Isolation of Type I and Type II Pericytes from Mouse Skeletal Muscles
Summary
This work describes a FACS-based protocol that allows for easy and simultaneous isolation of type I and type II pericytes from skeletal muscles.
Abstract
Pericytes are perivascular multipotent cells that show heterogeneity in different organs or even within the same tissue. In skeletal muscles, there are at least two pericyte subpopulations (called type I and type II), which express different molecular markers and have distinct differentiation capabilities. Using NG2-DsRed and Nestin-GFP double-transgenic mice, type I (NG2-DsRed+Nestin-GFP–) and type II (NG2-DsRed+Nestin-GFP+) pericytes have been successfully isolated. However, the availability of these double-transgenic mice prevents the widespread use of this purification method. This work describes an alternative protocol that allows for the easy and simultaneous isolation of type I and type II pericytes from skeletal muscles. This protocol utilizes the fluorescence-activated cell sorting (FACS) technique and targets PDGFRβ, rather than NG2, together with the Nestin-GFP signal. Following isolation, type I and type II pericytes show distinct morphologies. In addition, type I and type II pericytes isolated with this new method, like those isolated from the double-transgenic mice, are adipogenic and myogenic, respectively. These results suggest that this protocol can be used to isolate pericyte subpopulations from skeletal muscles and possibly from other tissues.
Introduction
Muscular dystrophy is a muscle-degenerative disorder that has no effective treatments so far. The development of therapies that promote tissue regeneration has always been of great interest. Tissue regeneration and repair after damage depend on resident stem cells/progenitor cells1. Satellite cells are committed myogenic precursor cells that contribute to muscle regeneration2,3,4,5,6,7. Their clinical use, however, is hampered by their limited migration and low survival rate after injection, as well as by their decreased differentiation capability after in vitro amplification8,9,10,11. In addition to satellite cells, skeletal muscles also contain many other cell populations with myogenic potential12,13,14,15,16, such as platelet-derived growth factor receptor-beta (PDGFRβ)-positive interstitial cells. There is evidence showing that muscle-derived PDGFRβ+ cells are able to differentiate into myogenic cells and improve muscle pathology and function14,17,18,19,20. PDGFRβ predominantly labels pericytes21, which are perivascular cells with pluripotency22,23. In addition to PDGFRβ, many other markers, including Neuron-Glial 2 (NG2) and CD146, are also used to identify pericytes21. It should be noted, however, that none of these markers is pericyte-specific21. Recent studies revealed two subtypes of muscle pericytes, called type I and type II, which express different molecular markers and carry out distinct functions19,24,25. Biochemically, type I pericytes are NG2+Nestin–, while type II pericytes are NG2+Nestin+19,24. Functionally, type I pericytes can undergo adipogenic differentiation, contributing to fat accumulation and/or fibrosis, whereas type II pericytes can differentiate along the myogenic pathway, contributing to muscle regeneration19,24,25. These results demonstrate that: (1) type I pericytes may be targeted in the treatment of fatty degenerative disorders/fibrosis, and (2) type II pericytes have great therapeutic potential for muscular dystrophy. Further investigation and characterization of these populations require an isolation protocol that enables the separation of type I and type II pericytes at a high level of purity.
Currently, the isolation of pericyte subpopulations relies on NG2-DsRed and Nestin-GFP double-transgenic mice19,24. The availability of NG2-DsRed mice and the quality of most NG2 antibodies limit the widespread use of this method. Given that all NG2+ pericytes also express PDGFRβ in skeletal muscles19,20,24, we hypothesize that NG2 can be replaced by PDGFRβ for the isolation of pericytes and their subpopulations. This work describes a FACS-based protocol that uses PDGFRβ staining and the Nestin-GFP signal. This method is less demanding for investigators because: (1) it does not require the NG2-DsRed background and (2) it uses commercially available PDGFRβ antibodies, which are well-characterized. In addition, it enables the simultaneous isolation of type I and type II pericytes at high purity, allowing for further investigation into the biology and therapeutic potential of these pericyte subpopulations. Following purification, these cells can be grown in culture, and their morphologies can be visualized. This work also shows that type I and type II pericytes isolated using this method, like those purified from double-transgenic mice, are adipogenic and myogenic, respectively.
Protocol
Wildtype and Nestin-GFP transgenic mice were housed in the animal facility at the University of Minnesota. All experimental procedures were approved by the Institutional Animal Care and Use Committee at the University of Minnesota and were in accordance with the NIH Guide for the Care and Use of Laboratory Animals.
1. Muscle Dissection and Single-cell Isolation
- Euthanize adult mice (6-10 weeks, both male and female) with tribromoethanol (250 mg/kg, i.p.) and sterilize their abdomen skin with 70% ethanol.
NOTE: Tribromoethanol was used here instead of ketamine for anesthesia/euthanasia, as ketamine is known to interact with the NMDA receptors, which could potentially have an impact on the study. - Place the mice in the supine position and use a scalpel to make a horizontal incision on the abdominal skin. Peel off the skin by hand, pulling in opposite directions to expose the hindlimb muscles.
- Collect the muscles from both hindlimbs using forceps and scissors. Store them in sterile phosphate-buffered saline (PBS) supplemented with 1% penicillin-streptomycin (P/S) on ice.
- Wash the dissected hindlimb muscles in ice-cold PBS supplemented with 1% P/S two times and transfer them to a sterile 10 cm plate.
- Carefully dissect out nerves, blood vessels, and connective tissue from the muscles using forceps and scissors under a dissection microscope at 2X magnification.
- Finely chop and mince the muscles into small pieces (1-2 mm3) using sterile scissors and blades. If necessary, add a small amount of Dulbecco's Modified Eagle Medium (DMEM) to ensure that the muscles are not dried out.
- Mechanically break down the tissue by pipetting up and down through a 10 mL serological pipette 10 times.
- Add freshly made digestion solution (DMEM supplemented with 0.2% Type 2 collagenase) to the mixture. Incubate at 37 °C for 2 h with gentle agitation at 35 revolutions/min.
- Triturate using an 18 G needle to homogenize the mixture. Then, centrifuge at 500 x g for 5 min. Discard the supernatant and then resuspend the pellet in 0.25% trypsin/EDTA. Incubate at 37 °C for 10 min. Repeat the centrifugation step two more times.
- Add 10 mL of DMEM supplemented with 20% fetal bovine serum (FBS) to the solution and centrifuge at 500 x g for 5 min. Discard the supernatant and resuspend the pellet in red blood cell lysis buffer (155 mM NH4Cl, 10 mM KHCO3, and 0.1 mM EDTA).
- Centrifuge at 500 x g for 5 min. Discard the supernatant and resuspend the pellet in sorting buffer (20 mM HEPES, pH 7.0; 1 mM EDTA; and 1% BSA in Ca/Mg2+-free PBS, pH 7.0). Filter the mixture through a 40 µm cell strainer to obtain a single-cell suspension.
- Centrifuge at 500 x g for 5 min. Discard the supernatant and resuspend the pellet in 1 mL of sorting buffer.
- Count the cell number with a hemocytometer and dilute the single-cell suspension to 5 x 106/mL in sorting buffer.
2. Cell Staining and Sorting
- Prepare controls and the sample as described in Table 1. Stain the single-cell suspension with the respective antibodies on ice for 30 min, as described in Table 1.
- Centrifuge at 500 x g for 5 min and wash the pellets twice with sorting buffer.
- Add DAPI to the single-cell solutions, as indicated in Table 1. Use 5 µg/mL DAPI (final concentration) for the DAPI single-color control and 1 µg/mL DAPI for the PDGFRβ-PE-FMO control and the sample. Keep all tubes on ice throughout the experiment.
- Turn on the sorter and the software. Scan and insert a 100 µm sorting chip when prompted.
- Perform the automatic setup (i.e., chip alignment, droplet calibration, side stream calibration, and sort delay calibration) by loading the automatic setup beads when prompted.
- When automatic setup is complete, go to the "Experiment" tab, click on "New," and select the "Blank Template" from "Public Templates."
- Under “Measurement Settings,” enter “DAPI” for “FL1,” “Nestin-GFP” for “FL2,” and “PDGFRβ” for “FL3.” Uncheck the boxes for “FL4”-“FL6”.
- Check the boxes to activate the 405, 488, and 561 lasers and click on "Create New Experiment."
- Select the "Start Compensation Wizard" option and follow the "Compensation Wizard" software prompts to set up the compensation.
- Load the unstained control and click "Start." Click "Detector & Threshold Settings" and adjust the sensor gain of the FSC and BSC detectors to place the population on the scale.
- Adjust the gain levels of the FL1-FL3 fluorescence channels to place the negative populations on the left side of the histograms. Click the "Record" button to record the data.
- Load single-color controls one by one when prompted. Click "Start and Record" to record the data. Adjust the gates for the positive populations on the histograms. Click "Next."
- Go to "Calculate Matrix" on the "Compensation" tab and click "Calculate" in the "Calculate Compensation Settings" panel to perform the compensation. Click "Finish" to exit the "Compensation Wizard."
- Load the PDGFRβ-PE-FMO control and click "Start." Draw a polygon gate (Gate A) around the cells of interest under the "All Events" plot.
- Double-click inside Gate A to create a child plot. Change the Y-axis to DAPI and draw a polygon gate (Gate B) around live (DAPIlow) cells. Double-click inside Gate B to create a child plot. Change the X-axis to FSC-H and the Y-axis to FSC-W and draw a polygon gate (Gate C) around the singlets to eliminate doublets.
- Double-click inside Gate C to create a child plot. Change the X-axis to Nestin-GFP and the Y-axis to PDGFRβ-PE. Click the “Record” button to record the data.
- Load the sample and repeat steps 2.11-2.12. After recording, click on "Pause" to preserve the sample.
- Define the gating boundaries for PDGFRβ+ and Nestin-GFP+ cells based on the PDGFRβ-PE-FMO control. Draw gates for the PDGFRβ+Nestin-GFP– and PDGFRβ+Nestin-GFP+ populations.
- Under “Sorting Method,” select “2-way Tubes” and assign “PDGFRβ+Nestin-GFP–” and “PDGFRβ+Nestin-GFP+” cells to the left and right collecting tubes, respectively. Mount the sorting buffer-filled 15 mL collecting tubes on the collection stage and click the “Load Collection” button".
- Click the "Resume" button to keep the sample running. Click "Start Sort" to collect PDGFRβ+Nestin-GFP– (type I pericytes) and PDGFRβ+Nestin-GFP+ (type II pericytes) cells.
3. Post-sorting Analyses
- Centrifuge the sorted cells at 500 x g for 5 min, resuspend the pellet in 1 mL of pericyte medium (see Materials Table), and count the cell density using a hemocytometer.
- Seed type I and type II pericytes on poly-D-lysine (PDL)-coated coverslips at ~1 x 104 cells/cm2. Grow in pericyte medium for 3 days at 37 °C with 5% CO2.
- On day 3, examine the pericyte morphology (under phase contrast) and endogenous Nestin-GFP expression using a fluorescent microscope (excitation laser: 488 nm, excitation filter: 470/40 nm, and emission filter: 515/30 nm). Take images under a 20X objective (0.45 NA).
- Replace the pericyte medium with adipogenic (mouse MSC basal medium + adipogenic stimulatory supplement) and myogenic (DMEM + 2% horse serum) medium to initiate adipogenic and myogenic differentiation, respectively, as described previously20. Change the medium every 2-3 days.
- Fix the cells on day 17 (14 days after adipogenic/myogenic differentiation) in 4% paraformaldehyde (PFA) for 20 min at room temperature.
NOTE: Caution, PFA is a carcinogen. - Perform immunocytochemistry against perilipin (adipocyte marker) and S-myosin (mature myotube/myofiber marker), as described in previous publications19,20.
- Wash the fixed cells 3 times in PBS for 10 min at room temperature.
- Add blocking buffer (PBS supplemented with 5% donkey serum, 3% BSA, and 0.3% Triton X-100) and incubate at room temperature for 1 h.
- Incubate the cells with anti-perilipin (2 µg/mL) and/or anti-S-myosin (2 µg/mL) antibodies at 4 °C overnight.
- Wash the cells 3 times in PBS for 10 min at room temperature.
- Incubate the cells with Alexa 555 donkey anti-rabbit (4 µg/mL) and/or Alexa555 donkey anti-mouse (4 µg/mL) antibodies at room temperature for 1 h.
- Wash the cells 3 times in PBS for 10 min at room temperature.
- Mount the immunostained cells with mounting medium containing DAPI (see the table of materials). Examine perilipin and S-myosin expression using a fluorescent microscope (excitation laser: 543 nm; 540/45 nm excitation and 600/50 nm emission) and take images under a 40X objective (0.60 NA).
Representative Results
FACS parameters, including laser intensity and channel compensation, are corrected based on the results of unstained control and single-color controls. The PDGFRβ-PE-FMO control is used to set the gating for the PDGFRβ-PE+ population (Figure 1A). Among the PDGFRβ-PE– cells, two populations representing Nestin-GFP+ and Nestin-GFP– cells are clearly separated (Figure 1A). Gating boundaries for the PDGFRβ-PE+Nestin-GFP+ and PDGFRβ-PE+Nestin-GFP– populations are set based on the gating for PDGFRβ-PE+ and Nestin-GFP+ (Figure 1A). These boundaries are used to define and sort PDGFRβ-PE+Nestin-GFP+ (type II pericytes) and PDGFRβ-PE+Nestin-GFP– (type I pericytes) cells from the sample (Figure 1B). Isolated PDGFRβ-PE+Nestin-GFP– and PDGFRβ-PE+Nestin-GFP+ cells account for 9.5% and 2.1% of the total cells in the single-cell solution, respectively.
FACS-isolated type I and type II pericytes demonstrate morphological differences after three days in culture. Type I pericytes display amoeboid morphology, with round cell bodies and short processes (Figure 2). Type II pericytes, however, show ramified morphology, characterized by small cell bodies and long, thin processes (Figure 2). At this time, most type II pericytes remain as Nestin-GFP+, while type I pericytes are Nestin-GFP– (Figure 2).
In addition, type I, but not type II, pericytes differentiate into perilipin-expressing adipocytes after 14 days in adipogenic medium (Figure 3). Type II, but not type I, pericytes differentiate into S-myosin-expressing myotubes after 14 days in myogenic medium (Figure 4). These results strongly indicate that type I pericytes are adipogenic, while type II pericytes are myogenic.
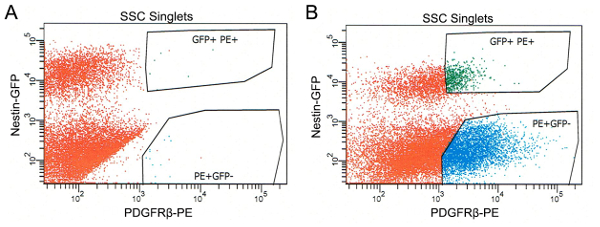
Figure 1: Gating boundaries and representative sorting. (A) Fluorescent plot of the PDGFRβ-PE-FMO control, demonstrating the PDGFRβ-PE+Nestin-GFP– and PDGFRβ-PE+Nestin-GFP+ gating boundaries. (B) Representative fluorescent plot of the sample showing the distribution of the PDGFRβ-PE+Nestin-GFP– (type I pericytes) and PDGFRβ-PE+Nestin-GFP+ (type II pericytes) cells. Please click here to view a larger version of this figure.
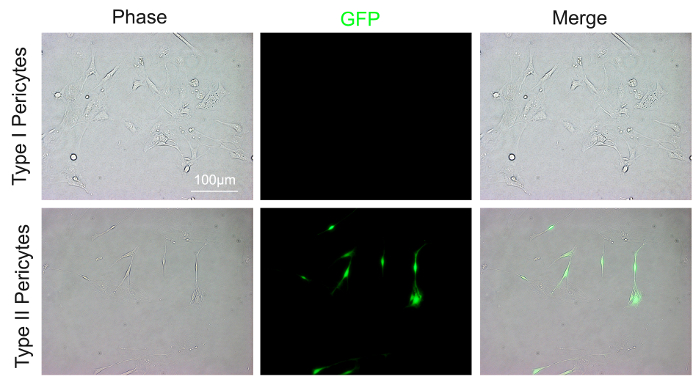
Figure 2: Morphology and Nestin-GFP expression in sorted type I and type II pericytes. Sorted type I and type II pericytes were seeded on coverslips and grown in pericyte medium for 3 days. Type I pericytes showed round cell bodies, with short processes, and no endogenous GFP signal under a fluorescent microscope (excitation Laser: 488 nm; excitation and emission filters: 470/40 nm and 515/30 nm, respectively). Type II pericytes demonstrated small cell bodies, with long and thin processes, and a strong endogenous GFP signal under a fluorescent microscope under the same settings. Scale bar = 100 µm Please click here to view a larger version of this figure.
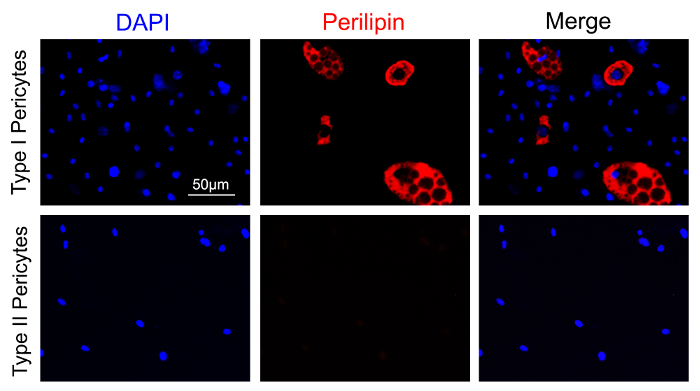
Figure 3: Adipogenic differentiation of sorted type I and type II pericytes. Sorted type I and type II pericytes were grown in pericyte medium for 3 days. They were then differentiated in adipogenic medium for 14 days. The cells were fixed and immunostained with anti-perilipin antibody followed by Alexa555 donkey anti-rabbit antibody. Immunocytochemistry showed that type I, but not type II, pericytes expressed the adipocyte marker perilipin (red) under a fluorescent microscope (excitation laser: 543 nm; excitation and emission filters: 540/45 nm and 600/50 nm, respectively). Scale bar = 50 µm Please click here to view a larger version of this figure.
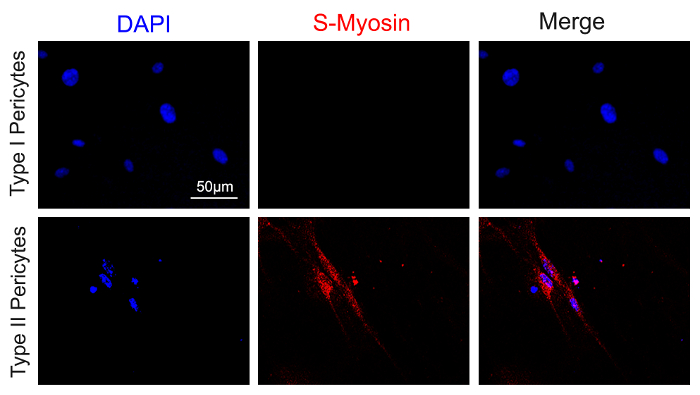
Figure 4: Myogenic differentiation of sorted type I and type II pericytes. Sorted type I and type II pericytes were grown in pericyte medium for 3 days and were then differentiated in myogenic medium for 14 days. The cells were fixed and immunostained with anti-S-myosin antibody and Alexa555 donkey anti-mouse antibody. Immunocytochemistry showed that type II, but not type I, pericytes expressed the mature myotube/myofiber marker S-myosin (red) under a fluorescent microscope (excitation laser: 543 nm; excitation and emission filters: 540/45 nm and 600/50 nm, respectively). Scale bar = 50 µm Please click here to view a larger version of this figure.
| Tubes | Staining |
| Unstained control | Single cell suspension from wildtype mice |
| DAPI single-color control (dead cell exclusion) | Single cell suspension from wildtype mice + DAPI (5 μg/mL) |
| GFP single-color control | Single cell suspension from Nestin-GFP mice |
| PE single-color control | OneComp eBeads + PDGFRβ-PE antibody (4 μg/mL) |
| PE-FMO control | Single cell suspension from Nestin-GFP mice + DAPI (1 μg/mL) |
| Sample | Single cell suspension from Nestin-GFP mice + PDGFRβ-PE antibody (4 μg/mL) + DAPI (1 μg/mL) |
Table 1: Staining protocol for the single-color controls, PDGFRβ-PE-FMO control, and muscle sample.
Discussion
Pericytes are multipotent perivascular cells22,23 located on the abluminal surface of capillaries21,26. In skeletal muscles, pericytes are able to differentiate along the adipogenic and/or myogenic pathways19,20,24. Recent studies revealed two subpopulations of pericytes, with different marker expression and distinct differentiation potentials19,24,25. Type I (NG2+Nestin–) pericytes are adipogenic, while type II (NG2+Nestin+) pericytes are myogenic. The isolation of these subpopulations for in vitro studies requires the NG2-DsRed and Nestin-GFP double-transgenic mouse line. The dependence of this purification protocol on double-transgenic mice significantly limits its application and thus research on the biology of pericyte subpopulations.
This video article illustrates an alternative method for the isolation of type I and type II pericytes from mouse skeletal muscles. This new method still relies on a FACS-based technique for cell separation. However, instead of utilizing transgenic NG2-DsRed fluorescence, it targets the endogenous PDGFRβ signal. This is based on the observation that NG2-DsRed expression co-localizes with PDGFRβ in skeletal muscle19. Compared to the original method, this protocol has several advantages. First, it does not require the NG2-DsRed genetic background, although the Nestin-GFP background is still needed. Second, instead of targeting NG2, this protocol uses PDGFRβ, a well-characterized marker with validated and commercially available antibodies. Third, it allows for the simultaneous isolation of both type I and type II pericytes. For example, PDGFRβ-PE+Nestin-GFP– and PDGFRβ-PE+Nestin-GFP+ cells isolated using this protocol demonstrate distinct differentiation capabilities. Specifically, PDGFRβ-PE+Nestin-GFP–, but not PDGFRβ-PE+Nestin-GFP+, cells differentiate into adipocytes in adipogenic condition, whereas PDGFRβ-PE+Nestin-GFP+, but not PDGFRβ-PE+Nestin-GFP–, cells undergo myogenic differentiation under myogenic condition. These data are consistent with previous reports showing that NG2-DsRed+Nestin-GFP– cells (type I pericytes) are adipogenic and NG2-DsRed+Nestin-GFP+ cells (type II pericytes) are myogenic19,24, suggesting that isolated PDGFRβ-PE+Nestin-GFP– and PDGFRβ-PE+Nestin-GFP+ cells are indeed type I and type II pericytes, respectively. Together, these results suggest that this new protocol allows for the relatively easy isolation/purification of pericyte subpopulations from mouse skeletal muscles, and possibly other tissues. This will promote studies on pericyte biology and their therapeutic translation for various disorders.
It should be noted, however, that there is a limitation of this method due to the use PDGFRβ. It has been shown in a previous study that PW1+ interstitial cells (PICs) also express PDGFRβ20. Therefore, isolated PDGFRβ-PE+Nestin-GFP– and PDGFRβ-PE+Nestin-GFP+ populations may contain PICs. However, the contribution of these PICs to pericyte differentiation is limited, given that pericytes outnumber PICs in skeletal muscles20 and that type I pericytes do not undergo myogenesis19,25.
Using this method, type I and type II pericytes were obtained at yields of 9.5% and 2.1%, respectively. These numbers were comparable to the reported yields of 2.8% and 3.4%, respectively, in the double-transgenic mice19. The slight difference may be due to different gating strategies or digestion protocols. These results again suggest that the proposed protocol can be used to isolate subtypes of pericytes from skeletal muscles.
The critical steps of this protocol include the following points: (1) Make sure that the skeletal muscles are fully minced and able to easily pass through a 10 mL serological pipette. Large muscle blocks interfere with enzymatic digestion and significantly reduce yield. (2) Use freshly made collagenase solution for muscle digestion and allow gentle agitation (35 revolutions/min) during incubation. (3) Keep the controls and sample on ice throughout the staining and sorting steps. (4) Use FMO controls to set up gating boundaries.
In addition to differentiation capability, type I and type II pericytes also show different morphology. Specifically, type I pericytes have round cell bodies, with short processes and relatively large nuclei. Type II pericytes, on the other hand, usually have small cell bodies, with long and thin processes and small nuclei. The morphological differences suggest that type I and type II pericytes are intrinsically different, which is consistent with the heterogeneous nature of pericytes21. What causes/maintains the difference between type I and type II pericytes, as well as the underlying molecular mechanisms, remain unclear and require further investigation. This isolation protocol will help to answer these important questions.
Divulgazioni
The authors have nothing to disclose.
Acknowledgements
This work was partially supported by a Fund-A-Fellow grant from the Myotonic Dystrophy Foundation (MDF-FF-2014-0013) and the Scientist Development Grant from the American Heart Association (16SDG29320001).
Materials
| Cell Sorter | Sony | SH800 | |
| Automatic Setup Beads | Sony | LE-B3001 | |
| DMEM | Gibco | 11995 | |
| Avertin | Sigma | T48402 | |
| Pericyte Growth Medium | ScienCell | 1201 | |
| MSC Basal Medium (Mouse) | Stemcell Technologies | 5501 | |
| Adipogenic Stimulatory Supplement (Mouse) | Stemcell Technologies | 5503 | |
| Fetal Bovine Serum | Gibco | 16000 | |
| Horse Serum | Sigma | H1270 | |
| Collagenase Type 2 | Worthington | LS004176 | |
| 0.25% Trypsin/EDTA | Gibco | 25200 | |
| Penicillin/Streptomycin | Gibco | 15140 | |
| PDL | Sigma | P6407 | |
| PDGFRβ-PE Antibody | eBioscience | 12-1402 | |
| Perilipin Antibody | Sigma | P1998 | |
| S-Myosin Antibody | DSHB | MF-20 | |
| Alexa 555-anti-rabbit antibody | ThermoFisher Scientific | A-31572 | |
| Alexa 555-anti-mouse antibody | ThermoFisher Scientific | A-31570 | |
| Mounting Medium with DAPI | Vector Laboratories | H-1200 | |
| DAPI | ThermoFisher Scientific | D1306 | |
| HEPES | Gibco | 15630 | |
| EDTA | Fisher | BP120 | |
| BSA | Sigma | A2058 | |
| NH4Cl | Fisher Scientific | A661 | |
| KHCO3 | Fisher Scientific | P184 | |
| PBS | Gibco | 14190 | |
| 18G Needles | BD | 305196 | |
| 10ml Serological Pipette | BD | 357551 |
Riferimenti
- Rennert, R. C., Sorkin, M., Garg, R. K., Gurtner, G. C. Stem cell recruitment after injury: lessons for regenerative medicine. Regenerative medicine. 7 (6), 833-850 (2012).
- Sambasivan, R., et al. Pax7-expressing satellite cells are indispensable for adult skeletal muscle regeneration. Development. 138 (17), 3647-3656 (2011).
- Relaix, F., Zammit, P. S. Satellite cells are essential for skeletal muscle regeneration: the cell on the edge returns centre stage. Development. 139 (16), 2845-2856 (2012).
- von Maltzahn, J., Jones, A. E., Parks, R. J., Rudnicki, M. A. Pax7 is critical for the normal function of satellite cells in adult skeletal muscle. Proc Natl Acad Sci U S A. 110 (41), 16474-16479 (2013).
- Lepper, C., Partridge, T. A., Fan, C. M. An absolute requirement for Pax7-positive satellite cells in acute injury-induced skeletal muscle regeneration. Development. 138 (17), 3639-3646 (2011).
- Kuang, S., Charge, S. B., Seale, P., Huh, M., Rudnicki, M. A. Distinct roles for Pax7 and Pax3 in adult regenerative myogenesis. J Cell Biol. 172 (1), 103-113 (2006).
- Murphy, M. M., Lawson, J. A., Mathew, S. J., Hutcheson, D. A., Kardon, G. Satellite cells, connective tissue fibroblasts and their interactions are crucial for muscle regeneration. Development. 138 (17), 3625-3637 (2011).
- Morgan, J. E., Pagel, C. N., Sherratt, T., Partridge, T. A. Long-term persistence and migration of myogenic cells injected into pre-irradiated muscles of mdx mice. J Neurol Sci. 115 (2), 191-200 (1993).
- Beauchamp, J. R., Morgan, J. E., Pagel, C. N., Partridge, T. A. Dynamics of myoblast transplantation reveal a discrete minority of precursors with stem cell-like properties as the myogenic source. J Cell Biol. 144 (6), 1113-1122 (1999).
- Partridge, T. A. Invited review: myoblast transfer: a possible therapy for inherited myopathies. Muscle Nerve. 14 (3), 197-212 (1991).
- Montarras, D., et al. Direct isolation of satellite cells for skeletal muscle regeneration. Science. 309 (5743), 2064-2067 (2005).
- Asakura, A., Seale, P., Girgis-Gabardo, A., Rudnicki, M. A. Myogenic specification of side population cells in skeletal muscle. J Cell Biol. 159 (1), 123-134 (2002).
- Tamaki, T., et al. Skeletal muscle-derived CD34+/45- and CD34-/45- stem cells are situated hierarchically upstream of Pax7+ cells. Stem Cells Dev. 17 (4), 653-667 (2008).
- Dellavalle, A., et al. Pericytes resident in postnatal skeletal muscle differentiate into muscle fibres and generate satellite cells. Nat Commun. 2, 499 (2011).
- Mitchell, K. J., et al. Identification and characterization of a non-satellite cell muscle resident progenitor during postnatal development. Nat Cell Biol. 12 (3), 257-266 (2010).
- Pannerec, A., Formicola, L., Besson, V., Marazzi, G., Sassoon, D. A. Defining skeletal muscle resident progenitors and their cell fate potentials. Development. 140 (14), 2879-2891 (2013).
- Dellavalle, A., et al. Pericytes of human skeletal muscle are myogenic precursors distinct from satellite cells. Nat Cell Biol. 9 (3), 255-267 (2007).
- Kostallari, E., et al. Pericytes in the myovascular niche promote post-natal myofiber growth and satellite cell quiescence. Development. 142 (7), 1242-1253 (2015).
- Birbrair, A., et al. Role of pericytes in skeletal muscle regeneration and fat accumulation. Stem Cells Dev. 22 (16), 2298-2314 (2013).
- Yao, Y., Norris, E. H., Mason, C. E., Strickland, S. Laminin regulates PDGFRbeta(+) cell stemness and muscle development. Nat Commun. 7, 11415 (2016).
- Armulik, A., Genove, G., Betsholtz, C. Pericytes: developmental, physiological, and pathological perspectives, problems, and promises. Dev Cell. 21 (2), 193-215 (2011).
- Dore-Duffy, P. Pericytes: pluripotent cells of the blood brain barrier. Curr Pharm Des. 14 (16), 1581-1593 (2008).
- Dore-Duffy, P., Katychev, A., Wang, X., Van Buren, E. CNS microvascular pericytes exhibit multipotential stem cell activity. J Cereb Blood Flow Metab. 26 (5), 613-624 (2006).
- Birbrair, A., et al. Skeletal muscle pericyte subtypes differ in their differentiation potential. Stem Cell Res. 10 (1), 67-84 (2013).
- Gautam, J., Nirwane, A., Yao, Y. Laminin differentially regulates the stemness of type I and type II pericytes. Stem Cell Research & Therapy. 8 (1), 28 (2017).
- Birbrair, A., et al. Pericytes: multitasking cells in the regeneration of injured, diseased, and aged skeletal muscle. Front Aging Neurosci. 6, 245 (2014).

