Assessment of Blood-brain Barrier Permeability by Intravenous Infusion of FITC-labeled Albumin in a Mouse Model of Neurodegenerative Disease
Summary
In this study, we present an easy and efficient procedure for evaluating the disruption of the blood-brain barrier under neurodegenerative conditions. To achieve our objective, we infused high molecular weight fluorescein isothiocyanate labelled (FITC)-albumin into mouse jugular vein and evaluated its leakage into the brain parenchyma by fluorescence microscopy.
Abstract
Disruption of blood-brain barrier (BBB) integrity is a common feature for different neurological and neurodegenerative diseases. Although the interplay between perturbed BBB homeostasis and the pathogenesis of brain disorders needs further investigation, the development and validation of a reliable procedure to accurately detect BBB alterations may be crucial and represent a useful tool for potentially predicting disease progression and developing targeted therapeutic strategies.
Here, we present an easy and efficient procedure for evaluating BBB leakage in a neurodegenerative condition like that occurring in a preclinical mouse model of Huntington disease, in which defects in the permeability of BBB are clearly detectable precociously in the disease. Specifically, the high molecular weight fluorescein isothiocyanate labelled (FITC)-albumin, which is able to cross the BBB only when the latter is impaired, is acutely infused into a mouse jugular vein and its distribution in the vascular or parenchymal districts is then determined by fluorescence microscopy.
Accumulation of green fluorescent-albumin in the brain parenchyma functions as an index of aberrant BBB permeability and, when quantitated by using Image J processing software, is reported as Green Fluorescence Intensity.
Introduction
Homeostasis within the central nervous system (CNS) is a prerequisite for the proper communication and function of the neuronal cells. The CNS parenchyma is tightly sealed off from the periphery by the endothelial blood-brain barrier (BBB), which represents the interface between the peripheral bloodstream and the brain and plays a pivotal role in the cross-talk between these two districts1,2. The BBB is a complex and dynamic three-dimensional structure mainly composed of specialized micro-vessel endothelial cells (ECs) linked to each other through intercellular junctional complexes – tight junctions (TJs)- and surrounded by pericytes, neuron endings and astrocyte foot processes1,2.
Under physiological conditions, the extremely low permeability of the intact BBB ensures the strict regulation of the transport of nutrients and other molecules into and out of the brain, and provides the CNS with a unique protection from changes occurring in the composition of the blood that might influence neural activity and against potential peripheral insults1,2,3.
Disruption of BBB integrity and its enhanced permeability has long been known to constitute a key feature for many neurological and neurodegenerative disorders4 including Huntington's Disease (HD)5,6, however, whether such a dysfunction is a causative phenomenon or a propagative event in the course of the disease is still unclear. The timing of BBB breakdown also remains elusive, however, emerging evidence by our group and others indicates that disrupted BBB integrity does not represent a late event in the disease progression, but rather an early step6,7,8, which may have long-term consequences.
With this in mind, it is important to precociously reveal BBB breakdown in neurodegeneration in order to develop strategies useful to predict disease progression and brain damage and to develop alternative and more targeted interventions capable of successfully mitigating the clinical consequences of such a disruption. Reliable imaging of BBB impairment is, therefore, of major importance in both experimental research and clinical management of brain diseases.
In this paper, we describe a successful and simple procedure for the evaluation of BBB permeability in a HD mouse model by using the high molecular weight fluorescein isothiocyanate labelled-albumin (FITC-albumin). Extravasation of FITC-albumin, which normally cannot cross the barrier, into the brain parenchyma was measured as an index of BBB leakage. This technique is readily adaptable to rats and to other pathological conditions characterized by cerebrovasculature impairment9,10.
Protocol
All procedures on animals were approved by the IRCCS Neuromed Animal Care Review Board and by "Istituto Superiore di Sanità" (permit number: 1163/2015- PR) and were in accordance with the guidelines of the EU Directive 2010/63/EU for animal experiments.
1. Preparation of FITC-albumin Solution to be Injected into Jugular Vein
NOTE: All experiments were carried out in manifest (11-week old) HD R6/2 mice and in age and gender-matched wild-type (WT) control littermates.
- Dissolve FITC-albumin powder (see Table of Materials) in phosphate buffered saline (PBS) at 10 mg/ml.
NOTE: For optimal results, this should be prepared fresh for each labelling reaction.
2. Preparation of Equipment to Be Used During Surgery
- Lay out the previously autoclaved surgical instruments (see Table of Materials).
- Clean the surgical bench with alcohol (70% ethanol solution). Prepare the surgical area (45 cm x 45 cm) with a surgical sterile disposable towel drape and set up the operating stereomicroscope.
3. Surgical Procedure for FITC-albumin Infusion into the Jugular Vein
- Record mouse body weight for anesthesia.
- Anesthetize mouse with an intraperitoneal injection (i.p) of an appropriate dose of Ketamine (100 mg/kg) – xylazine (10 mg/kg) solution.
- Monitor animal sedation by gentle toe pinch withdraw reflex as demonstrated in Walantus et al.11,12.
- Place the anesthetized mouse in a face-up supine position and fix the four legs and the tail on the surgical workbench with adhesive tape.
- After the mouse is secured, carefully shave the appropriate surgical margin on the neck with an electric razor (see Table of Materials) and disinfect it with povidone-iodine solution and 70% ethanol.
- Dry the exposed shaved surface with cotton swabs.
- Gently pull up the shaved skin and make a 1.5 cm long longitudinal incision, by scalpel, in the midclavicular line starting midway on the thorax and extending to just below the neck.
- Carefully stretch apart connective tissue with forceps under microscopic observation (5X magnification) to expose the external jugular vein.
- Ensure a sufficient space on the right and on the left of the jugular vein to facilitate the insertion of the 30½ G needle for the infusion of the FITC-albumin solution.
- Inject the animal intravenously (right jugular vein) slowly (over 3 min) with 100 µL of 10 mL/kg FITC-albumin by using the 30½ G needle.
- After 15 minutes, euthanize the mouse by decapitation, rapidly remove the brain from the skull as previously described13, and immerse it for 15 min in at least 10x the volume of the brain itself of pre-chilled isopentane.
- Move the isopentane frozen brain into a pre-chilled tube and store it into a -80 °C freezer until histological sectioning.
4. Histological Sectioning of Brain Infused with FITC-albumin
- Embed frozen brain in optimal cutting temperature (OCT) compound (see Table of Materials) prior to cryostat sectioning.
- Serially cut the brain into 20 µm thick sections, with a cryostat (see Table of Materials), and mount them onto microscope glass slides.
- Perform the FITC-Albumin/laminin co-staining by using a specific anti-laminin antibody (see Table of Materials) as previously described6.
- Briefly, incubate sections with the primary antibody anti-laminin (pAb 1:1000) over night and proceed with appropriate secondary antibody conjugated to Cy3.
- Coverslip slides with mounting medium containing 4',6-diamidino-2-phenylindole (DAPI) (absorption peak = 360 nm; emission peak = 460 nm (see Table of Materials).
- Leave the slide to dry overnight at 4 °C in the dark.
- Observe the fluorescence staining under a fluorescence microscope (see Table of Materials) at 20X magnification equipped with both FITC (absorption peak = 495 nm; emission peak = 525 nm) and Cy3 filters (absorption peak = 550 nm; emission peak = 570 nm).
- To view the samples immediately after the staining, secure the coverslip at the corners using nail polish and examine them under the microscope as above (4.3.3).
5. Setting Up the Fluorescence Microscope and Acquisition of Colour Images
- Set up the fluorescence microscope as follows.
- Turn on the fluorescence lamp about 10 minutes before use. Turn on the microscope, the connected computer, and the digital camera. Run the image analysis software (see Table of Materials).
- Use the appropriate objective for optimal signal collection and spatial resolution, and select appropriate filters.
- Acquisition of Colour Images
- Select the [live] command on the image analysis software (see Table of Materials) and set up the optimal illumination parameters for each filter.
- Select the [freeze] command to take the single channel colour image and add the scale bar ('Edit>Burn Scale')
- Save each image in the '.tiff' format.
- Merge channels into a single image by selecting 'File>Merge channel' and save it in the
- tiff' format.
- Acquire a minimum of four twenty-four bit color pictures (2200 µm x 3400 µm) per brain slice (6 sections per slide).
6. Assessment of FITC-albumin Extravasation
- Analyze the fluorescence intensity for each single channel by using the freely available ImageJ14,15.
- Normalize the total fluorescent signal intensity of the FITC-albumin by the total CY3-Laminin fluorescent intensity per each image and report the extravasated albumin outside the vessels as Green Fluorescence Intensity.
Representative Results
Proper infusion of FITC-albumin into the jugular vein results in the extravasation of the green fluorescence tracer from the bloodstream into the brain parenchymawhen the BBB is compromised6. Under physiological conditions, distribution of infused fluorescent albumin is restricted to the inside of the brain blood vessels and no signal in the surrounding perivascular area and/or brain parenchyma is detectable (Figure 1A, micrographs on the top). Conversely, when the BBB is compromised under neuro-pathological circumstances, FITC-albumin shows a diffuse fluorescence pattern along perivascular spaces and in the brain parenchyma (Figure 1A, micrographs on the bottom). Co-staining with the vascular marker, laminin, is used to clearly localize site of FITC-albumin accumulation (Figure 1A). Increased green fluorescence intensity emitted by FITC-albumin in compromised BBB was assessed by using an image processing software (see Table of Materials) and reported as Green Fluorescence Intensity (N = 3; 0.3112 vs 0.5765; ****, p = 0.0004, Unpaired T-test) (Figure 1B). FITC-albumin intensity was normalized as reported above.
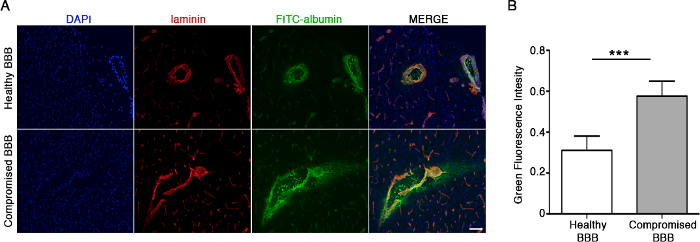
Figure 1: FITC-Albumin extravasation as a marker of BBB damage. (A) Representative fluorescence micrographs of brain cryosections showing differential distribution of green fluorescent albumin under both physiological (top) and pathological conditions (bottom). Red staining represents the vascular marker laminin. Exposure times: DAPI 20 ms, FITC 500 ms, Tx Red 60 ms. Scale bar = 100 µm (merge monograph). (B) This graph displays the quantification of the green fluorescence emitted by FITC-albumin, which is reported as the Green Fluorescence Intensity (see step 6.2), in both physiological (healthy BBB) and pathological conditions (compromised BBB). N = 3. Data are represented as mean ± SD, *** p = 0.0004 (Unpaired T-test). Please click here to view a larger version of this figure.
Discussion
The technique we describe here is primarily useful for detecting BBB leakage under brain disease conditions. BBB dysfunction is gaining attention as a common feature of diverse neurologic disorders4. We previously used this approach to describe the early derangement of BBB integrity in a mouse model of a rare neurodegenerative disease like HD6.
This method takes advantage of the relative simplicity and effectiveness of the procedure, which provides accurate and reliable results. It represents a highly sensitive preclinical research tool and is useful for the speed and immediacy it offers in qualitatively assessing BBB disruption by simple visualization of green fluorescence within sectioned brain tissue. However, when combined with biochemical and molecular analysis, the technique is successful in providing quantitative information about the structural and functional integrity of vascular endothelial TJs and permits analysis of the correlation of BBB disruption with brain homeostasis.
Although it cannot provide any information about the BBB ultrastructure, the protocol described here may result in less expensive and time-demanding procedures in comparison with other methods such as high resolution electron microscopy16,17. Moreover, due to its easy application and reproducibility, the procedure may be useful for monitoring the progressive lesions of brain vasculature which characterize neurodegenerative disorder pre-clinical models.
A great strength of assessing BBB disruption by a fluorescence-based approach lies in the potential to investigate the role of subtle changes in BBB function in common and rare neurological disorders and eventually to directly test in real time the effect of BBB-influencing drugs or endogenous molecules when applied from the blood or brain compartment. Although the procedure cannot yet be translated to humans, it has the potential to provide indications for the development of new brain tracers to be used in clinical practice in the future.
Divulgazioni
The authors have nothing to disclose.
Acknowledgements
This work was supported by "Fondazione Neuromed" and funded by the Italian Ministry of Health "Ricerca Corrente" to V.M.
Materials
| Albumin-fluorescin isothiocyanate conjiugate | SIGMA | A9771-100MG | |
| pAb anti-Laminin | Novus Biologicals | NB300-144 | |
| CY3 anti-rabbit made in goat | MILLIPORE | AP132 | |
| SUPERFROST PLUS | Thermo Scientific | J1800AMNZ | |
| Cover Slips 24 X 50 mm | Thermo Scientific (DIAPATH) | 61050 | |
| Kilik Optimal Cutting Temperature (OCT) compound | Bio Optica | 05-9801 | |
| VECTASHIELD Mounting Media | VECTOR | H-1500 | Mounting media with DAPI |
| iNSu/Light Insulin Disposible Syringe | RAYS Health &Safety | INS1ML26G13 | |
| 30G 1/2" | BD Microlance | 304000 | Needle for Insulin disposible Syringe |
| Scalpel Handle | F.S.T. | 91003-12 | |
| #22 Disposable Scalpel blads | F.S.T. | 10022-00 | |
| Fine Iris scissors 10.5 cm | F.S.T. | 14094-11 | |
| Dumont Forceps #5745 45° 0.10 x 0.06 mm | F.S.T. | 11251-35 | |
| Graefe Forceps 10 cm | F.S.T. | 11051-10 | |
| Dumont Forceps #5 0.1 X 0.06 mm | F.S.T. | 11251-20 | |
| Medical patch | Medicalis | 34788 | |
| Sterile disposable towel drape | Dispotech | TVO50VE | |
| Stereoscopic Microscope | NIKON | SMZ 745 T | |
| Optic Illuminator LED light (C-FLED2) | NIKON | 1003167 | Optic Illuminator for Stereoscopic Micrscope |
| Eclipse Ni-U Microscope | Nikon | 932162 | Epifluorescence Microscope |
| Microscope digital Camera | Nikon | DS-Ri2 | Microscope camera |
| Intenslight | Nikon | C-HGFI | Microscope lamp |
| NIS-Elements 64 bit | Nikon | AR 4.40.00 | Analysis Software |
| Electric Razor | Gemei | GM-3007 |
Riferimenti
- Obermeier, B., Verma, A., Ransohoff, R. M. The blood-brain barrier. Handb Clin Neurol. 133, 39-59 (2016).
- Serlin, Y., Shelef, I., Knyazer, B., Friedman, A. Anatomy and physiology of the blood-brain barrier. Semin Cell Dev Biol. 38, 2-6 (2015).
- Moretti, R., et al. Blood-brain barrier dysfunction in disorders of the developing brain. Front Neurosci. 9, 40 (2015).
- Zhao, Z., Nelson, A. R., Betsholtz, C., Zlokovic, B. V. Establishment and Dysfunction of the Blood-Brain Barrier. Cell. 163 (5), 1064-1078 (2015).
- Drouin-Ouellet, J., et al. Cerebrovascular and blood-brain barrier impairments in Huntington’s disease: Potential implications for its pathophysiology. Ann Neurol. 78 (2), 160-177 (2015).
- Di Pardo, A., et al. Impairment of blood-brain barrier is an early event in R6/2 mouse model of Huntington Disease. Sci Rep. 7, 41316 (2017).
- Lecler, A., Fournier, L., Diard-Detoeuf, C., Balvay, D. Blood-Brain Barrier Leakage in Early Alzheimer Disease. Radiology. 282 (3), 923-925 (2017).
- van de Haar, H. J., et al. Blood-Brain Barrier Leakage in Patients with Early Alzheimer Disease. Radiology. 282 (2), 615 (2017).
- Fernandez-Lopez, D., et al. Blood-brain barrier permeability is increased after acute adult stroke but not neonatal stroke in the rat. J Neurosci. 32 (28), 9588-9600 (2012).
- Yang, Y., Rosenberg, G. A. Blood-brain barrier breakdown in acute and chronic cerebrovascular disease. Stroke. 42 (11), 3323-3328 (2011).
- Walantus, W., Castaneda, D., Elias, L., Kriegstein, A. In utero intraventricular injection and electroporation of E15 mouse embryos. J Vis Exp. (6), e239 (2007).
- Szot, G. L., Koudria, P., Bluestone, J. A. Transplantation of pancreatic islets into the kidney capsule of diabetic mice. J Vis Exp. (9), e404 (2007).
- Gage, G. J., Kipke, D. R., Shain, W. Whole animal perfusion fixation for rodents. J Vis Exp. (65), (2012).
- McCloy, R. A., et al. Partial inhibition of Cdk1 in G 2 phase overrides the SAC and decouples mitotic events. Cell Cycle. 13 (9), 1400-1412 (2014).
- Burgess, A., et al. Loss of human Greatwall results in G2 arrest and multiple mitotic defects due to deregulation of the cyclin B-Cdc2/PP2A balance. Proc Natl Acad Sci U S A. 107 (28), 12564-12569 (2010).
- Krueger, M., Hartig, W., Reichenbach, A., Bechmann, I., Michalski, D. Blood-brain barrier breakdown after embolic stroke in rats occurs without ultrastructural evidence for disrupting tight junctions. PLoS One. 8 (2), e56419 (2013).
- Hirano, A., Kawanami, T., Llena, J. F. Electron microscopy of the blood-brain barrier in disease. Microsc Res Tech. 27 (6), 543-556 (1994).

