation and purification) in which the user has to manually inject the solution into the hydrogenator, collect the fractions, set up the drying procedure, redissolve the product in buffer and filter it. During the filtering step it is easy to lose a large amount of radioactive material in the walls of the vials. Thus, it is important to try to collect all the liquid prior to filtering. Using a greater amount of buffer to dissolve may improve the yield of recovery but its use is discouraged because it will require injecting a larger volume on the HPLC, causing the peak to broaden and increase the volume of the final dose.
In order to troubleshoot and optimize the procedure is important to keep track of the yields of each step. For most steps this is done simply by measuring the amount of radioactivity before and after any step. In the case of the reaction the yields can be calculated through quantification of the HPLC peaks. Table 1 in the Results Section shows the typical yields for each step. Table 2 below lists many of the commonly encountered failures with potential reasons for the failure and how to correct them.
Finally, even though the procedure demonstrated here is specific for the synthesis of [18F]3F4AP, the general workflow and many of the individual steps are common to the synthesis of other compounds17. In this article we also demonstrated the typical QC tests performed on any PET tracer.
Divulgazioni
The authors have nothing to disclose.
Acknowledgements
This project was supported by grants NIH/NIBIB 1K99EB020075 to Pedro Brugarolas and an Innovation Fund Award from the Chicago Innovation Exchange to Brian Popko and Pedro Brugarolas. Prof. Brian Popko is gratefully acknowledged for his mentorship and financial support to the project. Prof. Chin-Tu Chen and the Integrated Small Animal Imaging Research Resource at the University of Chicago are acknowledged for generously sharing laboratory space and equipment. IBA is acknowledged for sponsoring open-access of this article.
Materials
| Cyclotron produced [18F]fluoride | House supplied/Zevacor | IBA Cyclone 18 | 100-200 mCi |
| Integrated fluid processor for production FLT/FDG | ABX | K-2715SYN | Cassette used for nucleophilic substitution |
| Anhydrous acetonitrile | Janssen | 36431-0010 | Transfer under nitrogen |
| Methanol | Janssen | 67-56-1 | |
| ultrapure water | house supplied | Millipore MilliQ system | |
| TBA-HCO3 | ABX | 808.0000.6 | abx.de |
| QMA | Waters | WAT023525 | Quaternary methyl ammonium: Anion exchange solid phase extraction cartridge for trap and release of 18F- from the target water |
| Sodium bicarbonate | ABX | K-28XX.03 | Prefilled 5 mL syringes |
| Alumina-N | Waters | WAT020510 | Alumina-N solid phase extraction cartridge (for trapping unreacted 18F-) |
| 3-fluoro-4-nitropyridine N-oxide | Synthonix | 76954-0 | Store in desicator. Precursor |
| 3-fluoro-4-aminopyridine | Sigma Aldrich | 704490-1G | Reference standard |
| Oxalic acid | Sigma Aldrich | 75688-50G | |
| Sodium phosphate monobasic | Fisher Scientific | S80191-1 | |
| Triethyl amine | Fisher Scientific | 04885-1 | |
| Ethanol | Decon Labs | DSP-MD.43 | USP |
| Final product vial | ABX | K28XX.04 | |
| Millex Filter Syringe | Millex | SLGVR04NL | |
| 10% Pd/C cartridge | Sigma Aldrich | THS-01111-12EA | |
| 11 mm vials + crimp seals | Fisher Scientific | 03-250-618, 06-451-117, or equivalent | |
| 13 mm vials + crimp seals | Fisher Scientific | 06-718-992, 06-718-643, or equivalent | |
| HPLC vials | Fisher Scientific | 03-391-16, 03-391-17, or equivalent | |
| SEMIPREP C18 column | Agilent | 990967-202 | |
| V-vials | Alltech | ||
| Syringes: 1, 3, 10 mL | Fisher Scientific | 14-829-10D, 14-829-13Q, 14-829-18G, or equivalent | |
| Compressed gases: N2, He, H2 | Airgas | UHP N300, UHP HE300, UHP H300, or equivalent | |
| TLC plates | Sigma Aldrich | Z193275, or equivalent | |
| Name | Company | Catalog Number | Comments |
| Equipment | |||
| Synthera automated synthesizer | IBA SA, Belgium, iba-worldwide.com | Synthera, 250.001 | Automatic synthesis unit |
| In-house hydrogenator | See picture | See text description | |
| Hot cells | Comecer | For manipulating radioactive materials | |
| RadioTLC scanner | Eckert and Ziegler | For handling sterile materials | |
| HPLC | Dionex | Ultimate 3000 | |
| Dose calibrator | Capintec | CRC15 | Or equivalent |
| Gamma counter | Capintec, 7 Vreeland Road, Florham Park, NJ 07932 | CRC 15, PET-CRC25, or equivalent | For measuring radioactivity |
| Personal dosimeters | Packard | Cobra II | For measuring gamma spectrum |
| Personal radiation badges and rings | Atlantic Nuclear | Rados Rad-60 Electronic Dosimeter, or equivalent | |
| Rotavap + vacuum pump | Landauer | ||
| Lead pigs + syringe shields | Heidolph | Or equivalent | |
| Geiger counters | Pinestar | ||
| Ludlum | Model 3 + Pancake GM detector, 4801605, 47-1539, or equivalent |
Riferimenti
- Valk, P. E. . Positron emission tomography : basic science and clinical practice. , (2003).
- Phelps, M. E. . PET: molecular imaging and its biological applications. , (2004).
- Ametamey, S. M., Honer, M., Schubiger, P. A. Molecular imaging with PET. Chem Rev. 108 (5), 1501-1516 (2008).
- Oriuchi, N., et al. Present role and future prospects of positron emission tomography in clinical oncology. Cancer Sci. 97 (12), 1291-1297 (2006).
- Heiss, W. D., Herholz, K. Brain receptor imaging. J Nucl Med. 47 (2), 302-312 (2006).
- Brugarolas, P., Freifelder, R., Cheng, S. -. H., DeJesus, O. Synthesis of meta-substituted [18F]3-fluoro-4-aminopyridine via direct radiofluorination of pyridine N-oxides. Chemical Communications. , (2016).
- Jones, R. E., Heron, J. R., Foster, D. H., Snelgar, R. S., Mason, R. J. Effects of 4-aminopyridine in patients with multiple sclerosis. J Neurol Sci. 60 (3), 353-362 (1983).
- Davis, F. A., Stefoski, D., Rush, J. Orally administered 4-aminopyridine improves clinical signs in multiple sclerosis. Ann Neurol. 27 (2), 186-192 (1990).
- Goodman, A. D., et al. Sustained-release oral fampridine in multiple sclerosis: a randomised, double-blind, controlled trial. Lancet. 373 (9665), 732-738 (2009).
- Brugarolas, P., et al. . Abstracts Of Papers Of The American Chemical Society. , (2016).
- Brugarolas, P., et al. Development of a PET tracer for MS. J Nucl Med Meeting Abstracts. 55 (1), 1124 (2014).
- Brugarolas, P., et al. Fluorinated 4-aminopyrdines as PET tracers for MS. Journal of Nuclear Medicine. 56, 493 (2015).
- Yoswathananont, N., Nitta, K., Nishiuchi, Y., Sato, M. Continuous hydrogenation reactions in a tube reactor packed with Pd/C. Chem Comm. (1), 40-42 (2005).
- Stöcklin, G., Pike, V. W. . Radiopharmaceuticals for Positron Emission Tomography-Methodological Aspects. 24, (1993).
- Liu, Z., et al. Preclinical evaluation of a high-affinity 18F-trifluoroborate octreotate derivative for somatostatin receptor imaging. J Nucl Med. 55 (9), 1499-1505 (2014).
- Liu, Z., et al. 18F-trifluoroborate derivatives of [des-arg(10)]kallidin for imaging bradykinin b1 receptor expression with positron emission tomography. Mol Pharm. 12 (3), 974-982 (2015).
- Scott, P. J. H., Hockley, B. G., Kilbourn, M. R. . Radiochemical Syntheses, Volume 1: Radiopharmaceuticals for Positron Emission Tomography. , (2012).

